Académique Documents
Professionnel Documents
Culture Documents
Chapter8 PhaseDiagram Handouts
Transféré par
wagdy87Description originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Chapter8 PhaseDiagram Handouts
Transféré par
wagdy87Droits d'auteur :
Formats disponibles
Chapter 8 Phase Diagrams
A phase in a material is a region that differ in its microstructure and or composition from another region
Al2CuMg
H2O(solid, ice) in H2O (liquid) 2 phases homogeneous in crystal structure and atomic arrangement have same chemical and physical properties throughout have a definite interface and able to be mechanically separated from its surroundings
Al
Chapter 8 in Smith & Hashemi Additional resources: Callister, chapter 9 and 10 Chapter 8
Phase diagram and degrees of freedom
A phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically-distinct phases; or to show what phases are present in the material system at various T, p, and compositions
equilibrium is important: phase diagrams are determined by using slow cooling conditions no information about kinetics
Degree of freedom (or variance) F is the number of variables (T, p, and composition) that can be changed independently without changing the phases of the system
Phase diagram of CO2 Chapter 8
8.1 Phase Diagram of Water
3 phases: solid, liquid, vapour Triple point:
4.579 Torr (~603Pa), 0.0098oC
Field 1 phase Line phase coexistence, 2 phases Triple point 3 phases
Chapter 8
8.2 Gibbs Phase Rule
Gibbs' phase rule describes the possible # of degrees of freedom (F) in a closed system at equilibrium, in terms of the number of separate phases (P) and the number of chemical components (C) in the system (derived from thermodynamic principles by Josiah W. Gibbs in the 1870s) F+P=C+2
F is # of degrees of freedom or variance P is # of phases C is # of components Component is the minimum # of species necessary to define the composition of the system H2O C=1 (i) P=1, F=2; (ii) P=2, F=1; (iii) P=3, F=0
Chapter 8 4
8.3 How to construct phase diagrams? Cooling curves
Cooling curves: used to determine phase transition temperature record T of material vs time, as it cools from its molten state through solidification and finally to RT (at a constant pressure!!!) The cooling curve of a pure metal BC: plateaue or region of thermal arrest; in this region material is in the form of solid and liquid phases CD: solidification is completed, T drops
Chapter 8
Cooling curve for pure iron @ 1atm
As T : melted iron (liquid) bcc Fe, (solid) fcc Fe, (solid) bcc Fe, (RT)
Chapter 8
8.4 Binary systems (C = 2)
F+P=C+2=4 F=4-P
Degrees of freedom (F): p, T, composition At p = const (or T=const)
F=3-P
0 composition
100% A
weight % of B
100%
100% B
1. Two components are completely mixable in liquid and solid phase (form a solid state solution), and dont react chemically 2. Two components (A and B) can form stable compounds or alloys (for example: A, A2B, A3B, B) Chapter 8 7
Binary Isomorphous Alloy System (C=2)
Isomorphous: Two elements are completely soluble in each other in solid and liquid state; substitutional solid state solution can be formed; single type of crystal str. exist
Reminder: Hume-Rothery rules: (1) atoms have similar radii; (2) both pure materials have same crystal structure; (3) similar electronegativity (otherwise may form a compound instead); (4) solute should have higher valence
Example: Cu-Ni phase diagram (only for slow cooling conditions) Liquidus line: the line connecting Ts at which liquid starts to solidify under equilibrium conditions Solidus: the temperature at which the last of the liquid phase solidifies Between liquidus and solidus: P =2
Chapter 8
53 wt% Ni 47 wt% Cu at 1300oC
P=1 F=3P=2
P=2;F=3P=1
contains both liquid and solid phases neither of these phases can have average composition 53 wt% Ni 47 wt% Cu draw a tie line at 1300oC from the graph: composition of liquid phase wL = 45% and solid phase wS = 58% at 1300oC
Chapter 8
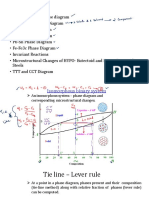
8.5 The Lever Rule
The weight percentages of the phases in any 2 phase region can be calculated by using the lever rule Consider the binary equilibrium phase diagram of elements A and B that are completely soluble in each other
Co
Mass fraction of B
Let x be the alloy composition of interest, its mass fraction of B (in A) is C Let T be the temperature of interest at T alloy x consists of a mixture of liquid (with CL - mass fraction of B in liquid) and solid (CS - mass fraction of B in solid phase)
Chapter 8
10
Lever Rule (cont.)
