Académique Documents
Professionnel Documents
Culture Documents
Galling
Transféré par
hrh_pogcCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Galling
Transféré par
hrh_pogcDroits d'auteur :
Formats disponibles
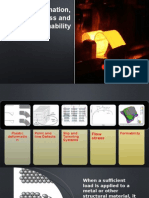
Figure 1 An electron microscope image
shows transferred sheet-material
accumulated on a tool surface during
sliding contact under controlled laboratory
conditions. The outgrowth of material or
localized, roughening and creation of
protrusions on the tool surface is
commonly referred to as a lump.
Figure 2 The damage on the metal sheet,
wear mode, or characteristic pattern shows
no breakthrough of the oxide surface layer,
which indicates a small amount of
adhesive material transfer and a flattening
damage of the sheet's surface. This is the
first stage of material transfer and galling
build-up.
Galling
From Wikipedia, the free encyclopedia
For the botanical concept, see bark-galling.
Galling is a form of wear caused by adhesion between
sliding surfaces. When a material galls, some of it is
pulled with the contacting surface, especially if there is a
large amount of force compressing the surfaces together.
Galling is caused by a combination of friction and
adhesion between the surfaces, followed by slipping and
tearing of crystal structure beneath the surface. This will
generally leave some material stuck or even friction
welded to the adjacent surface, while the galled material
may appear gouged with balled-up or torn lumps of
material stuck to its surface.
Galling is most commonly found in metal surfaces that
are in sliding contact with each other. It is especially
common where there is inadequate lubrication between
the surfaces. However, certain metals will generally be
more prone to galling, due to the atomic structure of
their crystals. For example, aluminum is a metal which
will gall very easily, whereas annealed (softened) steel is
slightly more resistant to galling. Steel that is fully
hardened is very resistant to galling.
Galling is a common problem in most applications
where metals slide while in contact with other metals.
This can happen regardless of whether the metals are the
same or of different kinds. Metals such as brass are often
chosen for bearings, bushings, and other sliding
applications because of their resistance to galling, as
well as other forms of mechanical abrasion.
Contents
1 Introduction
2 Mechanism
3 Incidence and location
4 Prevention
5 Clarification and limitations
6 See also
7 References
Page 1 of 6 Galling - Wikipedia, the free encyclopedia
2014/05/10 http://en.wikipedia.org/wiki/Galling
Figure 3 The damage on the metal sheet or
characteristic pattern illustrates continuous
lines or stripes, indicating a breakthrough
of the oxide surface layer. This type of
contact can, in different proportions, be
found simultaneously with the pattern
found in Figure 4. Both characteristic
patterns found in figure 3 and 4 arise as a
consequence and are sequential to the
pattern in figure 2.
Figure 4 The damage on the metal sheet or
characteristic pattern illustrates an "uneven
surface", a change in the sheet material's
plastic behaviour and involves a larger
deformed volume compared to flattening
of the surface oxides seen in figure 2. This
type of contact is associated and usually
found in different proportions
simultaneously with the pattern in figure 3.
Introduction
Galling is adhesive wear. Galling is caused by
macroscopic transfer of material between metallic
surfaces, during transverse motion (sliding). Galling
occurs frequently whenever metal surfaces are in
contact, sliding against each other, especially with poor
lubrication. Galling often occurs in high load, low speed
applications, but also occurs in high-speed applications
with very little load. Galling is a common problem in
sheet metal forming, bearings and pistons in engines,
hydraulic cylinders, air motors, and many other
industrial operations. Galling is distinctive from gouging
or scratching in that galling involves the visible transfer
of material as it is adhesively pulled (mechanically
spalled) from one surface, leaving it stuck to the other in
the form of a raised lump. Unlike other forms of wear,
galling is usually not a gradual process, but occurs
quickly and spreads rapidly as the raised lumps induce
more galling.
Galling can often occur in screws and bolts, causing the
threads to seize and tear free from either the fastener or
the hole. In extreme cases, the bolt may lock-up to the
point where all turning force is used by the friction,
which can lead to breakage of the fastener or the tool
turning it. Threaded inserts of hardened steel are often
used in metals like aluminum or stainless steel that can
gall easily.
