Académique Documents
Professionnel Documents
Culture Documents
Fracture Dependence On Heat Treatment: Table 1-Dependence of Absorbed Energy (For Crack Propagation) Over Temperature
Transféré par
lokomoko1Description originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Fracture Dependence On Heat Treatment: Table 1-Dependence of Absorbed Energy (For Crack Propagation) Over Temperature
Transféré par
lokomoko1Droits d'auteur :
Formats disponibles
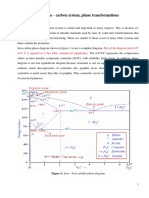
Table 1-dependence of absorbed energy (for crack propagation) over temperature
The table above shows the fracture behavior of Fe-0.4wt% carbon as a function of
temperature. The temperature is in the range of 20C (room temperature) and
-70C.
Heat treatment Energy(J)
Normalized 20C 50
Heated to 950C and quenched 2
heated to 950C, quenched then tempered to
680C and allowed to cool in air
123
Table 2-dependence of fracture behavior over heat treatment
The table shows how the energy absorption changes when different heat
treatment is applied to the samples. On sample 2 the low absorption of energy is
due to the martensite formed which is extremely brittle. After tempering, some
20, 50
0, 39
-20, 30
-40, 29
-70, 26
0
10
20
30
40
50
60
-80 -70 -60 -50 -40 -30 -20 -10 0 10 20 30
E
n
e
r
g
y
J
Temperature C
Fracture dependence on heat treatment
Specimen (Number) Temperature (C) Energy (J)
1 20 50
2 0 39
3 -20 30
4 -40 29
5 -70 26
the carbon has formed Fe
3
C. This process allows the material to become more
ductile and as a result more energy is needed during propagation of cracks.
Percentage carbon in steel Energy (J)
0.1 wt% 114
0.4 wt% 50
Table 3- dependence of energy absorption on percentage carbon in steels at room temperature
Ferrite - iron
It is a solid solution of carbon and iron, where the iron is the main element. Ferrite has a body-
centered cubic crystal structure (packing fraction 0.68) which explains the medium strength (=
280 N/mm
2
). This structure is stable at low temperature and remains in this phase up to 910 C.
Then it transforms to austenite (
iron) with FCC structure. Ferrite contains very little carbon due
to the size of the C atoms (twice as big as Fe atoms) as carbon atoms are interstitial impurities in
Fe structure. So at 0 C there is only 0.005 wt% C and the highest amount of C that can be
dissolved is 0.022 wt%, reached at 727 C. Other key property is that ferrite is hard (80 kgf/m
2
)
and ductile. It also has high electrical resistance and high magnetic permeability.
Cementite - Fe
3
C
Is a chemical compound with crystal lattice structure. Its carbon content exceeds 6.67 wt%. This
compound could be formed during cooling of austenite or tempering of martensite. Due to the
high amount of carbon this compound is very hard and brittle. Also it is solid and inert as a
result it could withstand crushing forces, chemical erosion as well as very high temperatures.
This intermetallic compound is metastable, it remains as a compound at room T, but
decomposes (very slowly, within several years) into -Fe and C (graphite) at 650 - 700 C
Pearlite
Pearlite is formed when cooling austenite below 727C with 0.77 wt% of carbon-an eutectoid
reaction. At that point when the temperature drops, austenite transforms to a particular
mixture of cementite (11%) and ferrite (89%). The process requires diffusion of all carbon
atoms. Thus the formation of pearlites takes time and the process is considered relatively slow.
This material is extremely strong (due to the small grain size of the ferrite) and brittle.
2
a) Ferrite starts to form at 880C
b) Austenite (37%) and ferrite (63%)
c) Pearlite- 11% Fe
3
C and 89% ferrite
3 Martensite
Martensite is formed when austenite of high temperature is cooled very quickly so the carbon
does not have time to diffuse during the transformation and as a result it remains trapped in a
low temperature ferrite structure. This iron phase is called martensite. The carbon inside the
structure creates a lot of disturbances and impacts the movement of dislocations. Once unable
to distort the material becomes extremely strong, hard and brittle.
a) 0.8 wt% carbon steel is known to be unweldable. This is because its CEV is over 0.4 wt%.
