Académique Documents
Professionnel Documents
Culture Documents
Userguide 35.en
Transféré par
Edward PittsTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Userguide 35.en
Transféré par
Edward PittsDroits d'auteur :
Formats disponibles
1
Electron Spectroscopy of Surfaces
1. General remarks
Electrons are well suited probes for the investigation of electronic and geometrical properties
of clean and adsorbate covered surfaces. The use of electrons includes various techniques as:
Low Energy Electron Diffraction (LEED) for the investigation of the surface geometry in
the reciprocal space.
Electron Microscopy for structure investigations in real and (at the resolution limit) in the
reciprocal space.
Electron Energy Loss Spectroscopy (EELS) and High Resolution Electron Energy Loss
Spectroscopy (HREELS) for the investigation of electronic and vibrational excitations.
Electron Stimulated Desorption (ESD) for studies of the microscopic processes related with
irradiation damage
Photoelectron Spectroscopy (PES) and Auger Electron Spectroscopy (AES) for investiga-
tions of electronic properties and for surface analysis.
It is the purpose of this experiment to make you familiar with these last two techniques, PES
and AES.
The main reason for the widespread use of electrons as probes in surface science is their short
inelastic mean free path (Fig.1). For electrons between 10 and 1000 eV kinetic energy it is
only a few atomic layers. In a typical photoemission experiment, only electrons from a narrow
region at the solid-vacuum interface will reach the detector without energy loss. This makes
electrons very surface sensitive probes.
Fig.1: Inelastic mean free path of electrons as a function of their kinetic energy, for various
elements.
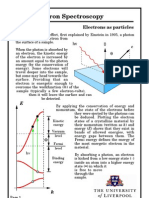
2. Photoelectron Spectroscopy
2.1. Basics
The basis of PES is the photoeffect, which was experimentally discovered by Hallwachs in
1887 and theoretically explained by Einstein in 1905. Although well known for a long time, it
2
took more than 60 years of experimental development until Siegbahn and coworkers suc-
ceeded in establishing PES as a standard analytical method in atomic, molecular, and solid
state physics.
The basic process is simple: A photon of well known energy h is absorbed by an electronic
systems, promoting an electron from an occupied electronic level into an unoccupied state. If
this unoccupied state lies beyond the vacuum level, the electron can escape into the vacuum
and can be detected by an energy sensitive analyzer. The energy balance is as follows:
) ( ) ( sys E E sys E
f kin i
+ = + h
E
i
(sys) and E
f
(sys) are the total energies of the system (atom, molecule, cluster, or solid)
before and after photon absorption, respectively. E
kin
is the kinetic energy of the photoelec-
tron. In a one electron picture (i.e. neglecting the energy of mutual electron interaction) the
difference of E
f
(sys) - E
i
(sys) can be understood as the binding energy of the ejected electron:
kin i f B
E sys E sys E E = = h ) ( ) (
Recording the number of photoelectrons as a function of their kinetic energy yields a spec-
trum of distinct lines, which in first approximation reflect the occupied orbitals of the elec-
tronic system under investigation. Because the number and binding energies of these occupied
orbitals are different for each chemical element, the PE spectra differ as well. They can serve
as "fingerprints" of the different elements, making PES a very useful analytical tool.
Fig.2: Schematic of the photoionization process (left). The total width of the photoelectron
spectrum is equal to h-
sample
(right side).
Depending on the photon energy we discriminate core electron (or: inner shell, E
b
~40 eV)
and valence electron (outer shell) PES (0 E
b
~40 eV). The latter is termed UPS
(ultraviolet photoelectron spectroscopy) because UV photons from gas discharge sources or
UV synchrotron beamlines are commonly used. PE spectroscopy with soft x-rays is labeled
XPS (x-ray photoelectron spectroscopy). Common light sources are x-ray tubes with or
without an additional monochromator, and soft x-ray synchrotron beamlines. At this
3
experiment you will perform XPS measurements with the characteristic soft x-ray light from
an aluminium anode (Al K radiation: h = 1486.6 eV).
2.2. Quantitative aspects of PES
2.2.1. Energetic shifts (chemical shifts and satellites)
2.2.1.1 Chemical shifts of core electron lines
If we compare the binding energies of core electrons of a distinct atom in different systems
(i.e., in different molecules or solids), we find that the exact value of the binding energy de-
pends of the chemical environment of the atom. These energy shift are termed "chemical
shifts". By comparison with data from well known "standard" substances the values of these
shifts can be used for the determination of the nature of the chemical bond encountered by the
atom in many cases. This application of XPS, which is expressed by the alternative name
ESCA (electron spectroscopy for chemical analysis), is an important application of inner shell
PE in materials research.
Initial state effects
Ground state effects
Ground state effects ar those contributions to the observed energy shifts which are due to
variations of the distribution of valence electrons depending on the chemical environment, i.e.
which are due to the electronic part of the potential. Experimentally observed chemical shift
are only partly due to ground state effects. So called final state effects (see below) contribute
as well.
