Académique Documents
Professionnel Documents
Culture Documents
Fourth Periodical Examination: Taligaman National High School
Transféré par
Melvin CabonegroDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Fourth Periodical Examination: Taligaman National High School
Transféré par
Melvin CabonegroDroits d'auteur :
Formats disponibles
TALIGAMAN NATIONAL HIGH SCHOOL
Butuan City
SCIENCE III
FOURTH PERIODICAL EXAMINATION _______________________________________________________________________
Choose the letter that corresponds to your answer. 1. Which of the following does not describe a gas? a. compressible b. widely separated
(S.Y. 2013-2014)
c. no fixed volume; d. higher density 2. In comparison to solid and liquids gases have!!!!!!!!!!!!!!!!!densities due to the amount of space between the molecules. a. no b. lower c. high d. higher ". #ccording to $inetic %olecular &heory 'inetic energy of a substance ( a. increases during fusion c. increases with increasing temperature b. decreases during conduction d. decreases with increasing temperature ). What happens to the average 'inetic energy of ideal gas molecules when the volume of gas is increased by decreasing the pressure at constant temperature? a. $inetic energy increase. c. $inetic energy decreases b. *o change in 'inetic energy d. $inetic energy changes by a factor of +., ,. Which of the following assumptions of $inetic %olecular &heory of gases is correct? a. -as particles strongly attract each other. b. -as particles travel in curved path. c. &he volume of gas particles prevents random motion. d. .nergy may be transferred between colliding particles. /. &he following are 0ostulates of $inetic %olecular &heory. I. -as molecules move in straight lines in all directions. II. 1ollisions with other molecules changes the direction of motion. III. -as molecules occupy all regions of the container. Why does a gas will evenly fill any container of any shape? a. I only b. II only c. III only d. I II and III 2. What is the process of spreading out spontaneously to uniformly occupy a space? a. expansion b. volume c. diffusion d. pressure 3. In standard temperature and pressure 45&06 the average 'inetic energy of the molecules is proportional to the temperature of the gas in $elvin. &emperature in $elvin can be converted into degrees 1elsius; by a. dividing 22" from the temperature c. subtracting 22" from the temperature b. multiplying 22" from the temperature d. adding 22" from the temperature 7. Which of the following will substantially change in volume if the pressure exerted on it is doubled? a. gas b. liquid c. solid d. all of these 1+. If the volume of one mole of gas molecules remains constant lowering the temperature will ma'e the pressure? a. increase b. increase then decrease c. decrease d. decrease then increase 11. If the pressure of a confined gas at constant temperature is tripled what will happen to the volume? a. the volume will be tripled c. the volume will remain unchanged b. the volume will be reduced to one third the original d. the volume will be doubled 12. &he volume of a mole of any ideal gas varies !!!!!!!! at constant pressure. a. directly with the radius of glass molecules b. inversely with the radius of the gas molecules c. directly with the $elvin 4absolute6 temperature d. inversely with the $elvin 4absolute6 temperature 1". 8or a fixed mass of gas at constant temperature the volume of the gas 9 is inversely proportional to its pressure 0. Which of the following mathematical equation expresses this relationship?
a. 09 : constant b. 0 : 9
c.
1). Which of the following indicates the amount of gas particles? a. 1+.+ ; b. 2/+ torr c. 22" $ d. ".+ mole of <e 1,. # sealed metal container was heated at constant volume and at constant amount of gas. What happened when pressure increased? a. boiling point is the same c. boiling point increases b. boiling point decreases d. boiling point cannot be determined 1/. -iven the equation 0191 : 0292? which of the following is the formula for final temperature? &1 &2 a. &2 : 02 x 92 x &1 c. &2 : 01 x 91 x &1 01 9 1 02 9 2 1 b. &2 : 01 x 92 02 9 1 x &1 d. &2 : &1 x 91 x &1 01 02
P = constant V V = constant d. P
12. Which of the following laws made up the combined gas law ? a. =oyle>s ;aw -ay ? ;ussac>s ;aw and #vogadro>s ;aw b. 1harles> ;aw #vogadro>s ;aw and -raham>s ;aw c. -ay ? ;ussac>s ;aw 1harles ;aw and =oyle>s ;aw d. -raham>s ;aw Ideal -as ;aw and 1harles> ;aw 13. # child>s balloon is filled with helium to give a volume of ,.+ ; in an air conditioned store at 21@1. &he child ta'es the balloon outdoors where the air temperature is "3@1. What happens to the balloon? a. the volume increases c. the volume decreases b. there is no change in the volume d. the pressure will change 17. <aAel is blowing a balloon and its volume increases. What will happen if it is punctured? a. volume increases as some air lea's out c. volume decreases as some air lea's out b. volume increases as more air is added d. volume decreases as some air is added 2+. #t constant pressure which curve below shows the relationship between the volume of an ideal gas and its absolute temperature? a. # b. = c. 1 d. B # = 9olume in ;iters 1
&emperature in $elvin 21. Which plot describes the volume?pressure relationship in gases at constant & if 9 and 0 are the C and D
axes respectively?