In this case, the cooling rate of the steel is very high and allowed to cool a hard and
brittle phase will easily develop. The formation of martensite may lead to failure of the
steel when load is applied. However, there are ways to make 0.8 wt% carbon steel more
receptive to welding. The first one is preheating. Preheating protects the parent metal
by helping prevent hardening of the weld as brittle phases form. So creating softer, more
ductile structure better resists cracking. Another method of improving 0.8 wt% carbon
steel is by post heating. When the martensite has formed, tempering will result in
increase of toughness as the amount of carbon trapped in the structure forms cementite
and hence increase the ductility of the HAZ.
b) 0.1% carbon steel is highly weldable. Its CE value is under 0.4 wt% following a formation
of strong and very ductile material in the heat affected zones, if welded. Because of all it
properties it could be used in nearly all welding processes with high quality results.
c) The 300 series of austenic stainless steel are the most weldable of the stainless steels.
There are two things to be considered when welding the 300 series. The first is
avoidance of solidification of cracks and the next is preservation of corrosion resistance
of the weld in the HAZ. The 300 series of stainless steel are modified and they contain
max of 0.03 wt% Sulfur and 0.04 wt% Phosphorus since increased amount of S and P
increases the material brittleness. So containing low amounts of P and S in the
composition decreases the likelihood of solidification cracking. As far as the corrosion
resistance is concerned, there are two approaches. One is lowering the carbon amount
so that not much Cr would react with C and in this way disturb the Cr layer that prevents
corrosion. So the range of C in the 300 series is from 0.01-0.08 wt%.The other method is
to add elements that react more readily with C than Cr does. Such elements are Niobium
and Titanium. Finally, the amount of Cr must be greater so that the losses would not
affect the percentage of the Cr to be over 11wt%. The Cr range in the 300 series is 17-26
wt%.
4 Youngs modulus
Hookes law of elasticity defines Youngs modulus as the ratio of stress to corresponding strain
below the elastic (proportional) limit
. The load obtain in this experiment are at the
yield load (the point at which plastic deformation starts) and at the tensile load (the maximum
load that the sample can withstand).The yield load is measured as 0.2% offset proves. That
point is determined by human who leaves great possibility for errors. Also the point is in the
plastic region which makes the data even more invaluable to determine Youngs modulus.
Moreover, in the experiment we measure the length and the cross section area of the sample
before and after the tensile test. This gives as the overall extension whereas for the Youngs
modulus we are interested in extension in the linear-elastic part of the stress strain graph. To
enable the experiment output data to be manipulated in order to obtain result for youngs
modulus the apparatus should enable us to take several measurement of the load and the
extension of the sample in the elastic region.
Vous aimerez peut-être aussi
- Solutions Manual to accompany Engineering Materials ScienceD'EverandSolutions Manual to accompany Engineering Materials ScienceÉvaluation : 4 sur 5 étoiles4/5 (1)
- Metal and Metal Alloys NotesDocument18 pagesMetal and Metal Alloys Notesrutuja75% (4)
- Heat-Treatment of High Carbon Steel Wire - PatentingDocument4 pagesHeat-Treatment of High Carbon Steel Wire - Patentingعزت عبد المنعم100% (1)