Reference level effects:
Shifts of the reference levels for the kinetic energy scale may also contribute to experimen-
tally obtained initial state shift. In order to be able to compare E
b
values, all PE data have to
be measured with respect to identical reference levels. This can be difficult for atoms in dif-
ferent environments. For gas phase measurement E
b
values are commonly referred to the vac-
uum level, whereas for solid state investigations the Fermi level is the commonly adopted
zero point of the E
b
scale. If gas phase and solid state data are compared one has to know the
difference between the vacuum and the Fermi level (= the work function).
Final state effects:
Relaxation:
A very important final state effect is the relaxation of the electronic system as an answer to
the creation of a core hole (= the vacancy in the inner shell). The core hole constitutes a posi-
tive charge acting on the residual electrons. By the thus induced polarization of the electronic
system the core hole is screened. This screening effect consists of an intra atomic and an extra
atomic contribution. The intra atomic part is due to the relaxation of the electrons of the ex-
cited atom itself. For isolated atoms it is the only contribution. For atoms bound in molecules
or solids the electrons of the neighboring atoms are polarized by the creation of the core hole
as well, although to a lesser extent, giving rise to the extra atomic contributions of relaxation.
Because of energy conservation, screening reduces the apparent E
b
of the core hole. Because
screening depends on the electronic environment, relaxations shifts differ from system to
system for a specific core hole. This difference in combination with the initial state effects
(see above) constitute the main contributions to the chemical shift.
Multiplet splitting
Removal of a core electron may create different final state configurations with different spin
and orbital angular momentum, and therefore different binding energies in PES (= different
lines in the spectrum). For N1s photoionization of isolated NO, e.g., two lines are obtained
4
which correspond to parallel or anti parallel orientation of the residual 1s electron's spin with
respect to the unpaired electron in the molecular 2 orbital (triplet and singlet final states,
respectively). Even for photoemission from closed shell systems (e.g.: rare gases) multiplet
splitting is obtained for parallel or anti parallel orientation of spin and orbit (e.g.: 2p
3/2
and
2p
1/2
). For open shell systems the situation is even more complicated.
Multi electron excitations and satellites.
The reorganisation of the electrons upon the creation of the core hole can lead to electroni-
cally excited final states. Because of energy conservation this excitation energy is missing in
the kinetic energy of the photoelectron, i.e., we find a redshifted satellite line. We can dis-
criminate different processes. We encounter a shake-up satellite, if an additional electron is
promoted from an occupied into a bound unoccupied state (means: a state below the vacuum,
but above the Fermi level (for solids), or (for molecules) from the HOMO (highest occupied
molecular orbital) to the LUMO (lowest unoccupied MO, as far as this state lies below the
vacuum level). If the second electron is promoted into a continuum state above the vacuum
level, we speak of a shake-off satellite. Other possible satellites are plasmon satellites (core
ionisation + plasmon excitation = collective oscillation of electrons with respect to the posi-
tively charged ion cores), or inter band satellites in solids corresponding to additional excita-
tions of electrons into unoccupied states above "Fermi". The latter may lead to discrete lines
in some cases, but may also show up as an asymmetric broadening of the low energy edge of
the photoemission peaks on the kinetic energy scale.
2.2.2. Intensity
The interaction of electromagnetic radiation with an atom is described by the Hamiltonian:
'
2
1
0
2
H H V e A
c
e
p
m
H + = + +
+ =
r
r
with:
V p
m
H + =
2
0
2
1 r
and:
( )
2
2
2
2 2
' A
mc
e
e p A p A
mc
e
H
r
r
r
r
r
+ + + =
By assuming source free space and appropriate gauge transformations (Coulomb gauge:
0 , 0 = = A div
r
) and neglecting higher orders of A we obtain:
p A
mc
e
H
r
r
2
' =
A
r
is the vector potential of the electromagnetic radiation defining the direction of polariza-
tion, and p is the momentum operator. With Fermi's golden rule we obtain in first order
perturbation theory the transition rate P
fi
from the initial state
i
with Energy E
i
to the final
state
f
with energy E
f
:
5
( ); '
2
2
h E E H P
i f i f fi
=
h
And for the angle integrated cross section:
); ( ~
2
h E E M
dE
d
i f fi
f
with the matrix element
i f fi
p A M =
r
r
and the sum over all initial and final states which are
allowed by energy conservation (= for which the argument of the delta function is zero).
Because the matrix element in general does not depend on the chemical environment, the
intensity of the emission line of an atom can be used for quantitative analysis.
The absolute intensity I of a PE line from an element A per solid angle is equal to:
dV
E
z
z y x N z y x J d E y x T
d
d
d
dI
A B
A A
A A
) cos
) (
exp( ) , , ( ) , , ( ) , , , (
with the transmission function of the electron analyzer T, the incident photon flux J, the den-
sity of atoms A N
A
, and the inelastic mean free path of electrons with the energy E
A
in
medium B
B
(E
A
). is the polar angle between analyzer axis and surface normal.