22. 1alculate the pressure of a gas if it has ,++ m; of volume at 22 @1 if there are " moles of the gas present. 1onvert the volume and temperature according to the units provided in the gas constant as follows; E : +.+32 atm.; mole.$ a. +.+1"" atm b. 1".23 atm c. 1)2./ atm d. )7".7 atm 2". What happen to one>s lung when one relaxes? a. 9olume of lungs decreases causing air pressure in lungs to be greater than atmospheric pressure. b. 9olume of lungs increases causing air pressure in lungs to be greater than atmospheric pressure. c. &emperature in lungs decreases causing air pressure in lungs to be greater than atmospheric pressure. d. &emperature in lungs decreases causing air pressure in lungs to be greater than atmospheric pressure. 2). 0ressure coo'er saves time. What happen when one coo's food in it? a. Water in the pressure coo'er will boil at lower 1 temperature than 1++ @1 and food coo's faster. b. Water in the pressure coo'er will boil at lower temperature than 1++ @1 and food coo's slower. c. Water in the pressure coo'er will boil at higher temperature than 1++ @1 and food coo's slower. d. Water in the pressure coo'er will boil at higher temperature than 1++ @1 and food coo's faster.
2,. # syringe with a sealed tip contains +.1 g of gas at room temperature. #s the plunger of the syringe is pushed down which of the statement does not happen? a. &he pressure of the gas increases. c. &he molecules come closer to each other b. &he molecules move faster. d. &he volume of the gas decreases. 2/. What happens when a reaction ta'es place endothermically? a. &he temperature of the system drops. b. &he temperature of the surroundings drops. c. &he temperature of the surroundings rises. d. &he temperature of the surroundings is constant. 22. Which of the following changes is exothermic? a. steaming of fish c. melting of ice b. photosynthesis in plants d. burning of alcohol 23. In industry catalysts are added to chemical reaction. What will be the correct statement below that best describes the role of catalyst in a chemical reaction? a. it increases the temperature of reaction c. it increases the amount of products formed b. it enables the products to be separated d. it increases the speed of formation of products 27. Which of the following laws states that the total mass of the reactants is equal to the total mass of the product? a. ;aw of definite composition c. ;aw of conservation of mass b. ;aw of conservation of energy d. ;aw of inertia "+. Balton>s #tomic &heory explains the laws of chemical changes. Which of Balton>s assumptions explains the ;aw of 1onservation of %ass? a. #n atom is the smallest particle of an element. b. In ordinary chemical reactions no new atoms are created and no old ones are destroyed. c. #toms of a given element have the same properties of siAe shape and mass. d. #toms combine in ratios of small whole numbers. "1. &he atomic mass of carbon is 12 amu while that of hydrogen is 1 amu. &he compound methane has the formula 1< ) and the molar mass is 1/ gFmole. What is the percentage composition of 1< )? a. ,+G 1; ,+G < c. 2,G 1; 2,G < b. 12G 1; 1/G < d. 7+G 1; 1+G < "2. What is the chemical formula of the compound formed when %g reacts with 5? a. %g52 c. %g5 b. %g2 d. %g25" "". What is the correct formula of iron4III6 sulfate? a. 8e5 c. 8e245H)6" b. 8e"45H)62 d. 8e5H) "). What products form when potassium nitrate decomposes? a. 0otassium nitrate and oxygen c. 0otassium oxygen and nitrogen b. 0otassium nitrite and oxygen d. 0otassium oxide and nitrogen ",. What type of reaction is shown by the equation below? In J 2 *aH< <2 J *a2InH2 a. combination b. decomposotion d. FOR ITEMS 36-37 -iven these two chemical reactionsK I. 2 $*H" 2 $*H2 J H2 II. 8e J 5 8e5 "/. What type of chemical reactions are in I? a. combination reaction b. displacement reaction "2. What type of chemical reactions are in II? a. combination reaction b. displacement reaction c. double displacement reaction d. decomposition reaction c. double displacement reaction d. decomposition reaction
c. single displacement double replacement
"3. What 'ind of reaction does the following general equation applies to? # J = #= a. combination b. decomposition c. oxidation d. "7. Which reaction below is not a combination reaction? a. 2 *a J 1l2 2 *a1l b. 2 <2 J H2 2 <2H c. ) 8e J " H2 2 8e2H" 12 d. 1a1H" 1aH J 1H
displacement
)+. Which of the following illustrates single replacement reaction?