- Material Flow ChartDocument49 pagesMaterial Flow Charttayyab aliPas encore d'évaluation
- Engineering Metallurgy Chapter-8 Ref: Introduction To Physical MetallurgyDocument34 pagesEngineering Metallurgy Chapter-8 Ref: Introduction To Physical MetallurgyMD Al-AminPas encore d'évaluation
- TTT 11Document55 pagesTTT 11MrDOTPas encore d'évaluation
- 8.heat TreatmentDocument7 pages8.heat Treatmentrohan_n_desai100% (2)
- Fe CDocument34 pagesFe CZaza ArifinPas encore d'évaluation
- Heat TreatmentDocument56 pagesHeat TreatmentAakarsh RastogiPas encore d'évaluation
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarPas encore d'évaluation
- Iron Carbon DiagramDocument9 pagesIron Carbon DiagramNagamuthu PandianPas encore d'évaluation
- Iron-Carbon Phase Diagram (A Review) See Callister Chapter 9Document34 pagesIron-Carbon Phase Diagram (A Review) See Callister Chapter 9Zefa Erliana YullahPas encore d'évaluation
- Theoretical Part: Chapter-1Document10 pagesTheoretical Part: Chapter-1hayder1920Pas encore d'évaluation
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- 08 Transformasi Fasa - UASDocument35 pages08 Transformasi Fasa - UASDinta PratiwiPas encore d'évaluation
- Iron Carbon DiagramDocument8 pagesIron Carbon Diagramashok pradhanPas encore d'évaluation
- BG2802 Heat Treatment and Mechanical Properties of SteelsDocument11 pagesBG2802 Heat Treatment and Mechanical Properties of SteelsVenus LimPas encore d'évaluation
- Plain Iron Carbon SteelsDocument5 pagesPlain Iron Carbon Steelsروشان فاطمة روشانPas encore d'évaluation
- Materi Kuliah Heat TreatmentDocument16 pagesMateri Kuliah Heat TreatmentGama Kus RohkmatullohPas encore d'évaluation
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongPas encore d'évaluation
- Pure Iron, Allotropy:: Steels Are Alloys of Iron and Carbon With Carbon Content Up To 2%Document13 pagesPure Iron, Allotropy:: Steels Are Alloys of Iron and Carbon With Carbon Content Up To 2%Abhishek SakatPas encore d'évaluation
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanPas encore d'évaluation
- TEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ADocument5 pagesTEST 1marking Guide: Mass Effect of Heat-Treatment. This Eliminates The More Costly Need To Quench-Harden With ARhea GaiaPas encore d'évaluation
- Tempered Martensite: H. K. D. H. BhadeshiaDocument20 pagesTempered Martensite: H. K. D. H. BhadeshiaBahaa Eldin Sayed BahaaPas encore d'évaluation
- Hardening From The Liquid StateDocument5 pagesHardening From The Liquid StateSinhrooPas encore d'évaluation
- ANSWER Welding MetallurgyDocument3 pagesANSWER Welding MetallurgyShaikhan Nadzemi100% (1)
- 03 - Iron - Iron CarbideDocument35 pages03 - Iron - Iron CarbidebotobotoakbarPas encore d'évaluation
- Heat Treatment of MetalsDocument18 pagesHeat Treatment of MetalsalikytrnPas encore d'évaluation
- Applying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartsDocument5 pagesApplying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartssathishelakkiyaPas encore d'évaluation
- EMM LectureDocument38 pagesEMM Lecturelatendra kumar srivastavPas encore d'évaluation
- Martensite and The Control of Retained AusteniteDocument6 pagesMartensite and The Control of Retained AusteniteMarcoTulioFonsecaPas encore d'évaluation
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Iron and SteelDocument65 pagesIron and SteelSunny PatilPas encore d'évaluation
- Fe-C Phase DiagramDocument34 pagesFe-C Phase DiagramYoung-long Choi100% (1)
- Martensite and Retained AusteniteDocument4 pagesMartensite and Retained Austenitemp87_ing100% (1)