In most cases, calibration of absolute intensities based on this formula is not possible. In
practical experiments, measurements of relative intensities are important, for instance for the
detection of relative surface coverages by adsorbates or contaminants.
Fig.3: Schematic of the detection geometry
6
2.2.3 Line shapes
Experimentally obtained line shapes are convolutions of different contributions. They result
i) from the equipment used in the experiment; and ii) from the photoemission process itself.
To i) contribute the line width and the line shape of the photon source which is used for the
excitation, and the energy resolution (= the transfer function) of the electron analyzer. Type ii)
are broadening due to the limited lifetime of the core hole, and broadening by coupling to the
nuclear motion (intra molecular vibrations including rapidly dissociating antibonding states,
and phonons). Lifetime broadening causes Lorentzian line shapes. We have to replace the
delta function in the golden rule by a Lorentzian:
( ) [ ]
2 2
+
=
h E E
L
i f
with energy broadening
h
; (= half width half maximum: HWHM). Vibrational broad-
ening, on the other hand, causes Gaussian line shapes. For metals we obtain additional asym-
metric line broadening by electron hole excitations at the Fermi edge. These lead to broaden-
ing of the low energy edge of the photo emission peak.
2.2.4 Inelastic contributions to the photoelectron spectrum
Passing through the bulk of a solid, electrons can suffer inelastic energy losses on their way to
the analyzer. These inelastic processes show up as a stepwise increase of the background in-
tensity at the low kinetic energy side of each photoemission peak. For the correct determina-
tion of peak areas, peak positions and peak widths this background has to be subtracted. Par-
ticularly at low kinetic energies (< 100 eV) this background is very large due to the increasing
number of secondary electrons, and the evaluation of the spectra may be difficult.
3. Auger Electron Spectroscopy (AES)
Photoionization of an inner shell level creates an electronically excited state. This excited
states can decay via two different routes:
a) The hole in the inner shell X is filled by an electron from an outer shell Y, and a photon is
emitted. This is the well known x-ray emission process or fluorescent decay:
) ( ) ( Y E X E h
f i
=
E
i
(X), E
f
(Y) are total energies of the system with holes in the shells X and Y, respectively.
b) The hole in the inner shell X is filled by an electron from an outer shell Y as for the above
case, but the excess energy is transferred to a third electron from an outer shell Z which is
emitted. The final state is a doubly ionized state (Auger effect). The kinetic energy of the
emitted electron is (with respect to E
F
):
) , ( ) (
,
Z Y E X E E
f i F kin
= ;
with E
i
(X) and E
f
(Y,Z) as total energies of the system with one hole in shell X, and two holes
in shells Y and Z, respectively. This radiationless decay is of course only permitted for
E
kin,F
> 0 which limits the number of possible X,Y,Z combinations.
7
The branching ratio of processes a) and b) is governed by the respective decay cross sections.
For the E
b
range below 1.5 keV as in our experiment, the radiationless decay dominates by
far.
In most cases, core ionization and core decay can be treated as independent (2 step process).
Apart from photoionization we can create the primary core hole also by inelastic electron
scattering. We discriminate XAES (x-ray induced Auger electron spectroscopy) and EAES
(electron induced Auger spectroscopy), depending if the primary hole has been created by
photoionization or by electron scattering. Excitation by electrons is very popular, because
electron sources are much cheaper than photon sources. Moreover, electron guns can have
very small beam spots enabling element specific Auger electron microscopy. Core ionization
with photons (XAES), on the other hand, has the advantage that the concomitant rate of va-
lence excitations is lower than in the EAES case. Valence excitations, however, are the main
sources for irradiation damage, i.e., XAES is less destructive. Because of this, XAES is an
important technique for the investigation of radiation sensitive layers on surfaces.
We obtain in AES as in the PES case spectra with discrete, element specific lines (see Fig.4
for a survey of different processes). As for PES, the spectra can be used for element specific
analysis. The nomenclature of Auger lines follows the electron shells which contribute to the
decay. An Auger line labeled XYZ would correspond to a primary hole in the X shell which
decays into final holes in the Y and the Z shell (see Fig.5). Commonly the x-ray nomenclature
is used for the identification of the holes: The K shell corresponds to 1s electrons, L
1
,L
2
,L
3
to
2s, 2p
1/2
, and 2p
3/2
electrons, respectively, etc. The decay cross section is maximum if one of
the two final holes has the same principal quantum number (= is from the same shell) as the
primary hole. These XXZ processes are called Coster Kronig transitions. For super Coster
Kronig all participating holes have identical principal quantum numbers (e.g.: N
4
N
6
N
6
for
tungsten).
Fig.4: Elements of electron spectra: Photoionization of d) valence levels; of b) core levels; c)
Auger decay; a) inelastic loss processes.