a. b. c. d.
*<)H< J <1l 0b4*H"62 J 2 $=r 8e J 1u5H) *aH< J <1l
*<)1l J <2H 0b=r2 J 2 $*H" 8e5H) J 1u *a1l J <H<
)1. 0owdered copper when heated in an atmosphere of oxygen produces 1opper 4II6 oxide according to the chemical equationK 2 1u J H2 2 1uH Which of the following conclusion will be formed about the mass of 1opper 4II6 oxide that forms when all reactants were combined? a. &he same as the mass of copper that combines with oxygen. b. &wice the mass of oxygen that combines with copper. c. &he sum of the masses of copper and oxygen that combined. d. &he sum of twice the mass of copper and the mass of oxygen that combined )2. What is the first step in balancing chemical equation? a. Identify and write the word equation of its reactant and product. b. Writing the s'eleton equation from the given word equation. c. 0utting of coefficient numbers to the given compounds in both the reactant and the product. d. Betermine the number of atoms of the elements that comprises the reactant and the product. )". Which of the following statement is the final step in balancing chemical equation? a. Identify and write the word equation of its reactant and product. b. Writing the s'eleton equation from the given word equation. c. 0utting of coefficient numbers to the given compounds in both the reactant and the product. d. Betermine the number of atoms of the elements that comprises the reactant and the product. 8HE I&.%5 )) ?)/ -iven a chemical equationK 0b4*H"62 J 2 $=r 0b=r2 J 2 $*H"?
)). Which of the following indicates that there are / atoms of Hxygen are present in the given chemical equation? a. Its subscript numbers c. its subscript numbers in the parenthesis b. Its coefficient numbers d. its subscript and coefficient numbers in the parenthesis ),. Which statement is true about the number of atoms of =romine in the given equation? a. It is not correctly balanced with its corresponding number subscripts and coefficients. b. It is balanced with its corresponding number subscripts and coefficients. c. Its subscript number in the product is not equal to its coefficient numbers in the reactant. d. Its coefficient number in the reactant is not equal to its subscript number in the product.
)/. What statement below is not true in describing the given equation?
a. b. c. d. &he given equation is a double replacement reaction. &he given equation shows two elements displacing each other. &he given equation is not correctly balanced. &he given equation is correctly balanced.
)2. &he following equation are not correctly balance except one What chemical equation below is correctly balanced? a. <2 J H2 <2H c. %g J 2 <1l %g1l2 b. 5H2 J H2 5H" d. In J 2 <25H) In5H) J 2<2 )3. Which of the following equations describes the complete balanced decomposition of potassium chlorate? a. 2 $1lH" 2 $1l J 2 H2 c. 2 $1lH2 2 $1l J ) H2 b. 2 $1lH" 2 $1l J " H2 d. 2 $1lH 2 $1l J H2 )7. Which is the correct coefficient of oxygen in the equation 1 "<3 J H2 " 1H2 J ) <2H a. " b. ) c. , d. / ,+. Which of the following is not correctly balanced? a. *a1l J #g*H" b. ) 8e J "H2 *a*H" J #g1l 2 8e2H" c. *2 J <2 d.2 *a J 2 <2H *<" 2 *aH< J <2
GOOD LUCK!!!