Chapter 8
11
Q.: A Cu-Ni alloy contains 47 wt % Cu and 53% of Ni and is at 1300oC. Use Fig.8.5 and answer the following: A. What is the weight percent of Cu in the liquid and solid phases at this temperature? B. What weight percent of this alloy is liquid and what weight percent is solid?
Chapter 8
12
8.6 Nonequilibrium Solidification of Alloys
constructed by using very slow cooling conditions
Atomic diffusion is slow in solid state; as-cast microstructures show core structures caused by regions of different chemical composition
As-cast 70% Cu 30% Ni alloy showing a cored structure Chapter 8 13
Nonequilibrium Solidus
Solidification of a 70% Ni-30%Cu alloy
Fig. 8.9, Smith
Schematic microstructures at T2 and T4
Fig.8.10, Smith
each core structure will have composition gradient 1-7 additional homogenization step is often required (annealing <T7)
Chapter 8 14
8.7 Binary Eutectic Alloy System
Components has limited solid solubility in each other Example: cooling 60%Pb 40%Sn system
_T Liquid eutectic a solid solution + b solid solution
This eutectic reaction is called an invariant reaction occurs under equilibrium conditions at specific T and alloy composition F=0 at eutectic point
Chapter 8
15
Solubility Limit: Water-Sugar
Changing T can change # of phases: path A to B. Changing Co can change # of phases: path B to D
B (100,70) 10 0
Temperature (C)
1 phase
D(100,90)
2 phases
80 60 40 20 0
0
L
(liquid)
L
(liquid solution
i.e., syrup)
+ S
(solid sugar)
A(70, 20 )
2 phases
20 40 60 70 80 C o =Composition (wt% sugar)
Chapter 8
10 0
Adapted from Callister 16
Binary Eutectic Alloy System
Figure 8.13, Smith
Chapter 8
17
Q: A lead-tin (Pb Sn) alloy contains 64 wt % proeutectic () and 36% eutectic + at 183oC T. Using Figure 8.13, calculate the average composition of this alloy.
Chapter 8
18
8.8 Binary Peritectic Alloy System
The melting points of the two components are quite different A liquid phase reacts with the solid phase to form a new and different solid phase Liquid +
Chapter 8
19
Binary Peritectic Alloy System (cont.)
Chapter 8
20
8.9 Binary monotectic systems
Monotectic reaction: a liquid phase transforms into a solid phase and another liquid phase L1 + L2
Chapter 8
21
8.10 Invariant Reactions
To summarize: 5 invariant reactions (F = 0) 1. Eutectic Liquid 2. Eutectoid 3. Peritectic Liquid + 4. Peritectoid + 5. Monotectic L1
+ + + L2
The eutectic and eutectoid reactions are similar in that they both involve the decomposition of a single phase into two solid phases. The oid suffix indicates that a solid, rather than liquid, phase is decomposing.