[1]
The tendency of a material to gall is affected by the
ductility of the material. Typically, hardened materials
are more resistant to galling whereas softer materials of
the same type will gall more readily. The propensity of a
material to gall is also affected by the specific
arrangement of the atoms, because crystals arranged in a
face-centered cubic (FCC) lattice will usually allow
material-transfer to a greater degree than a body-
centered cubic (BCC). This is because a face-centered
cubic has a greater tendency to produce dislocations in
the crystal lattice, which are defects that allow the lattice
to shift, or "cross-slip," making the metal more prone to
galling. However, if the metal has a high number of
stacking faults (a difference in stacking sequence between atomic planes) it will be less apt to cross-
slip at the dislocations. Therefore, a material's resistance to galling is usually determined by its
stacking-fault energy. A material with high stacking-fault energy, such as aluminum or titanium, will
be far more susceptible to galling than materials with low stacking-fault energy, like copper, bronze,
or gold. Conversely, materials with a hexagonal close packed (HCP) structure, such as cobalt-based
alloys, are extremely resistant to galling.
[2]
Page 2 of 6 Galling - Wikipedia, the free encyclopedia
2014/05/10 http://en.wikipedia.org/wiki/Galling
In engineering science and in other technical aspects, the term galling is widespread. The influence
of acceleration in the contact zone between materials have been mathematically described and
correlated to the exhibited friction mechanism found in the tracks during empiric observations of the
galling phenomenon, (see figures 1,2,3 and 4). Due to problems with previous incompatible
definitions and test methods, better means of measurements in coordination with greater
understanding of the involved frictional mechanisms, have led to the attempt to standardize or
redefine the term galling to enable a more generalized use. ASTM International has formulated and
established a common definition for the technical aspect of the galling phenomenon in the ASTM
G40 standard: "Galling is a form of surface damage arising between sliding solids, distinguished by
microscopic, usually localized, roughening and creation of protrusions, (i.e. lumps, see figure 1),
above the original surface".
[3]
Mechanism
When two metallic surfaces are pressed against each other the initial interaction and the mating
points are the asperities or high points found on each surface. An asperity may penetrate the
opposing surface if there is a converging contact and relative movement. The contact between the
surfaces, initiates friction or plastic deformation and induces pressure and energy in a small area or
volume called the contact zone.
The elevation in pressure increases the energy density and heat level within the deformed volume.
This leads to greater adhesion between the surfaces which initiate material transfer, galling build-up,
lump growth and creation of protrusions above the original surface. An example of accumulated
transferred material or lump growth on a tool surface can be seen in figure 1. The initial
asperity/asperity contact and surface damage on the opposing sheet-metal surface can be seen in
figure 2.
If the lump (or protrusion of transferred material to one surface) grows to a height of several
micrometers, it may penetrate the opposing surface oxide layer and cause damage to the underlying
bulk material. Damage in the bulk material is a prerequisite for plastic flow that is found in the
deformed volume which surrounds the lump. The geometry and the nominal sliding velocity of the
lump defines how the flowing material will be transported, accelerated and decelerated around the
lump. This torrent or material flow is critical when defining the contact pressure, energy density and
developed temperature during sliding. The mathematical function describing acceleration and
deceleration of flowing material is thereby defined by the geometrical constraints, deduced or given
by the lump's surface contour. The contact damage from deformation of bulk material is seen in
figure 3.
If the right conditions are met, such as geometric constraints of the lump that cause less energy
transfer away from the contact zone than what is added by movement and plastic deformation, an
accumulation of energy can cause a clear change in the sheet materials contact and plastic behaviour;
generally this increases adhesion and the friction force needed for further advancement. The sheet
damage from this type of high energy contact can be seen in figure 4.
In dynamic contact and sliding friction, increased compressive stress is proportionally equal to a rise
in potential energy and temperature within the contact zone or "the system of the mechanics". The
reasons for accumulation of energy during sliding can be the lesser loss of energy away from the
contact zone due to a small surface area on the system boundary and low heat conductivity. Another
reason is the amount of energy that is continuously forced into the system, which is a product of the
Page 3 of 6 Galling - Wikipedia, the free encyclopedia
2014/05/10 http://en.wikipedia.org/wiki/Galling
acceleration of mass and developed pressure. In cooperation these mechanism allows a constant
accumulation of energy and increased energy density and temperature in the contact zone during
sliding.