1
TALIGAMAN NATIONAL HIGH SCHOOL
Butuan City
SCIENCE III
THIRD PERIODICAL EXAMINATION
(S.Y. 2013-2014)
ANSWER KEY
1.D 2.B 3.C 4.B 5.D .D !.C ".# $.A 10.# 11.b 12.C 13.A 14.D 15.C 1 .A 1!.C 1".A 1$.C 20.C 21.A 22.C 23.A 24.D 25.B 2 .B 2!.D 2".D 2$.C 30.B 31.C 32.C 33.C 34.B 35.C 3 .D 3!.A 3".A 3$.D 40.C 41.C 42.A 43.C 44.D 45.B 4 .C 4!.D 4".B 4$.C 50.C
________________________________________________________________________________ _________________
TALIGAMAN NATIONAL HIGH SCHOOL
Butuan City
SCIENCE III
THIRD PERIODICAL EXAMINATION
(S.Y. 2011-2012)
ANSWER KEY
1.D 2.B 3.C 4.B 5.D .D !.C ".D $.A 10.A 11.A 12.C 13.A 14.D 15.C 1 .A 1!.C 1".A 1$.C 20.C 21.A 22.C 23.A 24.D 25.B 2 .B 2!.D 2".D 2$.C 30.B 31.C 32.C 33.C 34.B 35.C 3 .D 3!.A 3".A 3$.D 40.C 41.C 42.A 43.C 44.D 45.B 4 .C 4!.D 4".B 4$.C 50.C
Vous aimerez peut-être aussi
- I. Multiple Choice. Choose The Letter of The Answer That Best Corresponds To The Given Question. Write Your Answer in Capital LetterDocument2 pagesI. Multiple Choice. Choose The Letter of The Answer That Best Corresponds To The Given Question. Write Your Answer in Capital LetterJaenicaPaulineCristobal64% (11)
- 24.physical ChemistryDocument15 pages24.physical ChemistryJames BalanaPas encore d'évaluation
- Physical Chemistry 2Document10 pagesPhysical Chemistry 2Clara MazangoPas encore d'évaluation
- Fourth Quarter Final Exam G9 and G10Document17 pagesFourth Quarter Final Exam G9 and G10Sylvs EnongPas encore d'évaluation
- Science 10 Q4 Module 1Document29 pagesScience 10 Q4 Module 1Maki Tuna100% (2)
- Grade 10 Fourth PeriodicalsDocument3 pagesGrade 10 Fourth PeriodicalsSalve Gregorio AguirrePas encore d'évaluation
- Grade 10first TQDocument3 pagesGrade 10first TQJerbs PacundoPas encore d'évaluation
- Che 205 Practice Questions 1Document9 pagesChe 205 Practice Questions 1Sunday EdemaPas encore d'évaluation
- Sci3 4thPTDocument5 pagesSci3 4thPTMelvin CabonegroPas encore d'évaluation
- Rustico Capahi Sr. Memorial Antional High SchoolDocument2 pagesRustico Capahi Sr. Memorial Antional High SchoolLorraine Donio100% (1)
- Unit Practice Test: Gas Laws: Multiple ChoiceDocument8 pagesUnit Practice Test: Gas Laws: Multiple Choiceanj pianoPas encore d'évaluation
- Chapter 5-6 Extra Practice Sheet Qs (G10 Chem)Document39 pagesChapter 5-6 Extra Practice Sheet Qs (G10 Chem)The TomatoPas encore d'évaluation
- Equilibrium MCQ W AnsDocument6 pagesEquilibrium MCQ W AnsHovan Tall Nut Tan100% (1)
- Quiz - Chapter 6Document5 pagesQuiz - Chapter 6dPas encore d'évaluation
- Genchem 2ND Q Summative 2021 2022Document4 pagesGenchem 2ND Q Summative 2021 2022Rizalyn Padua ReyPas encore d'évaluation
- 2nd Quarter Gen Chem 1 Module 3 and 4Document2 pages2nd Quarter Gen Chem 1 Module 3 and 4DavePas encore d'évaluation
- Department of Education: Parallel Assessment Grade 10 Fourth Quarter ScienceDocument3 pagesDepartment of Education: Parallel Assessment Grade 10 Fourth Quarter ScienceJanePas encore d'évaluation
- Test Ch.10: Multiple ChoiceDocument6 pagesTest Ch.10: Multiple ChoiceMj LeePas encore d'évaluation
- Introduction To General Organic and Biochemistry 11Th Edition Bettelheim Test Bank Full Chapter PDFDocument36 pagesIntroduction To General Organic and Biochemistry 11Th Edition Bettelheim Test Bank Full Chapter PDFarthur.hendricks257100% (13)