Chapter 8
22
8.11 Phase Diagrams with Intermediate Phases and Compounds
Terminal phase: a solid solution of one component in another for which one boundary of the phase field is a pure component Intermediate phase: a phase whose composition range is between those of terminal phases
Chapter 8
23
Ti-Si-O system
Experiment (700-1000oC) Ti + SiO2 Ti5Si3 and TiOy At equilibrium the system will be in TiSix TiOy SiO2 three phase region (from calculations) Ti5Si3 TiO SiO2 three phase region determined experimentally and remaining tie lines can be inferred
Chapter 8
24
8.12 Ternary Phase Diagram
F+P=C+2 For p = 1atm, T = const (isoterms)
Cr-Fe-Ni alloy stainless steel
Chapter 8
25
Three and four component system
AB + AC = 2A + BC G = (2GA + GBC) (GAB + GAC) If G <0, there is tie line between A and BC The remaining tie lines cannot cross
A
AB + AC + AD = 3A + BCD G = (3GA + GBCd) (GAB + GAC + GAD)
AB
AC
Two phase equilibrium is represented by a tie line If G <0, there is a tie line between A and BCD; otherwise plane connects AB-AC-AD
26
BC
Chapter 8
The Ti-Si-N-O quaternary phase diagram
Entire phase diagram can be calculated by taking into account all possible combinations of reactions and products 4 ternary diagrams of Ti-Si-N, Ti-N-O, Ti-Si-O and Si-N-O were evaluated additional quaternary tie lines from TiN to SiO2 and Si2N2O stable metallization bilayer of TiN and TiSi2 in contact with SiO2
A.S.Bhansali, et al., J.Appl.Phys. 68(3) (1990) 1043 Z.Chen, et al., Phys.Stat.Sol.B 241(10) (2004) 2253 Chapter 8 27
Vous aimerez peut-être aussi
- A Modern Course in Statistical PhysicsD'EverandA Modern Course in Statistical PhysicsÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Chapter 4-Phase DiagramDocument16 pagesChapter 4-Phase Diagramtky96Pas encore d'évaluation
- Binary Alloy Phase DiagramsDocument12 pagesBinary Alloy Phase Diagramseeng.ali651550% (2)
- Reviews in Computational ChemistryD'EverandReviews in Computational ChemistryAbby L. ParrillPas encore d'évaluation
- Ch8 PhaseDiagramDocument20 pagesCh8 PhaseDiagramThrishnaa BalasupurManiamPas encore d'évaluation
- Materials Science - Alloys System - 2Document16 pagesMaterials Science - Alloys System - 2Sumit JoshiPas encore d'évaluation
- EEAQ 2118 - 2 Phase Diagrams and Ferrous AlloysDocument52 pagesEEAQ 2118 - 2 Phase Diagrams and Ferrous AlloysOdhiambo AustinPas encore d'évaluation
- Eutectics: Lecture 3 Manufacturing TechnologyDocument24 pagesEutectics: Lecture 3 Manufacturing TechnologyAyush BhadauriaPas encore d'évaluation
- Phase DiagramDocument47 pagesPhase DiagramMuhammed MansPas encore d'évaluation
- PHASE DIAGRAMS AND MICROSTRUCTURESDocument38 pagesPHASE DIAGRAMS AND MICROSTRUCTURESNameIs RajPas encore d'évaluation
- MM235 - Phase Diagram - SMDocument18 pagesMM235 - Phase Diagram - SMUtkarsh MishraPas encore d'évaluation
- Phase Diagrams Chapter 9Document39 pagesPhase Diagrams Chapter 9eeng.ali6515Pas encore d'évaluation
- Chapt 08Document21 pagesChapt 08Jesse McClure100% (5)
- Phase DiagramsDocument21 pagesPhase DiagramsJnrPas encore d'évaluation
- Ch10 Phase DiagramsDocument79 pagesCh10 Phase DiagramsDhileepan KumarasamyPas encore d'évaluation
- Phase DiagramsDocument79 pagesPhase DiagramsArun V NairPas encore d'évaluation