The process and contact found in figure 4 can be compared to cold welding or friction welding,
because cold welding is not truly cold and the fusing points exhibit an increase in temperature and
energy density derived from applied pressure and plastic deformation in the contact zone.
Incidence and location
Galling or adhesive wear is often found between metallic surfaces where direct contact and relative
motion have occurred. Sheet metal forming, thread manufacturing and other industrial operations
may include moving parts or contact surfaces made of stainless steel, aluminium, titanium, and other
metals whose natural development of an external oxide layer through passivation increases their
corrosion resistance but renders them particularly susceptible to galling.
[4]
In metalworking that involves cutting (primarily turning and milling), galling is often used to
describe a wear phenomenon which occurs when cutting soft metal. The work material is transferred
to the cutter and develops a "lump". The developed lump changes the contact behavior between the
two surfaces, which usually increases adhesion and resistance to further advancement and, due to
created vibrations, can be heard as a distinct sound. An example of a change in material behavior can
be seen in figure 4.
Galling often occurs with aluminium compounds and is a common cause of tool breakdown.
Aluminium is a ductile metal, which means it possesses the ability for plastic flow with relative ease,
which presupposes a relatively consistent and large plastic zone. In comparison, brittle fractures
exhibit a momentary and unstable plastic zone around the cutter, which gives a discontinuous
fracture mechanism that deters the accumulation of heat.
High ductility and flowing material can be considered a general prerequisite for excessive material
transfer and galling build-up because frictional heating is closely linked to the constitution
(physique) of plastic zones around penetrating objects and, as mentioned, brittle fractures seldom
generate a great amount of heat.
Galling can occur even at relatively low loads and velocities because it is the real local pressure or
energy density in the system that induces a phase transition, which often leads to an increase in
material transfer and higher friction.
Prevention
Generally there are two major frictional systems which affect adhesive wear or galling. In terms of
prevention, they work in dissimilar ways and set different demands on the surface structure, alloys
and crystal matrix used in the materials:
Solid surface contact
Lubricated contact
In solid surface contact or unlubricated conditions, the initial contact is characterised by interaction
between asperities and the exhibition of two different sorts of attraction. Cohesive surface energy or
chemical attraction between atoms or molecules connect and adhere the two surfaces together,
Page 4 of 6 Galling - Wikipedia, the free encyclopedia
2014/05/10 http://en.wikipedia.org/wiki/Galling
notably even if they are separated by a measurable distance. Direct contact and plastic deformation
generates another type of attraction through the constitution of a plastic zone with flowing material
where induced energy, pressure and temperature allow bonding between the surfaces on a much
larger scale than cohesive surface energy.
In metallic compounds and sheet metal forming, the asperities are usually oxides and the plastic
deformation mostly consists of brittle fracture, which presupposes a very small plastic zone. The
accumulation of energy and temperature is low due to the discontinuity in the fracture mechanism.
However, during the initial asperity/asperity contact, wear debris or bits and pieces from the
asperities adhere to the opposing surface, creating microscopic, usually localized, roughening and
creation of protrusions (in effect lumps) above the original surface. The transferred wear debris and
created lumps penetrate the opposing oxide surface layer and cause damage to the underlying bulk
material, allowing continuous plastic deformation, plastic flow, and accumulation of energy and
temperature. With regard to the previously defined difference between the initial two types of
attraction in "solid surface contact" or unlubricated conditions, the prevention of adhesive material
transfer is accomplished by the following or similar approaches:
Less cohesive or chemical attraction between surface atoms or molecules.
Avoiding continuous plastic deformation and plastic flow, for example through a thicker oxide
layer on the subject material in sheet metal forming (SMF).
Coatings deposited on the SMF work tool, such as chemical vapor deposition (CVD) or
physical vapor deposition (PVD) and titanium nitride (TiN) or diamond-like carbon coatings
exhibit low chemical reactivity even in high energy frictional contact, where the subject
material's protective oxide layer is breached, and the frictional contact is distinguished by
continuous plastic deformation and plastic flow.
Lubricated contact sets other demands on the materials surface structure, and the main issue is to
retain the protective lubrication thickness and avoid plastic deformation. This is important because
plastic deformation raises the temperature of the oil or lubrication fluid and changes the viscosity.