8
Fig.5: Different types of Auger decay processes: a) KLL decay; b) LMM decay; c) Coster-
Kronig transition; d) Auger decay including valence levels supplies chemical information
Fig.6: Reduced KLL Auger energies from theory (lines) and experiment (points) as a function
of the atomic number Z. Low Z (left side): LS-coupling, high Z (right side): jj-coupling, in-
termediate coupling case between.
9
Satellite lines occur for AES as for PES. Multiplet features are even richer for AES than for
PES because of the doubly ionized final state, i.e. we have at least two holes which interact
(decay satellites, i.e. shake-up or shake-off processes upon decay make the situation even
more complicated). Depending on the atomic number Z, LS (for low Z) or jj coupling prevails
(for large Z). See Fig.6 for possible final states of KLL transitions.
Calculation of kinetic energies of Auger electrons according to the above formula can be dif-
ficult for electronic systems larger than isolated atoms. Approximations are then necessary.
One possibility is to take one electron binding energies for the initial and the two final holes
from PES with an additional term U which accounts for the interaction of the two holes in the
final state:
;
, , , ,
U E E E E
XPS
Z b
XPS
Y b
XPS
X b F kin
=
Neglecting U we obtain the energy range of E
kin
for a distinct transition. U can then be ob-
tained experimentally and supplies important information about the interaction energy of the
two holes (see Fig.6).
The intensity of an Auger line is governed by the local overlap of the final state wave func-
tions
Y
and
Z
with the wave function
X
of the initial hole. Because of this, AES enables
investigations of electron densities at the site of a specific core hole.
4. Adsorption
4.1 Preface
We call the process when particles from the gas phase become bound to a surface "adsorp-
tion". The energy released upon this process is called energy of adorption U
ad
or sometimes
also binding energy (not to mix up with the binding energy from PES).
The reverse process, i.e. the release of gas particles from the surface is called desorption. De-
sorption may be stimulated by heat (thermal desorption), by electrons (electron stimulated
desorption), or by photons or ions (photon, respectively ion stimulated desorption).
4.2. Types of adsorption
4.2.1 Physisorption
The particles are bound by Van der Walls forces to the surface. The adsorption energy U
ad
is
small, typically less then 40kJ/ mol. Formation of multilayers is possible (condensates).
4.2.2 Chemisorption
The particles are bound by chemical forces, e.g. transfer of electrons and/or formation of co-
valent bonds (common molecular orbitals). In most cases the adsorption energy is much larger
than 40 kJ/mole. Chemisorbates form typically only one layer (= direct neighborhood of sur-
face atoms and chemisorbate is required).
4.3. Adsorption order
First order adsorption: One particle from the gas phase yields one particle of the adsorbate,
i.e., molecules, which do not dissociate upon adsorption. Reversed process: First oder desorp-
tion.
Examples: CO/nickel CO chemisorbes on Ni surfaces molecularly with ~ 120 kJ/mole
Ar/Ruthenium Ar physisorbes on Ru with ~10 kJ/mole
10
Second order adsorption (dissociative adsorption):
A molecule dissociates upon adsorption. One particle in the gas phase yields two particles on
the surface. The reverse process is associative desorption or second order desorption
Example: H
2
/Nickel: H
2
dissociates into 2 H atoms on Ni (~ 90 kJ/mole).
Fig.7 shows typical potential curves of second order adsorption of H
2
on a transition metal
like Ni . The full line corresponds to the interaction of the intact molecule with the surface,
the broken line belongs to the chemisorptive bonds of the H atoms. At the intersection of both
curves the molecule dissociates. If this intersection lies at positive energy values, the disso-
ciation is activated. The activation energy E
A
has to be supplied by the particle approaching
the surface, e.g. by its kinetic energy. If the intersection of the two curves lies below zero en-
ergy, we have the case of non-activated adsorption.
Fig.7: Energy potential curves for 2
nd
order adsorption, e.g. for H
2
/Ni. E
DISS
: Energy of
dissociation; E
A
: Activation energy.
5. Nomenclature (common in surface science):
(Relative) coverage : Number of adsorbate particles N
ad
/ number of substrate partic-
les N
sub
Collision rate : Number of particles hitting the surface / time x area;
= p/(2mk
B
T)
1/2
; T: Temperature; m: Particle mass; p: Pres-
sure
Sticking coefficient s: Probability that a particle colliding with the surface becomes
adsorbed.
s = (N
ad
/dt)/(N
coll
/dt); 0 s 1;
11
Lifetime of an adsorbate : Time, an adsorbed particle stays at the surface before desorp-
tion.
= (k
0
)
-1
exp(U
ad
/k
B
T
surface
). k
0
: typically 10
13
/s
6. Experimental (details during the experiment):
Vacuum system: Ultra high vacuum (UHV) system with a base pressure in the 10
-8
range.
Pumps: Ion getter pump + titanium sublimation pump.
Sample: Tungsten ribbon; it can be heated by direct current up to 2500K.
X-ray source: Characteristic Al K radiation from an Al x-ray anode. Typical operation con-
ditions: U
anode
= 12 kV; I
emission
= 20 mA.