- CHAPTER 3 Phase Diagram TTT HT - 1stDocument25 pagesCHAPTER 3 Phase Diagram TTT HT - 1stAriff AziziPas encore d'évaluation
- Eng Mat Chapter 4Document126 pagesEng Mat Chapter 4VC Chua Yee LeongPas encore d'évaluation
- New civil module5 PDF notesDocument18 pagesNew civil module5 PDF notesDrMohan KumarPas encore d'évaluation
- Phase Diagram ExDocument23 pagesPhase Diagram ExTey KaijingPas encore d'évaluation
- Phase DiagramsDocument50 pagesPhase DiagramsIbrahim MalikPas encore d'évaluation
- Chapter 9: Phase Diagrams: Issues To Address..Document38 pagesChapter 9: Phase Diagrams: Issues To Address..yunlu0705Pas encore d'évaluation
- Phase Diagrams: Lecture 2 (Manufacturing Technology)Document21 pagesPhase Diagrams: Lecture 2 (Manufacturing Technology)Ayush BhadauriaPas encore d'évaluation
- Chapter 9: Phase Diagrams: Issues To Address..Document41 pagesChapter 9: Phase Diagrams: Issues To Address..Faiz AkhtarPas encore d'évaluation
- Chapter 8 - Phase Diagram PART2Document26 pagesChapter 8 - Phase Diagram PART2Mohd IqbalPas encore d'évaluation
- Unit 1 MsDocument126 pagesUnit 1 MsHarishPas encore d'évaluation
- Phase Diagrams TutorialDocument5 pagesPhase Diagrams TutorialUmmi Kalthum MohamadPas encore d'évaluation
- Phase Diagrams Material ScienceDocument46 pagesPhase Diagrams Material ScienceSabir Ali100% (1)
- Chapter 9Document57 pagesChapter 9Pavan PonnadaPas encore d'évaluation
- Phase Rule 1Document62 pagesPhase Rule 1arpitpandey494Pas encore d'évaluation
- Lecture06and07S Oct12Document22 pagesLecture06and07S Oct12ali_b1367Pas encore d'évaluation
- Phase Diagrams ExplainedDocument222 pagesPhase Diagrams ExplainedFAIQPas encore d'évaluation
- 02 Phase DiagramsDocument24 pages02 Phase DiagramsPalash SwarnakarPas encore d'évaluation
- Phase Diagrams & Heat Treatment of Carbon SteelDocument84 pagesPhase Diagrams & Heat Treatment of Carbon SteelTanmay DuttaPas encore d'évaluation
- 10.phase Diagrams PDFDocument24 pages10.phase Diagrams PDFMumpuniLuthfiPas encore d'évaluation
- PHASE RULE: IntroductionDocument48 pagesPHASE RULE: Introductiontenguria samriddhPas encore d'évaluation
- Ceramic Phase Equilibrium Diagrams PDFDocument42 pagesCeramic Phase Equilibrium Diagrams PDFPelita Mu'minatus SholihahPas encore d'évaluation
- Week 9 10 - 1 - IPE 2203-LecturesDocument86 pagesWeek 9 10 - 1 - IPE 2203-LecturesMD Al-AminPas encore d'évaluation
- Lecture 1 - Phase EquilibriumDocument73 pagesLecture 1 - Phase EquilibriumAliah Izzah0% (1)
- 98materials Phase DiagramsDocument27 pages98materials Phase DiagramsHari PrasathPas encore d'évaluation
- Ch. 9 Phase DiagramsDocument9 pagesCh. 9 Phase Diagramsravi hargassnerPas encore d'évaluation
- CHE 414 PPTDocument21 pagesCHE 414 PPTLooking forwardPas encore d'évaluation
- L8 Binary Phase Diagrams PDFDocument78 pagesL8 Binary Phase Diagrams PDFSudeepta MondalPas encore d'évaluation
- (Chapter 8) LC 8Document44 pages(Chapter 8) LC 8venosyah devanPas encore d'évaluation
- 4Document63 pages4ARJUN PESARU 19BME0161Pas encore d'évaluation
- Issues To Address... : - When We Combine Two Elements... - in Particular, If We Specify... Then..Document34 pagesIssues To Address... : - When We Combine Two Elements... - in Particular, If We Specify... Then..arif_ashraf94Pas encore d'évaluation