Any eventual material transfer or creation of protrusions above the original surface will also reduce
the ability to retain a protective lubrication thickness. A proper protective lubrication thickness can
be assisted or retained by:
Surface cavities (or small holes) can create a favourable geometric situation for the oil to retain
a protective lubrication thickness in the contact zone.
Cohesive forces on the surface can increase the chemical attraction between the surface and
used lubrication and enhance the lubrication thickness.
Oil additives may reduce the tendency for galling or adhesive wear.
Clarification and limitations
Galling should not be confused with other cases of attraction between surfaces which do not result in
plastic deformation. These latter types of attraction involve adhesive surface forces or surface energy
theories. Different energy potentials at the surfaces can develop adhesive bonds or cohesive forces
Page 5 of 6 Galling - Wikipedia, the free encyclopedia
2014/05/10 http://en.wikipedia.org/wiki/Galling
that may hold the two surfaces together. Surface energy and the cohesive force phenomenon are not
the same as galling, and are only partially correlated. Galling necessarily involves plastic
deformation of at least one surface.
However, the present research generally fails to make a clear distinction between energy derived
from plastic deformation and the cohesive surface forces with the adjacent counterpart, and chemical
attraction between atoms or surface molecules. The latter is likely to be involved in the initial
material transfer, as shown in figure 2, where only surface-oxide asperities are in contact. But it is
hard to distinguish these adhesive forces from more severe attractions caused by accumulated energy
and increased pressure from plastic deformation. Oxides are brittle and it is probable that most of the
energy in the fracture mechanism is consumed in brittle fracture, but the created wear debris will
instantaneously penetrate the opposing surface. This means that the transferred oxide material will
instantly act as a penetrating body and the concentration of energy, pressure and frictional heating is
immediate. Without this accumulation of energy, the tendency for material transfer will certainly
decrease.
The formation and constitution (physique) of plastic zones around penetrating objects are arguably a
prerequisite and the main factor for excessive material transfer, lump growth and galling build-up
even in the initial contact process (see figure 2).
See also
Tribology
Rheology
Surface engineering
Pin on disc tribometer
References
^ Mechanical Fastening Joining Assembly By James A. Speck -- Marcell Dekker 1997 Page 128 1.
^ Surface Engineering for Corrosion and Wear Resistance By J. R. Davis -- ASM International 2001
Page 76
2.
^ ASTM standard G40 (2006) 3.
^ "Stainless Steel Galling / Locking Up / Freezing
Up" (http://www.estainlesssteel.com/gallingofstainless.html). Estainlesssteel.com. Retrieved 2013-11-04.
4.
Retrieved from "http://en.wikipedia.org/w/index.php?title=Galling&oldid=585195141"
Categories: Tribology Materials degradation Mechanical engineering Materials science
Surface chemistry
This page was last modified on 8 December 2013 at 23:01.
Text is available under the Creative Commons Attribution-ShareAlike License; additional
terms may apply. By using this site, you agree to the Terms of Use and Privacy Policy.
Wikipedia is a registered trademark of the Wikimedia Foundation, Inc., a non-profit
organization.