X-ray lines: Al K
1,2
: 1486.6 eV + K
3,4
: 1496 eV (10% intensity of K
1,2
). Linewidth of the
K
1,2
line: HWFM = 0.78 eV (0.43 eV multiplet splitting + 0.47 eV lifetime broadening).
Electron analyzer: Electrostatic 180
0
hemisperical analyzer. An electrostatic lens between the
sample and the entrance slit of the analyzer focuses the photoelectrons from the sample onto
the entrance slit. The 180
0
analyzer is doubly focusing: A point source at its entrance is
imaged into a point image at its exit. Its energy resolution is:
E/E
p
= d/2r + ; E: Energy window; E
p
: Pass energy; d: geometrical average of entrance
and exit slit widts; r: average radius of hemispheres; : 1/2 of angle of acceptance.
Fig.8: Schematic of potentials and voltages
12
We operate the analyzer with constant E
p
to obtain constant absolute resolution E (i.e., an
energy window with constant width over our energy scan). To enable scans over arbitrary
energy ranges, the electrons are retarded (accelerated) by a potential difference R inside the
lens between the sample and the analyzer (Fig.8). The kinetic energy of the electrons which
reach the electron multiplier ( a chenneltron) is therefore:
E
kin,F
= R +E
p
+
A
; the work function of the analyzer
A
enters the equation because the
electrons feel the potential in front of the analzer's electrodes, i.e., we have to consider the
work function of the analyzer, but not the work function of the sample. To keep
A
constant,
all electrodes are covered with a chemically inert material (gold or graphite; in our case
graphite). To prevent perturbation of the electron trajectories by stray magnetic fields, the
analyzer region is shielded by a -metal shield inside the vacuum vessel.
7. Literature:
S. Hfner, Photoelectron spectroscopy; principles and applications (Springer, Berlin 2003).
J.C.Fuggle: High resolution Auger spectroscopy of solids and surfaces, in: Electron spectros-
copy, Theory, Techniques, and Applications, Vol.4, eds: C.R.Brundle and A.D.Baker (Aca-
demic press, London 1981).
The x-ray data booklet, by Lawrence Berkeley National Laboratory (with many valuable ta-
bles on binding energies and x-ray emission lines): http://xdb.lbl.gov/
D. Briggs and M.P. Seah (eds.): Practical surface analysis, Vol.1: Auger and x-ray photoelec-
tron spectroscopy (Wiley, New York 1990).
C.S. Fadley: Basic concepts of x-ray spectroscopy, in: Electron spectroscopy, Theory, Tech-
niques, and Applications, Vol.2, eds: C.R.Brundle and A.D.Baker (Academic press, London
1978).
K.D.Sevier, Low energy electron spectroscopy (Wiley, New York 1972).
8. Tables
8.1 Electron binding energies for all elements: http://xdb.lbl.gov/Section1/Table_1-1.pdf
8.2 X-ray energy emission lines for all elements: http://xdb.lbl.gov/Section1/Table_1-2.pdf
8.3 Photoionization cross sections: http://xdb.lbl.gov/Section1/Sec_1-5.pdf
13
8.4 Main Auger lines (see also: http://xdb.lbl.gov/Section1/Sec_1-4.html):
14
9. Exercises
1. Get an introduction by your advisor.
2. The tungsten ribbon has been cleaned by your advisor. Take a survey spectrum
(100 E
kin
1500 eV; E
p
= 50 eV). Try to assign all maxima.
3. Record spectra of the W 4f levels with different E
p
(2, 5, 10, 20, 50 eV).
4. Record PE spectra of the W valence band (E
p
= 50 eV). Determine
A
.
5. Record spectra for 200 E
kin
530 with the x-ray source operated at an anode voltage
of 1000 V and an emission current of 6 mA (consult advisor).
6. Your advisor will now cover your sample with a Mg film:
Record a survey spectrum from 50 to 1500 eV (E
p
= 50 eV).
Try to assign all features of this spectrum.
7. Record the Mg Auger spectrum in the range from 1000 eV to 1250 eV (E
p
= 20 eV) for:
a) (nearly) grazing, and
b) (nearly) normal electron exit.
8. Record O1s PE (50 eV E
p
). Any features?
9. The Mg layer will be oxidized by your advisor; repeat 8. Any changes? Mg still pre-
sent? If so:
10. Repeat 7 (the end).
10. Questions (to be addressed in the evaluation):
1. Assign all lines from 9.2
2. Compare measured and theoretically expected values for the constituents of the 4d
(from 9.2) and the 4f dublett (from 9.3). Plot the 4f intensity as a function of E
p
. Inter-
pretation for the variation?
3. Plot the experimentally obtained HWFM for the two constituents of the 4f dublett as a
function of E
p
. Which limits for their lifetime values do you obtain? Assume a convo-
lution of lifetime width and experimental line width.