- Pretest On Gas Laws: Multiple Choice: Choose The Letter of The Best AnswerDocument6 pagesPretest On Gas Laws: Multiple Choice: Choose The Letter of The Best AnswerClarence Mike BorjaPas encore d'évaluation
- Which Statement Is True About Chemical Reactions at Equilibrium?Document9 pagesWhich Statement Is True About Chemical Reactions at Equilibrium?Abdusalam IdirisPas encore d'évaluation
- Chemical Equilibrium Multiple Choice QuestionsDocument4 pagesChemical Equilibrium Multiple Choice QuestionsCarol Mae Celis100% (5)
- Department of Education Region VII, Central Visayas Division of Talisay City Talisay City, CebuDocument2 pagesDepartment of Education Region VII, Central Visayas Division of Talisay City Talisay City, CebuElue Sañal EtnaledPas encore d'évaluation
- Exam3 Practice ExamDocument4 pagesExam3 Practice ExamMatthew FernandezPas encore d'évaluation
- Fourth Quarter TestDocument5 pagesFourth Quarter TestLorraine Calvez Donio100% (4)
- Multiple Choice: Choose The Letter of The Best Answer.: B. Inversely ProportionalDocument7 pagesMultiple Choice: Choose The Letter of The Best Answer.: B. Inversely ProportionalClarence Mike BorjaPas encore d'évaluation
- 2009 Introduction To General Organic and Biochemistry 9th Edition Test BankDocument38 pages2009 Introduction To General Organic and Biochemistry 9th Edition Test BankaureliahoavunwPas encore d'évaluation
- GasesDocument2 pagesGasesJason TaburnalPas encore d'évaluation
- Phat (Necro) Ho - 5-00 Gases Unit Pack - 2021Document8 pagesPhat (Necro) Ho - 5-00 Gases Unit Pack - 2021Just an Abnormal SIMPPas encore d'évaluation
- Second Self-Assessment TestDocument3 pagesSecond Self-Assessment TestAbhay VishwakarmaPas encore d'évaluation
- (Percdc) Multiple Choice Questions in General Engineering and Applied SciencesDocument145 pages(Percdc) Multiple Choice Questions in General Engineering and Applied SciencesMaria Dhanita David Alfaro100% (2)
- Eastern Visayas State University: Education DepartmentDocument5 pagesEastern Visayas State University: Education DepartmentMichelle EscalientePas encore d'évaluation
- Chemical Equilibrium 26FFDocument26 pagesChemical Equilibrium 26FFLeul KassaPas encore d'évaluation
- Ch.1-Matter in Our Surroundings 9th SolvedDocument50 pagesCh.1-Matter in Our Surroundings 9th SolvedVikash SharmaPas encore d'évaluation
- QuizDocument2 pagesQuizHelma Jabello AriolaPas encore d'évaluation
- Q4 Sci10 Assessment2-2Document2 pagesQ4 Sci10 Assessment2-2Jaezean Jules B. GomezPas encore d'évaluation
- TDDocument22 pagesTDPankaj KumarPas encore d'évaluation
- Introduction To General Organic and Biochemistry 9th Edition Question AnswersDocument38 pagesIntroduction To General Organic and Biochemistry 9th Edition Question AnswerstaupaypayPas encore d'évaluation
- 4Q Sci10 Las2 - Charles' LawDocument4 pages4Q Sci10 Las2 - Charles' Lawrectoann08Pas encore d'évaluation
- Rates of Reaction TestDocument10 pagesRates of Reaction TestSaya MenangPas encore d'évaluation
- Chem 102 FinalDocument12 pagesChem 102 FinalAlex GampelPas encore d'évaluation
- Equilibrium Reactions and Equilibrium ConstantsDocument58 pagesEquilibrium Reactions and Equilibrium ConstantsRoger WangPas encore d'évaluation
- Chemistry (Long Quiz 1 Finals)Document7 pagesChemistry (Long Quiz 1 Finals)Francis TayagPas encore d'évaluation