Page 6 of 6 Galling - Wikipedia, the free encyclopedia
2014/05/10 http://en.wikipedia.org/wiki/Galling
Vous aimerez peut-être aussi
- GAGESDocument156 pagesGAGESUNIISCRIBDPas encore d'évaluation
- ASMEDocument16 pagesASMEviswabvPas encore d'évaluation
- IADC/SPE 99074 A Re-Examination of Drillpipe/Slip Mechanics: F F D D K K L LDocument8 pagesIADC/SPE 99074 A Re-Examination of Drillpipe/Slip Mechanics: F F D D K K L LJaaTa Da shaanPas encore d'évaluation
- Tone ReportDocument58 pagesTone ReportchuchisPas encore d'évaluation
- Corrosion and Materials in Hydrocarbon Production: A Compendium of Operational and Engineering AspectsD'EverandCorrosion and Materials in Hydrocarbon Production: A Compendium of Operational and Engineering AspectsPas encore d'évaluation
- Sour Service TestingDocument14 pagesSour Service TestingHazim KenobiPas encore d'évaluation
- Sucker Rod Failure Analysis Brochure V4 CompressedDocument24 pagesSucker Rod Failure Analysis Brochure V4 CompressedKoray YilmazPas encore d'évaluation
- Team Pipe RepairDocument24 pagesTeam Pipe RepairDavid Rios CruzPas encore d'évaluation
- Analysis of Steam Turbine Blade Failure LOW PRESSURE TURBINEDocument7 pagesAnalysis of Steam Turbine Blade Failure LOW PRESSURE TURBINEpoojaPas encore d'évaluation
- Drill String InspectionsDocument13 pagesDrill String Inspectionsamin peyvandPas encore d'évaluation
- Case Study of Bolt FailureDocument13 pagesCase Study of Bolt FailureJB MadeleinePas encore d'évaluation
- Plastic Deformation, Flow Stress and FormabilityDocument35 pagesPlastic Deformation, Flow Stress and FormabilityAnna100% (1)
- Form of Corrosion Illustration Form of Corrosion IllustrationDocument2 pagesForm of Corrosion Illustration Form of Corrosion IllustrationkhalesnabilPas encore d'évaluation
- On Oxygen-Induced Corrosion of An Oil Refinery Condensate Fraction at Ion UnitDocument17 pagesOn Oxygen-Induced Corrosion of An Oil Refinery Condensate Fraction at Ion UnitAzmi Mohammed NorPas encore d'évaluation
- Top Tensioned RiserDocument1 pageTop Tensioned RiserShen YenPas encore d'évaluation
- ReviewofWearandGallingCharacteristicsofStainlessSteel 9006Document0 pageReviewofWearandGallingCharacteristicsofStainlessSteel 9006fahreezPas encore d'évaluation
- Mechanism of Failure by Hydrogen-Induced Cracking PDFDocument216 pagesMechanism of Failure by Hydrogen-Induced Cracking PDFsankhadipPas encore d'évaluation
- Method of Statement For Electrical Works-UPDATEDocument35 pagesMethod of Statement For Electrical Works-UPDATEWaleed AbdulsattarPas encore d'évaluation
- Guidelines For Selecting Packer Elements and Seals: SectionDocument7 pagesGuidelines For Selecting Packer Elements and Seals: SectionMohamed Shafie100% (1)
- Matrix APDDocument1 pageMatrix APDQHSE BTGPas encore d'évaluation
- The Forms of Corrosion-Part2Document71 pagesThe Forms of Corrosion-Part2quiron2010100% (1)
- Corrosion Predictions and Risk Assessment in Oilfield Production SystemsDocument37 pagesCorrosion Predictions and Risk Assessment in Oilfield Production SystemsMuhammad AwaisPas encore d'évaluation
- Failure Analysis of Stress Corrosion Cracking of 316L Structured Packing in A Distillation TowerDocument10 pagesFailure Analysis of Stress Corrosion Cracking of 316L Structured Packing in A Distillation TowerJohanPas encore d'évaluation
- Bor1991 - Effect of Pearlite Banding On Mechanical Properties of Perlitic SteelDocument12 pagesBor1991 - Effect of Pearlite Banding On Mechanical Properties of Perlitic SteelAlejandroAcuñaMaureiraPas encore d'évaluation
- ControlFlow GateValveDocument29 pagesControlFlow GateValvehrh_pogcPas encore d'évaluation