4. Explain the results of 9.5. Consider x-ray emission from contaminants on the Al anode.
5. Assign all lines from 9.6.
6. Explain all details of 9.7. Consider plasmon losses (see, e.g., solid state physics by
Kittel). Select a transition of your choice and calculate the hole-hole interaction energy
15
U. Is the value you got as expected by you? Explain differences seen (if any) for the two
different exit angles.
7. Compare 9.8 and 9.9. Explanation?
8. Compare results from 9.7 and 9.10. Interpretation?
9. After which time at a pressure of 10
-5
Pa of oxygen will an originally clean metal sur-
face be covered with one monolayer of O atoms (assume s = 1 and 2
nd
order adsorp-
tion; make reasonable assumption about the density of the substrate atoms).
10. Assume a hemisherical analyzer with radii r
i
(inner) and r
o
(outer). Which potential
difference has to be applied to the hemispheres in order to obtain a trajectory with
r = (r
i
+ r
o
)/2 for electrons with the energy E
p
?
Vous aimerez peut-être aussi
- Feynman Lectures Simplified 2A: Maxwell's Equations & ElectrostaticsD'EverandFeynman Lectures Simplified 2A: Maxwell's Equations & ElectrostaticsPas encore d'évaluation
- ESR For TY As HandoutDocument16 pagesESR For TY As HandoutPavitra JonesPas encore d'évaluation
- Atomic Physics NotesDocument89 pagesAtomic Physics Notesawais33306Pas encore d'évaluation
- X-Ray Photoelectron Spectroscopy (XPS)Document19 pagesX-Ray Photoelectron Spectroscopy (XPS)caonguyenbanso100% (1)
- Gamma Ray Spectroscopy VerDocument12 pagesGamma Ray Spectroscopy VerLiberata MigloriaPas encore d'évaluation
- Introduction To Research and Research MethodsDocument46 pagesIntroduction To Research and Research MethodsStacie Simmons100% (4)
- Photoelectron SpectrosDocument24 pagesPhotoelectron SpectrosUmesh ChandraPas encore d'évaluation
- Unit - I P-N Junction Diode 1Document23 pagesUnit - I P-N Junction Diode 1Sandeep Babu VannempalliPas encore d'évaluation
- Principles of Solar Cells, LEDs and Related Devices: The Role of the PN JunctionD'EverandPrinciples of Solar Cells, LEDs and Related Devices: The Role of the PN JunctionPas encore d'évaluation
- 5.3 Photoelectron SpectrosDocument8 pages5.3 Photoelectron SpectrosShams Shams0% (1)
- Surface ChemistryDocument137 pagesSurface ChemistryMohammed86% (7)
- XPS IntroDocument35 pagesXPS IntroquzalbashPas encore d'évaluation
- X-Ray Photoelectron Spectroscopy (XPS) : Electron Spectroscopy For Chemical Analysis (ESCA)Document24 pagesX-Ray Photoelectron Spectroscopy (XPS) : Electron Spectroscopy For Chemical Analysis (ESCA)Jatin DarvePas encore d'évaluation
- Electron Spectroscopy1111Document47 pagesElectron Spectroscopy1111secatePas encore d'évaluation
- Photoelectron Spectroscopy Reveals Electronic StructureDocument16 pagesPhotoelectron Spectroscopy Reveals Electronic StructureAditiPas encore d'évaluation
- Bom XpsDocument22 pagesBom XpsAnonymous UYDJtUnPas encore d'évaluation
- Principle of XPS: Surface Analysis Using X-Ray Photoelectron SpectroscopyDocument4 pagesPrinciple of XPS: Surface Analysis Using X-Ray Photoelectron Spectroscopyonynho100% (1)
- X-Ray Photoelectron Spectroscopy (XPS) For Catalysts CharacterizationDocument12 pagesX-Ray Photoelectron Spectroscopy (XPS) For Catalysts CharacterizationHasan HadiPas encore d'évaluation
- tmp3327 TMPDocument11 pagestmp3327 TMPFrontiersPas encore d'évaluation
- Basic EPR Spectroscopy - TheoryDocument17 pagesBasic EPR Spectroscopy - TheoryDev DPas encore d'évaluation
- Electron Spin Resonance ExperimentDocument16 pagesElectron Spin Resonance ExperimentAtiq Ur RahmanPas encore d'évaluation
- XPS (X Ray Photoemission Spectroscopy) /ESCA (Electron Spectroscopy For Chemical Analysis)Document46 pagesXPS (X Ray Photoemission Spectroscopy) /ESCA (Electron Spectroscopy For Chemical Analysis)Serdar ArıcanPas encore d'évaluation
- Photoelectron Spectroscopy 6Document11 pagesPhotoelectron Spectroscopy 6alina.tlekkabylova270202Pas encore d'évaluation