- Diagram FasaDocument6 pagesDiagram Fasaolid_zonePas encore d'évaluation
- Mechanical Properties Improvement of Low Carbon Steel by Combined Heat TreatmentsDocument15 pagesMechanical Properties Improvement of Low Carbon Steel by Combined Heat TreatmentsReyza PrasetyoPas encore d'évaluation
- Austempering of Bearing Steel For Improved Mechanical PropertiesDocument4 pagesAustempering of Bearing Steel For Improved Mechanical PropertiesAhuti ChatterjeePas encore d'évaluation
- Effect of Heat Treatment On Microstructures and Mechanical Prop of Spring SteelDocument7 pagesEffect of Heat Treatment On Microstructures and Mechanical Prop of Spring SteelMahmood KhanPas encore d'évaluation
- Alloying Elements Effects On MartensiteDocument3 pagesAlloying Elements Effects On MartensiteMalcPas encore d'évaluation
- Heat Treatment - Critical Temperatures - Transformation On Heating / CoolingDocument23 pagesHeat Treatment - Critical Temperatures - Transformation On Heating / Coolingsamurai7_77Pas encore d'évaluation
- Presentation On Heat TreatmentDocument43 pagesPresentation On Heat Treatmentgosaye desalegnPas encore d'évaluation
- Capili Jefferson 10Document5 pagesCapili Jefferson 10Christian Al EncarnacionPas encore d'évaluation
- Heat Treatment of SteelsDocument41 pagesHeat Treatment of Steelsyaswanth1992Pas encore d'évaluation
- Time Temperature Transformation (TTT) Diagrams PDFDocument108 pagesTime Temperature Transformation (TTT) Diagrams PDFSerkan Apay100% (1)
- How The Material Built For Special PurposeDocument43 pagesHow The Material Built For Special PurposeDidi SudiprayitnaPas encore d'évaluation
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasPas encore d'évaluation
- Theory of Heat TreatmentDocument8 pagesTheory of Heat Treatmentayie740% (1)
- Bainite in SteelDocument12 pagesBainite in Steelsathyadevi konnurPas encore d'évaluation
- Seminarski Rad Fe Fe3C DijagramDocument10 pagesSeminarski Rad Fe Fe3C DijagramAlina OmerovicPas encore d'évaluation
- Carburization of SteelDocument9 pagesCarburization of SteelRtr Ahmed Abidemi CertifiedPas encore d'évaluation
- Heat TreatmentDocument179 pagesHeat TreatmentDebye101100% (1)
- The Metallurgy of Carbon SteelDocument103 pagesThe Metallurgy of Carbon SteelUmer AhsanPas encore d'évaluation
- Effect of Si Addition On The Creep Performance of A Ni Based SuperalloyDocument10 pagesEffect of Si Addition On The Creep Performance of A Ni Based SuperalloyDattatreya PatiPas encore d'évaluation
- Test2 SEMM2613 Nader Mohammed Ahmed Alkhulaifi A20EM9124: Red Region Is of Interest ForDocument4 pagesTest2 SEMM2613 Nader Mohammed Ahmed Alkhulaifi A20EM9124: Red Region Is of Interest ForNader MohammedPas encore d'évaluation
- TTT Phase DiagramDocument9 pagesTTT Phase Diagramhari krishnaPas encore d'évaluation
- Publication 4 11889 199Document9 pagesPublication 4 11889 199Mulia AridhoPas encore d'évaluation
- Welding the Inconel 718 Superalloy: Reduction of Micro-segregation and Laves PhasesD'EverandWelding the Inconel 718 Superalloy: Reduction of Micro-segregation and Laves PhasesPas encore d'évaluation
- Journal of Constructional Steel Research: Tayakorn Chandrangsu, Kim J.R. RasmussenDocument10 pagesJournal of Constructional Steel Research: Tayakorn Chandrangsu, Kim J.R. Rasmussenlokomoko1Pas encore d'évaluation
- Excalibur HSB 12mmDocument1 pageExcalibur HSB 12mmlokomoko1Pas encore d'évaluation
- Brett FillersDocument2 pagesBrett Fillerslokomoko1Pas encore d'évaluation
- Acrow Props PDFDocument3 pagesAcrow Props PDFlokomoko1Pas encore d'évaluation