- Use of Alloy 718 and 725 in Oil and Gas IndustryDocument9 pagesUse of Alloy 718 and 725 in Oil and Gas IndustryEddyWangPas encore d'évaluation
- Corrosion Handbook - Morris Place March 2008Document96 pagesCorrosion Handbook - Morris Place March 2008Bilal KhashanPas encore d'évaluation
- Galling InformationDocument3 pagesGalling Informationvp989Pas encore d'évaluation
- API RP 571 - Damage Mechanisms SpreadsheetDocument12 pagesAPI RP 571 - Damage Mechanisms SpreadsheetSoftware ManagerPas encore d'évaluation
- Hydrogen CrackingDocument26 pagesHydrogen CrackingmmkattaPas encore d'évaluation
- Scale PresentationDocument59 pagesScale PresentationMohamed SadekPas encore d'évaluation
- Introduction To OCTG Premium Connections Connectors Finite Element AnalysisDocument7 pagesIntroduction To OCTG Premium Connections Connectors Finite Element AnalysisHector BarriosPas encore d'évaluation
- ABS Group Presentation To NOIA G Burton R1Document10 pagesABS Group Presentation To NOIA G Burton R1jackiemaddy100% (1)
- 9365Document69 pages9365Kivanc NEROGLUPas encore d'évaluation
- Electrical Model of Thermal Power PlantDocument81 pagesElectrical Model of Thermal Power PlantMahesh KumbharPas encore d'évaluation
- Material Turbina A GásDocument47 pagesMaterial Turbina A GásGetúlio RibeiroPas encore d'évaluation
- Mat Spec, Astm A 694 F65 Low Alloy Steel Forgings: SubseaDocument14 pagesMat Spec, Astm A 694 F65 Low Alloy Steel Forgings: SubseaJones Pereira Neto100% (1)
- Metallurgy For Downhole Oilfield Equipment PDFDocument1 pageMetallurgy For Downhole Oilfield Equipment PDFYousuf MemonPas encore d'évaluation
- III 1 Epoxy Coatings and Cement Mortar Lining - CleanedDocument17 pagesIII 1 Epoxy Coatings and Cement Mortar Lining - CleanedKok WaiPas encore d'évaluation
- Pitting CorrosionDocument7 pagesPitting CorrosionAminPas encore d'évaluation
- T 18Document10 pagesT 18khuramluck100% (2)
- tn-16 Rate Process Method Projecting Pe PipeDocument8 pagestn-16 Rate Process Method Projecting Pe Pipeyrdna nawaiteos100% (1)
- PPL Su 1050 O.1Document56 pagesPPL Su 1050 O.1olalekanPas encore d'évaluation
- CO2 Corrosion NotesDocument9 pagesCO2 Corrosion NotesRony MayrizalPas encore d'évaluation
- Bridge Welding ProcessDocument151 pagesBridge Welding Processcentaury2013Pas encore d'évaluation
- World Oil CorrosionDocument4 pagesWorld Oil CorrosionmutemuPas encore d'évaluation
- American Fastener - ASTM, SAE, and ISO Grade MarkingsDocument6 pagesAmerican Fastener - ASTM, SAE, and ISO Grade MarkingsmameeranPas encore d'évaluation
- 06189G FrontmatterDocument11 pages06189G FrontmatterEd Marti100% (1)
- Shell - Corrosion FatigueDocument3 pagesShell - Corrosion FatiguexaploftPas encore d'évaluation
- Perforated PipesDocument1 pagePerforated PipeselcivilengPas encore d'évaluation
- A Review - Weight Loss Studies On The Corrosion Behavior of Some Metals in Various MediaDocument8 pagesA Review - Weight Loss Studies On The Corrosion Behavior of Some Metals in Various MediaRonald GarciaPas encore d'évaluation
- Iso 13679 Connection Testing: Test DatasheetDocument2 pagesIso 13679 Connection Testing: Test DatasheetMostafa HashemiPas encore d'évaluation
- Galling 2Document6 pagesGalling 2Ricardo MartinsPas encore d'évaluation
- 2.1) Technical Data Sheet - SS-COAT 909-Silver Zinc CoatDocument3 pages2.1) Technical Data Sheet - SS-COAT 909-Silver Zinc CoathaharameshPas encore d'évaluation
- Nyborg (2008) - Kjeller Field DataDocument5 pagesNyborg (2008) - Kjeller Field DataCharlyBoeingPas encore d'évaluation
- Dissimilar Metal Weldability Concepts Alber SadekDocument45 pagesDissimilar Metal Weldability Concepts Alber SadekMohammad AliPas encore d'évaluation