- Lectron PIN Esonance: Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles, Sec. 8.1 To 8.3Document11 pagesLectron PIN Esonance: Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles, Sec. 8.1 To 8.3FranciscoPas encore d'évaluation
- Photoelectron Spectroscopy Principles and ApplicationsDocument3 pagesPhotoelectron Spectroscopy Principles and ApplicationsElumalaiPas encore d'évaluation
- Ch7Spectroscopic ApproachesDocument17 pagesCh7Spectroscopic ApproachesAzabou MariamPas encore d'évaluation
- 5.2 Auger Electron SpectrosDocument7 pages5.2 Auger Electron SpectrosShams ShamsPas encore d'évaluation
- L. C. Andreani, OPTICAL TRANSITIONS, EXCITONS, AND POLARITONS IN BULK AND LOW-DIMENSIONAL SEMICONDUCTOR STRUCTURES, 1993Document56 pagesL. C. Andreani, OPTICAL TRANSITIONS, EXCITONS, AND POLARITONS IN BULK AND LOW-DIMENSIONAL SEMICONDUCTOR STRUCTURES, 1993vg51Pas encore d'évaluation
- M Icroscopic Quantum Interference Effects in The Theory of SuperconductivityDocument21 pagesM Icroscopic Quantum Interference Effects in The Theory of SuperconductivityAlonso CampoiPas encore d'évaluation
- Electron Energy-Loss Spectroscopy in The TEM: R F EgertonDocument25 pagesElectron Energy-Loss Spectroscopy in The TEM: R F EgertonImran KhanPas encore d'évaluation
- Working Principle of Solar EnergyDocument4 pagesWorking Principle of Solar EnergytamePas encore d'évaluation
- Simple Introduction To Angle Resolve Photo Emission Spectroscopy ARPESDocument7 pagesSimple Introduction To Angle Resolve Photo Emission Spectroscopy ARPESousolidPas encore d'évaluation
- AbhishekDocument11 pagesAbhishekNaresh kumar ChauhanPas encore d'évaluation
- Optical Processes in SemiconductorsDocument6 pagesOptical Processes in Semiconductorsvj.krlambaPas encore d'évaluation
- Soft X-Ray Photoionization of Atoms and Molecules Svensson2005Document19 pagesSoft X-Ray Photoionization of Atoms and Molecules Svensson2005jbmacielPas encore d'évaluation
- Modern Physics Dr:Yahia Elbashar ENG:Doaa Mohamed NAME:Abdallah Ibrahim Abdrabo ID:18194141Document17 pagesModern Physics Dr:Yahia Elbashar ENG:Doaa Mohamed NAME:Abdallah Ibrahim Abdrabo ID:18194141AboElnasr35Pas encore d'évaluation
- Ferroelectric PropertiesDocument39 pagesFerroelectric PropertiesMohanrajRajangamPas encore d'évaluation
- X-Ray Photoelectron Spectroscopy (XPS) : Electron Spectroscopy For Chemical Analysis (ESCA)Document24 pagesX-Ray Photoelectron Spectroscopy (XPS) : Electron Spectroscopy For Chemical Analysis (ESCA)Imran KhanPas encore d'évaluation
- CalTech Optical PumpingDocument18 pagesCalTech Optical PumpingJun-Han SuPas encore d'évaluation
- Topic 7 HandoutDocument7 pagesTopic 7 HandoutnattydreadfathelahPas encore d'évaluation
- Energy Bands and Charge Carriers in SemiconductorsDocument7 pagesEnergy Bands and Charge Carriers in SemiconductorsSushil KumarPas encore d'évaluation
- To Study Surface Magnetism and Critical Behavior: Capture and Emission of Spin-Polarized ElectronsDocument10 pagesTo Study Surface Magnetism and Critical Behavior: Capture and Emission of Spin-Polarized ElectronsSanghita BiswasPas encore d'évaluation
- ExcitonsDocument44 pagesExcitonsFei PuPas encore d'évaluation
- The Electron Density and Chemical Bonding in Organic Compounds by X-Ray DiffractionDocument58 pagesThe Electron Density and Chemical Bonding in Organic Compounds by X-Ray DiffractionJesus Isaac Castillo SanchezPas encore d'évaluation
- Franck-Hertz Experiment ExplainedDocument6 pagesFranck-Hertz Experiment ExplainedAndika MaulanaPas encore d'évaluation
- Photoelectron Spectroscopy: Electrons As ParticlesDocument2 pagesPhotoelectron Spectroscopy: Electrons As ParticlesRaees Ahmad100% (1)
- Photoelectric Effect ProblemsDocument1 pagePhotoelectric Effect ProblemsaminulahsanPas encore d'évaluation
- Epr - Interpretation Introduction Proportionality Factor (G-Factor) Hyperfine Interactions Easyspin Simulations References ProblemsDocument16 pagesEpr - Interpretation Introduction Proportionality Factor (G-Factor) Hyperfine Interactions Easyspin Simulations References Problemsdeepak_143Pas encore d'évaluation