- Research 6Document16 pagesResearch 6lokomoko1Pas encore d'évaluation
- EN 1991-1-4, NotesDocument9 pagesEN 1991-1-4, Noteslokomoko1Pas encore d'évaluation
- Application of Fourier SeriesDocument8 pagesApplication of Fourier Serieslokomoko1Pas encore d'évaluation
- BT 203 Basic Mechanical Engineering May 2019 PDFDocument2 pagesBT 203 Basic Mechanical Engineering May 2019 PDFKunta PatlePas encore d'évaluation
- AISI 1074 Carbon Steel (UNS G10740) : Topics CoveredDocument2 pagesAISI 1074 Carbon Steel (UNS G10740) : Topics CoveredAmrut Navratna Metal Corpn.Pas encore d'évaluation
- Chap-10 Materials and Fabrication SelectionDocument51 pagesChap-10 Materials and Fabrication SelectionSuprio KamalPas encore d'évaluation
- Abebe Amare Design of Clutch PDFDocument55 pagesAbebe Amare Design of Clutch PDFAbebe AmarePas encore d'évaluation
- 00 DOT Regulations (Portable Tank) Full Set - 20200812Document84 pages00 DOT Regulations (Portable Tank) Full Set - 20200812이훈Pas encore d'évaluation
- ASTM Welding Procedures A410 To A643Document8 pagesASTM Welding Procedures A410 To A643solrac4371Pas encore d'évaluation
- Astm F 492Document5 pagesAstm F 492DmitriyPas encore d'évaluation
- Unit 1 EmmDocument40 pagesUnit 1 EmmNagaaswin SPas encore d'évaluation
- Part 9 - Materials and Manufacturing ElementsDocument101 pagesPart 9 - Materials and Manufacturing ElementsREYNALD MILOPas encore d'évaluation
- Jerguson Spec GuideDocument30 pagesJerguson Spec GuideGanesh YadavPas encore d'évaluation
- Classification of Steel & Alloy SteelsDocument39 pagesClassification of Steel & Alloy SteelsNetaa sachinPas encore d'évaluation
- Lista MateriałówDocument7 pagesLista MateriałówVanessa KowalskaPas encore d'évaluation
- 2.2 - ICR DEC 05 - Fractured Kiln WeldingDocument3 pages2.2 - ICR DEC 05 - Fractured Kiln WeldingKreshnik StratiPas encore d'évaluation
- Module06 NewDocument206 pagesModule06 NewMiltiadis Zabelas0% (1)
- Balon Catalog PDFDocument96 pagesBalon Catalog PDFRudi Syarif Hidayat HarahapPas encore d'évaluation
- Machine Design Elements 6Document9 pagesMachine Design Elements 6ADOBOPas encore d'évaluation
- Microstructure of Metals and Materials PDFDocument70 pagesMicrostructure of Metals and Materials PDFAdriene SantosPas encore d'évaluation
- Roll Mill 1Document48 pagesRoll Mill 1klasiko bente tresPas encore d'évaluation
- 3.28 & 3.29 Kawat Las Nikko Steel 312 2,6 X 350 MM & 3,2 X 350 MMDocument1 page3.28 & 3.29 Kawat Las Nikko Steel 312 2,6 X 350 MM & 3,2 X 350 MMumarPas encore d'évaluation
- Heat TreatmentDocument44 pagesHeat TreatmentMastram HatheshPas encore d'évaluation
- Esco BROCHUREDocument18 pagesEsco BROCHUREJohn GonzalezPas encore d'évaluation
- J 403-Aisi 1060Document1 pageJ 403-Aisi 1060Cho thuê chung cư Cầu GiấyPas encore d'évaluation
- Agricultural Machinery Design - Construction MaterialsDocument4 pagesAgricultural Machinery Design - Construction Materialskirbey0% (1)
- AMS Tempering of Induction Hardened SteelsDocument30 pagesAMS Tempering of Induction Hardened SteelsThang DoPas encore d'évaluation
- Construction - Term 1Document19 pagesConstruction - Term 1Subhojeet SinhaPas encore d'évaluation
- Sae J1397-1992 PDFDocument11 pagesSae J1397-1992 PDFAdam GordonPas encore d'évaluation
- Inconel 625 - Stick (SMAW) and TIG - MIG Welding ProcedureDocument6 pagesInconel 625 - Stick (SMAW) and TIG - MIG Welding ProcedureAngga ErlanggaPas encore d'évaluation
- Design and Manufacturing of ECO Friendly Road Sweeper Machine Ijariie14789Document5 pagesDesign and Manufacturing of ECO Friendly Road Sweeper Machine Ijariie14789SANDIP PANDYAPas encore d'évaluation