- Chemistry (Practice Test) Name - Chapter 12 (The Gas Laws)Document4 pagesChemistry (Practice Test) Name - Chapter 12 (The Gas Laws)Cenando BodanioPas encore d'évaluation
- For Combined Gas LawDocument44 pagesFor Combined Gas LawApril Bartolome Flores100% (1)
- Chapter Test in Grade 10Document8 pagesChapter Test in Grade 10Maestro de Grapico100% (3)
- Rate ReactionDocument6 pagesRate ReactionAli r24Pas encore d'évaluation
- SCH4U Equilibrium Questions With SolutionsDocument28 pagesSCH4U Equilibrium Questions With SolutionsS P100% (1)
- Unit 7 - Chemical Equilibrium - SL - Paper 1 - 1.0 (Questions)Document12 pagesUnit 7 - Chemical Equilibrium - SL - Paper 1 - 1.0 (Questions)VedantPas encore d'évaluation
- Chapter 3 States of MatterDocument8 pagesChapter 3 States of Mattertconover145100% (1)
- Midterm Exam Review 2022Document7 pagesMidterm Exam Review 2022Graham MaddoxPas encore d'évaluation
- Exam Review Unit 3 - Energy Changes and Rates of ReactionDocument7 pagesExam Review Unit 3 - Energy Changes and Rates of ReactionrejymolPas encore d'évaluation
- 1st GENERAL CHEMISTRY 2 2nd QuarterDocument2 pages1st GENERAL CHEMISTRY 2 2nd QuarterSid Eleazar R. Gaffud100% (2)
- 201B Concept ThermoDocument3 pages201B Concept ThermoMrsriyansyahPas encore d'évaluation
- AP Chapter 13 MC Practice Questions With MC AnswersDocument9 pagesAP Chapter 13 MC Practice Questions With MC AnswersapantollanoPas encore d'évaluation
- Final Example: Answer: A. This Is Because in This Reaction, Cu Goes From 2+ in Cuo To 0 in Cu (S) - SoDocument9 pagesFinal Example: Answer: A. This Is Because in This Reaction, Cu Goes From 2+ in Cuo To 0 in Cu (S) - SoAlison JohnsonPas encore d'évaluation
- Unit 6 Gas Laws Test Review 2019-2020Document4 pagesUnit 6 Gas Laws Test Review 2019-2020Rachel PascucciPas encore d'évaluation
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterD'EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterÉvaluation : 5 sur 5 étoiles5/5 (1)
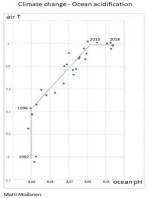
- Climate change - ocean acidity: Matemaattinen analyysiD'EverandClimate change - ocean acidity: Matemaattinen analyysiPas encore d'évaluation
- Daily Lesson LogDocument4 pagesDaily Lesson LogMelvin Cabonegro100% (2)
- Deped Order No. 42, S. 2016 Taligaman National High School Melvin C. CabonegroDocument6 pagesDeped Order No. 42, S. 2016 Taligaman National High School Melvin C. CabonegroMelvin CabonegroPas encore d'évaluation
- Taligaman National High School Taligaman, Butuan CityDocument1 pageTaligaman National High School Taligaman, Butuan CityMelvin CabonegroPas encore d'évaluation
- Grade 9: Name of Student School Last Attended Residence Address Contact Number RemarksDocument2 pagesGrade 9: Name of Student School Last Attended Residence Address Contact Number RemarksMelvin CabonegroPas encore d'évaluation
- School Base Management Dimensions A. Leadership and Governance B. Curriculum and LearningDocument2 pagesSchool Base Management Dimensions A. Leadership and Governance B. Curriculum and LearningMelvin CabonegroPas encore d'évaluation
- Grade 9: Name of Student School Last Attended Residence Address Contact Number RemarksDocument2 pagesGrade 9: Name of Student School Last Attended Residence Address Contact Number RemarksMelvin CabonegroPas encore d'évaluation