- Chapter 4Document68 pagesChapter 4Ermias GuragawPas encore d'évaluation
- Thermal Equilibrium DiagramDocument65 pagesThermal Equilibrium DiagramShohel RanaPas encore d'évaluation
- Phase Rule - Phase Diagram - Lever Rule - Microstructural Development During Slow CoolingDocument35 pagesPhase Rule - Phase Diagram - Lever Rule - Microstructural Development During Slow CoolingSiratullah ShahPas encore d'évaluation
- Phase Diagram 1Document23 pagesPhase Diagram 1Tony StarkPas encore d'évaluation
- Ch-7 Compatibility ModeDocument70 pagesCh-7 Compatibility Modedreamgurl9011Pas encore d'évaluation
- Phase DiagramsDocument80 pagesPhase DiagramsWilliams AkandiPas encore d'évaluation
- Module - 3, Iron - Carbon SystemDocument43 pagesModule - 3, Iron - Carbon SystemSayaf Khan PathanPas encore d'évaluation
- 1.1 Diagramas de FasesDocument60 pages1.1 Diagramas de FasesAlex Pelinco ApazaPas encore d'évaluation
- Phase DiagramDocument33 pagesPhase DiagramAlan TehPas encore d'évaluation
- 2008 ExamaaaaaaaaaaaaaaaDocument7 pages2008 ExamaaaaaaaaaaaaaaabbteenagerPas encore d'évaluation
- Chapter 6 (I-II) Phase DiagramDocument34 pagesChapter 6 (I-II) Phase Diagrammdipanwita48Pas encore d'évaluation
- 8 - Phase Diagram 0 PDFDocument28 pages8 - Phase Diagram 0 PDFAtif IrshadPas encore d'évaluation
- Chapter 8 (Heat Treatment)Document12 pagesChapter 8 (Heat Treatment)Shekinah KanindaPas encore d'évaluation
- Effects of Hot-Forging and SubsequentDocument14 pagesEffects of Hot-Forging and Subsequentwagdy87Pas encore d'évaluation
- Teach Yourself Phase DiagramsDocument25 pagesTeach Yourself Phase Diagramswagdy87Pas encore d'évaluation
- Effect of Annealing Treatment On MicrostructuralDocument11 pagesEffect of Annealing Treatment On Microstructuralwagdy87Pas encore d'évaluation
- Optimize Inventory Costs with EOQ and EPQ ModelsDocument40 pagesOptimize Inventory Costs with EOQ and EPQ Modelswagdy87Pas encore d'évaluation
- Testing of MaterialsDocument35 pagesTesting of MaterialsArvin ArviniPas encore d'évaluation
- RollingDocument18 pagesRollingwagdy87Pas encore d'évaluation
- N150 Wireless ADSL2+ Modem Router DGN1000: User ManualDocument107 pagesN150 Wireless ADSL2+ Modem Router DGN1000: User ManualGreg ScottPas encore d'évaluation
- Chapter 3b Miller IndicesDocument52 pagesChapter 3b Miller Indiceswagdy87Pas encore d'évaluation
- Ss 3Document12 pagesSs 3wagdy87Pas encore d'évaluation
- Tensile Strength of Mild SteelDocument11 pagesTensile Strength of Mild SteelPavan Ps50% (2)
- Solutions To Problem Set 1Document6 pagesSolutions To Problem Set 1wagdy87100% (2)
- Materials For Roads and Pavements: Standard Terminology Relating ToDocument4 pagesMaterials For Roads and Pavements: Standard Terminology Relating ToOscar VillaPas encore d'évaluation
- KF ChemicalsDocument52 pagesKF ChemicalsKevin FriasPas encore d'évaluation
- PWD 2018 Question PaperDocument12 pagesPWD 2018 Question PaperDhanjit SonowalPas encore d'évaluation
- Biochemistry Assignment-Week 1: NPTEL Online Certification Courses Indian Institute of Technology KharagpurDocument8 pagesBiochemistry Assignment-Week 1: NPTEL Online Certification Courses Indian Institute of Technology KharagpurSamarjeet Kumar SinghPas encore d'évaluation