- Elevated Temperature Erosive Wear of Metallic Materials 2006 ROYDocument24 pagesElevated Temperature Erosive Wear of Metallic Materials 2006 ROYLuiz Rafael R. SilvaPas encore d'évaluation
- NS-1-70. Hardness TestingDocument2 pagesNS-1-70. Hardness TestingWHWENPas encore d'évaluation
- BOPP - Type - GK-Ops-Maint Procedure RevisedDocument16 pagesBOPP - Type - GK-Ops-Maint Procedure RevisedTarasPas encore d'évaluation
- Corrosion Behavior of Steels in Gulf Seawater Environment...Document15 pagesCorrosion Behavior of Steels in Gulf Seawater Environment...DoctorAtomicPas encore d'évaluation
- SMS Casting BreakoutsDocument20 pagesSMS Casting BreakoutsShubham KaushikPas encore d'évaluation
- Equivalência Materiais - OTIMO PDFDocument40 pagesEquivalência Materiais - OTIMO PDFTúlio Barata FrançaPas encore d'évaluation
- ASME B16.47 Ser. A, Ser. B Industry Standard and AWWA Flanges Robert-James Sales, IncDocument25 pagesASME B16.47 Ser. A, Ser. B Industry Standard and AWWA Flanges Robert-James Sales, IncChairul AnwarPas encore d'évaluation
- 562Document98 pages562Kelvin Octavianus DjohanPas encore d'évaluation
- Gmaw Cladding PDFDocument20 pagesGmaw Cladding PDFMurad AlamPas encore d'évaluation
- Accelerated CoolingDocument7 pagesAccelerated CoolingHarikrishnan N SivaprasadPas encore d'évaluation
- Fluid Compatibility GuideDocument18 pagesFluid Compatibility GuideNazirul FariqPas encore d'évaluation
- Tubing SpecificationsDocument5 pagesTubing Specificationsrasnowmah2012Pas encore d'évaluation
- Advances in Research on the Strength and Fracture of Materials: An OverviewD'EverandAdvances in Research on the Strength and Fracture of Materials: An OverviewD M R TaplinPas encore d'évaluation
- Tuv Sud Asset Integrity Management PDFDocument2 pagesTuv Sud Asset Integrity Management PDFhrh_pogcPas encore d'évaluation
- Abrasive Wear of BallDocument15 pagesAbrasive Wear of Ballhrh_pogcPas encore d'évaluation
- 3 3 1 LC Desuperheater SolutionsDocument8 pages3 3 1 LC Desuperheater Solutionshrh_pogcPas encore d'évaluation
- Heat Resistant Insulation - 1 PageDocument1 pageHeat Resistant Insulation - 1 Pagehrh_pogcPas encore d'évaluation
- Tuv Sud Asset Integrity ManagementDocument4 pagesTuv Sud Asset Integrity Managementhrh_pogcPas encore d'évaluation
- A Study On Contact FatigueDocument33 pagesA Study On Contact Fatiguehrh_pogcPas encore d'évaluation
- Opac 10138Document42 pagesOpac 10138Maja RadojevićPas encore d'évaluation
- G 1020Document28 pagesG 1020hrh_pogcPas encore d'évaluation
- Swaging or Radial ForgingDocument3 pagesSwaging or Radial Forginghrh_pogcPas encore d'évaluation
- 3 3 1 LC Desuperheater SolutionsDocument8 pages3 3 1 LC Desuperheater Solutionshrh_pogcPas encore d'évaluation
- Petromaks DEMO 2000Document35 pagesPetromaks DEMO 2000hrh_pogcPas encore d'évaluation
- 3 3 1 LC Desuperheater SolutionsDocument8 pages3 3 1 LC Desuperheater Solutionshrh_pogcPas encore d'évaluation
- Drill USA: TEL: 713-594-7447 Rocksprings, TexasDocument4 pagesDrill USA: TEL: 713-594-7447 Rocksprings, Texashrh_pogcPas encore d'évaluation
- Forging PDFDocument18 pagesForging PDFhrh_pogcPas encore d'évaluation
- Laser NistDocument57 pagesLaser Nisthrh_pogcPas encore d'évaluation
- G02 WearNewsFall2009Document9 pagesG02 WearNewsFall2009hrh_pogcPas encore d'évaluation
- Johnzink-Flare Gas RecoveryDocument1 pageJohnzink-Flare Gas Recoveryhrh_pogcPas encore d'évaluation
- Inclined StressDocument28 pagesInclined StressAli El-Gazzar100% (1)
- Longitudinal Tooth ContactDocument6 pagesLongitudinal Tooth Contacthrh_pogcPas encore d'évaluation
- Elements of Metric Gear TechnologyDocument234 pagesElements of Metric Gear Technologyhrh_pogcPas encore d'évaluation
- Pilot Relief SarasinDocument44 pagesPilot Relief Sarasinhrh_pogcPas encore d'évaluation