- SHS 3 Years Work and Financial Plan WFPDocument8 pagesSHS 3 Years Work and Financial Plan WFPMelvin CabonegroPas encore d'évaluation
- Budget Proposal: March 29, 2016Document2 pagesBudget Proposal: March 29, 2016Melvin CabonegroPas encore d'évaluation
- Travel Authority: Taligaman National High SchoolDocument2 pagesTravel Authority: Taligaman National High SchoolMelvin CabonegroPas encore d'évaluation
- School Form 7 (SF7) School Personnel Assignment List and Basic ProfileDocument6 pagesSchool Form 7 (SF7) School Personnel Assignment List and Basic ProfileMelvin CabonegroPas encore d'évaluation
- Sf5 - 2017 - Grade 7 (Year I) - FrondaDocument3 pagesSf5 - 2017 - Grade 7 (Year I) - FrondaMelvin CabonegroPas encore d'évaluation
- BE Form 5 - RECORD OF DONATIONS RECEIVEDDocument1 pageBE Form 5 - RECORD OF DONATIONS RECEIVEDMelvin Cabonegro0% (1)
- BE Form 5 - RECORD OF DONATIONS RECEIVEDDocument1 pageBE Form 5 - RECORD OF DONATIONS RECEIVEDMelvin CabonegroPas encore d'évaluation
- Certificate of EmploymentDocument2 pagesCertificate of EmploymentMelvin CabonegroPas encore d'évaluation
- DEPED-Butuan City-Notice of Evaluation-SchoolsDocument1 pageDEPED-Butuan City-Notice of Evaluation-SchoolsMelvin CabonegroPas encore d'évaluation
- Tnhs Annual Report Finale 1Document44 pagesTnhs Annual Report Finale 1Melvin Cabonegro100% (1)
- Conduct of Meetings Policy 2015Document6 pagesConduct of Meetings Policy 2015Melvin CabonegroPas encore d'évaluation
- Awards and RecognitionDocument19 pagesAwards and RecognitionMelvin Cabonegro0% (1)
- Letterr NewDocument1 pageLetterr NewMelvin CabonegroPas encore d'évaluation
- Certificate of EmploymentDocument2 pagesCertificate of EmploymentMelvin CabonegroPas encore d'évaluation
- Cedula FormDocument14 pagesCedula FormMelvin CabonegroPas encore d'évaluation
- Second Quarter Butuan City CaragaDocument5 pagesSecond Quarter Butuan City CaragaMelvin CabonegroPas encore d'évaluation
- Revised School Based Management Assessment ToolDocument6 pagesRevised School Based Management Assessment ToolMelvin CabonegroPas encore d'évaluation
- A Resolution Adopted by The Supreme Student Government of Taligaman National High School During Its Special Session On December 4Document5 pagesA Resolution Adopted by The Supreme Student Government of Taligaman National High School During Its Special Session On December 4Melvin Cabonegro0% (1)
- Contact Details: Contact Details: Contact DetailsDocument2 pagesContact Details: Contact Details: Contact DetailsMelvin CabonegroPas encore d'évaluation
- School Improvement Project Title Project Objective Output For The Year Activitie S Person (S) Involve D Schedule/Venue Budge T Per Activit y Budget SourceDocument3 pagesSchool Improvement Project Title Project Objective Output For The Year Activitie S Person (S) Involve D Schedule/Venue Budge T Per Activit y Budget SourceMelvin CabonegroPas encore d'évaluation
- Brigada OpeningDocument2 pagesBrigada OpeningMelvin Cabonegro100% (4)
- Routes of Medication AdministrationDocument2 pagesRoutes of Medication AdministrationTracy100% (6)
- Transes - Male & Female GenitaliaDocument10 pagesTranses - Male & Female GenitaliacamatoviancaPas encore d'évaluation