- Benzalkonium ChlorideDocument1 pageBenzalkonium ChlorideVaibhav SankhePas encore d'évaluation
- Chemical Bonding New PDFDocument52 pagesChemical Bonding New PDFAniruddha KawadePas encore d'évaluation
- Graphene PropertiesDocument6 pagesGraphene PropertiesAwais BodlaPas encore d'évaluation
- Organic Chemistry II: 17 Basic Organic NamingDocument15 pagesOrganic Chemistry II: 17 Basic Organic NamingNahida HossainPas encore d'évaluation
- Sample Questions - Chapter 4Document6 pagesSample Questions - Chapter 4Rasel IslamPas encore d'évaluation
- GCSE CHEM Past Papers Mark Schemes Modified MayJune Series 2017 21801Document25 pagesGCSE CHEM Past Papers Mark Schemes Modified MayJune Series 2017 21801LubzPas encore d'évaluation
- Chem Unit 5 Transition Metals QuestionsDocument31 pagesChem Unit 5 Transition Metals Questionsareyouthere920% (1)
- EmulsionDocument42 pagesEmulsionShakeel IjazPas encore d'évaluation
- Sodium Sulfate PropertiesDocument5 pagesSodium Sulfate PropertiesPuji TharahPas encore d'évaluation
- Astm D3547 - 1 (En)Document2 pagesAstm D3547 - 1 (En)svvasin2013Pas encore d'évaluation
- G.O.C. Iws-1Document50 pagesG.O.C. Iws-1Lakshya ChandakPas encore d'évaluation
- HILCO Stick Electrodes at A GlanceDocument6 pagesHILCO Stick Electrodes at A Glanceziaee950Pas encore d'évaluation
- Chemical & Ionic Equilibrium - FDocument12 pagesChemical & Ionic Equilibrium - FAshwin BalajiPas encore d'évaluation
- MCPADocument142 pagesMCPAThein Zaw MinPas encore d'évaluation
- Advantages Disadvantages Soil Stabilization Using LimeDocument11 pagesAdvantages Disadvantages Soil Stabilization Using Limeamira hussein100% (2)
- United States Patent (10) Patent No.: US 9,096,564 B2Document6 pagesUnited States Patent (10) Patent No.: US 9,096,564 B2Ahmad InterestaPas encore d'évaluation
- Hydrogen-induced cracking of hardened steel fastenerDocument9 pagesHydrogen-induced cracking of hardened steel fastenerSinan ChenPas encore d'évaluation
- Thermodynamics and Calorimetry ConceptsDocument26 pagesThermodynamics and Calorimetry ConceptsCelape CabanesPas encore d'évaluation
- Protein Content Quantification by Bradfoed MethodDocument14 pagesProtein Content Quantification by Bradfoed MethodLê TùngPas encore d'évaluation
- RA 6235 Anti Hijacking Law - Cariaga HandoutsDocument2 pagesRA 6235 Anti Hijacking Law - Cariaga HandoutsKiara Chimi100% (1)
- CGFVHDocument6 pagesCGFVHvijayakumarPas encore d'évaluation
- Solution Polymerization of Methyl MethacrylateDocument3 pagesSolution Polymerization of Methyl MethacrylateMaten NasradinPas encore d'évaluation
- Polymer Practice ProblemsDocument11 pagesPolymer Practice ProblemspolypolyyPas encore d'évaluation
- IIT-JAM-2014 Chemistry QuestionsDocument8 pagesIIT-JAM-2014 Chemistry QuestionsMahendra GanuboyinaPas encore d'évaluation
- New Microsoft Office Word DocumentDocument6 pagesNew Microsoft Office Word DocumentVishvesh ShrivastavPas encore d'évaluation
- (Green Alternative Energy Resources) Shurong Wang, Zhongyang Luo-Pyrolysis of Biomass PDFDocument268 pages(Green Alternative Energy Resources) Shurong Wang, Zhongyang Luo-Pyrolysis of Biomass PDFWilliam R Vargas APas encore d'évaluation