- WJM Technologies: Excellence in Material JoiningDocument5 pagesWJM Technologies: Excellence in Material JoiningA K SinghPas encore d'évaluation
- SMAW Temper Bead Weld Repair Without GrindingDocument5 pagesSMAW Temper Bead Weld Repair Without Grindinghrh_pogcPas encore d'évaluation
- ASME InterpretationDocument4 pagesASME Interpretationhrh_pogcPas encore d'évaluation
- Testing and Inspection of WeldsDocument20 pagesTesting and Inspection of Welds7harma V1swaPas encore d'évaluation
- Wireless Selection GuideDocument50 pagesWireless Selection GuideShailesh KshatriyaPas encore d'évaluation
- Ericsson Essentials Health & Safety Plan Sample: Good For Smaller Projects and Bid QualificationsDocument18 pagesEricsson Essentials Health & Safety Plan Sample: Good For Smaller Projects and Bid QualificationsmohammedelrabeiPas encore d'évaluation
- Specification For Construction of Concrete ReservoirsDocument18 pagesSpecification For Construction of Concrete ReservoirsKeysha ApriliaPas encore d'évaluation
- Carbon Regeneration KilnsDocument3 pagesCarbon Regeneration KilnsLuis LabradorPas encore d'évaluation
- Tally Erp 9.0 Material Control Centre in Tally Erp 9.0Document27 pagesTally Erp 9.0 Material Control Centre in Tally Erp 9.0Raghavendra yadav KMPas encore d'évaluation
- Denmark Bye LawsDocument10 pagesDenmark Bye LawshimaniwatalPas encore d'évaluation
- Annex 1 - Air Dryer Duct DRG List - 140717Document1 pageAnnex 1 - Air Dryer Duct DRG List - 140717vemanreddy29Pas encore d'évaluation
- 2015f - CPCS 202 - Quiz 2 - SolutionDocument3 pages2015f - CPCS 202 - Quiz 2 - SolutionOsama MaherPas encore d'évaluation
- Ds 50 BrochureDocument2 pagesDs 50 BrochureRaulMesaPas encore d'évaluation
- Specification: CL 21 B 105 K A F N N N EDocument3 pagesSpecification: CL 21 B 105 K A F N N N EcometPas encore d'évaluation
- LucasFilm Sound Effects Library - Track & Index ListDocument22 pagesLucasFilm Sound Effects Library - Track & Index ListKALFER0% (1)
- Flapper Diverter Valve BrochureDocument4 pagesFlapper Diverter Valve BrochureRicardo Ramírez ZapataPas encore d'évaluation
- Artemis MK V PDFDocument2 pagesArtemis MK V PDFdeepsea74Pas encore d'évaluation
- K 1020189523hfjfjDocument3 pagesK 1020189523hfjfjCarlos Angel Vilcapaza CaceresPas encore d'évaluation
- Behringer EPR900 Powered Speaker SchematicsDocument14 pagesBehringer EPR900 Powered Speaker SchematicsJimPas encore d'évaluation
- Aircraft Construction, Repair & Modification Mock Board Exam 10Document5 pagesAircraft Construction, Repair & Modification Mock Board Exam 10July TadePas encore d'évaluation
- Owners Manual Dominar 250Document52 pagesOwners Manual Dominar 250RaghavPas encore d'évaluation
- Concrete Solutions-ProgramDocument18 pagesConcrete Solutions-ProgramEfthymios TatsisPas encore d'évaluation
- Build Your First Mobile Flex Application: Lab ExercisesDocument37 pagesBuild Your First Mobile Flex Application: Lab ExercisesSrdjan MarjanovicPas encore d'évaluation
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument24 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanPas encore d'évaluation
- Assignment 2 Ce Law Ethics Contracts Midterm - 103742Document13 pagesAssignment 2 Ce Law Ethics Contracts Midterm - 103742Myka SanchezPas encore d'évaluation
- Torre Sauter 0 - 5 - 320 - 025 - 4 PDFDocument27 pagesTorre Sauter 0 - 5 - 320 - 025 - 4 PDFGuiPas encore d'évaluation
- Qualcomm Extensible Diagnostic MonitorDocument2 pagesQualcomm Extensible Diagnostic MonitorGuilherme Pereira0% (1)
- Digital Joints Solutions - Sep-2016-CatalogueDocument102 pagesDigital Joints Solutions - Sep-2016-CatalogueiamlpPas encore d'évaluation