- Op Amp AssignmentDocument10 pagesOp Amp AssignmentJuan-Wian CoetzerPas encore d'évaluation
- The 5 TibetansDocument3 pagesThe 5 TibetansValentin100% (2)
- Hydraulics - MKM - DLX - Parts - Catalogue MAR 14 PDFDocument33 pagesHydraulics - MKM - DLX - Parts - Catalogue MAR 14 PDFRS Rajib sarkerPas encore d'évaluation
- Detailed Lesson Plan in Science Grade 10Document9 pagesDetailed Lesson Plan in Science Grade 10christian josh magtarayoPas encore d'évaluation
- ?????Document89 pages?????munglepreeti2Pas encore d'évaluation
- Criteria For Decorative Cosmetics PDFDocument3 pagesCriteria For Decorative Cosmetics PDFsamudra540Pas encore d'évaluation
- NSC Solution F2 enDocument8 pagesNSC Solution F2 ensaeidPas encore d'évaluation
- Hostel B Menu From 16 March To 31 March'2024Document4 pagesHostel B Menu From 16 March To 31 March'2024govindkauPas encore d'évaluation
- E9sht I C C I W D SDocument213 pagesE9sht I C C I W D SMAMBO95TLPas encore d'évaluation
- Truss Design GuidDocument3 pagesTruss Design GuidRafi HasanPas encore d'évaluation
- Post Graduate Diploma in Psychological CounselingDocument1 pagePost Graduate Diploma in Psychological CounselingAvalokiteswari KurupPas encore d'évaluation
- Defeat Cancer NaturallyDocument94 pagesDefeat Cancer NaturallyRknuviprasys Low100% (3)
- Ken Russell Revealed:: Still Images 1954 - 1957Document4 pagesKen Russell Revealed:: Still Images 1954 - 1957Adriana ScarpinPas encore d'évaluation
- A554-15 Standard Specification For Welded Stainless Steel Mechanical TubingDocument8 pagesA554-15 Standard Specification For Welded Stainless Steel Mechanical TubingChuthaPas encore d'évaluation
- Essential Oil ExtractionDocument159 pagesEssential Oil ExtractionAubrey Hernandez100% (4)
- 2022 Specimen Paper 1 Mark Scheme 1Document4 pages2022 Specimen Paper 1 Mark Scheme 1Herman HermanPas encore d'évaluation
- ENDOCRINE-BOARD REVIEW Dr. SchearDocument57 pagesENDOCRINE-BOARD REVIEW Dr. SchearNayara PataroPas encore d'évaluation
- Weirs: 2. Open Channel Flow 4. Flumes IndexDocument25 pagesWeirs: 2. Open Channel Flow 4. Flumes IndexlordsethdarknessPas encore d'évaluation
- DH-IPC-HDBW1231E: 2MP WDR IR Mini-Dome Network CameraDocument3 pagesDH-IPC-HDBW1231E: 2MP WDR IR Mini-Dome Network CameraDeltaz AZPas encore d'évaluation
- Astm D6321-98-2004Document3 pagesAstm D6321-98-2004Thyagu LingamurthyPas encore d'évaluation
- Object: Annex A, B, C DDocument74 pagesObject: Annex A, B, C DfjsdPas encore d'évaluation
- Chapter 1 - Physical Properties of Liquid PDFDocument43 pagesChapter 1 - Physical Properties of Liquid PDFrohit sharmaPas encore d'évaluation
- 3rd Quarter PHYSICAL SCIENCE ExamDocument19 pages3rd Quarter PHYSICAL SCIENCE ExamZhering RodulfoPas encore d'évaluation
- 4200 Magnetometer Interface Manual 0014079 - Rev - ADocument34 pages4200 Magnetometer Interface Manual 0014079 - Rev - AJose Alberto R PPas encore d'évaluation
- Cug, Ugdp, Pag-Asa, NurseryDocument5 pagesCug, Ugdp, Pag-Asa, NurseryRaymund Joshua Pre�aPas encore d'évaluation
- Distribution System ReliabilityDocument8 pagesDistribution System Reliabilityabera alemayehuPas encore d'évaluation
- SRS Cheat CodesDocument9 pagesSRS Cheat CodesnurhayatiPas encore d'évaluation
- 002-679e-08.19 v1.6.x KLDocument523 pages002-679e-08.19 v1.6.x KLChanon OnramoonPas encore d'évaluation