- Measure Energy of Charged ParticlesDocument6 pagesMeasure Energy of Charged ParticlesKcirtap ZkethPas encore d'évaluation
- tmp4383 TMPDocument9 pagestmp4383 TMPFrontiersPas encore d'évaluation
- Unit 1 Semiconductor Lecture NotesDocument22 pagesUnit 1 Semiconductor Lecture NotesMy Extra AccountPas encore d'évaluation
- Physics Unit 1Document131 pagesPhysics Unit 17csnty5wvgPas encore d'évaluation
- Structure and Bonding Fundamentals for Organic ChemistryDocument10 pagesStructure and Bonding Fundamentals for Organic Chemistry0Pas encore d'évaluation
- Semiconductor Physics-MCQsDocument18 pagesSemiconductor Physics-MCQsomkardeepak444Pas encore d'évaluation
- All India Senior Secondary School Certificate Examination (AISSCE-2013-14)Document10 pagesAll India Senior Secondary School Certificate Examination (AISSCE-2013-14)ScientistPas encore d'évaluation
- Photoelectric EffectDocument10 pagesPhotoelectric EffectScientistPas encore d'évaluation
- 978 1 4020 2575 4 - 7Document2 pages978 1 4020 2575 4 - 7Diya RoyPas encore d'évaluation
- ChemLab - Chemistry 6 - Spectrum of The Hydrogen Atom - Chemistry & BackgroundDocument13 pagesChemLab - Chemistry 6 - Spectrum of The Hydrogen Atom - Chemistry & BackgroundAt TanwiPas encore d'évaluation
- Hl.12.1 Electrons in AtomsDocument31 pagesHl.12.1 Electrons in AtomsJerry LouPas encore d'évaluation
- Applied ChemistryDocument11 pagesApplied ChemistryMaqsood Ahmad KhanPas encore d'évaluation
- A Study of The Electrochemical Oxidation and Polymerisation of 1,3 Dihydroxybenzene and 3-Hydroxybenzyl Alcohol in Acidic, Basic and Neutral Aqueous SolutionsDocument11 pagesA Study of The Electrochemical Oxidation and Polymerisation of 1,3 Dihydroxybenzene and 3-Hydroxybenzyl Alcohol in Acidic, Basic and Neutral Aqueous SolutionsEdward PittsPas encore d'évaluation
- 2008 Obc 4120Document5 pages2008 Obc 4120Edward PittsPas encore d'évaluation
- 2009 0628Document9 pages2009 0628Edward PittsPas encore d'évaluation
- Differential CalculusDocument44 pagesDifferential CalculuswkkchamaraPas encore d'évaluation
- EngDocument8 pagesEngEdward PittsPas encore d'évaluation
- Miscibility ChartDocument2 pagesMiscibility ChartEdward PittsPas encore d'évaluation
- Electrochemical Oxidation of Paracetamol Mediated by Nanoparticles Bismuth Oxide Modified Glassy Carbon ElectrodeDocument10 pagesElectrochemical Oxidation of Paracetamol Mediated by Nanoparticles Bismuth Oxide Modified Glassy Carbon ElectrodeEdward PittsPas encore d'évaluation
- 10-4985JP Published MainmanuscriptDocument21 pages10-4985JP Published MainmanuscriptEdward PittsPas encore d'évaluation
- 16 Solhan YahyaDocument5 pages16 Solhan YahyaEdward PittsPas encore d'évaluation
- Chemical Space Travel: Ruud Van Deursen and Jean-Louis ReymondDocument5 pagesChemical Space Travel: Ruud Van Deursen and Jean-Louis ReymondEdward PittsPas encore d'évaluation
- Differential Calculus: 61 Differentiation of ProcessesDocument67 pagesDifferential Calculus: 61 Differentiation of ProcessesEdward PittsPas encore d'évaluation
- 5990 7890en PDFDocument4 pages5990 7890en PDFEdward PittsPas encore d'évaluation
- Polymer Molecular Weight PropertiesDocument14 pagesPolymer Molecular Weight PropertiesJesus AmbrosioPas encore d'évaluation
- SolutionsHW9 MWDocument8 pagesSolutionsHW9 MWJunlene NgPas encore d'évaluation
- Topic 6Document5 pagesTopic 6Edward PittsPas encore d'évaluation
- Differential Calculus: 61 Differentiation of ProcessesDocument67 pagesDifferential Calculus: 61 Differentiation of ProcessesEdward PittsPas encore d'évaluation
- 8 and 15°C.: Stability ProgramsDocument1 page8 and 15°C.: Stability ProgramsEdward PittsPas encore d'évaluation
- Vocabuilder ThesisDocument32 pagesVocabuilder ThesisEdward PittsPas encore d'évaluation
- X 24Document10 pagesX 24Fawad Ali KhanPas encore d'évaluation
- 78 - 32120Document4 pages78 - 32120Edward PittsPas encore d'évaluation
- NitFix Book Chapter9Document27 pagesNitFix Book Chapter9Edward PittsPas encore d'évaluation
- SSRN Id1583304 PDFDocument18 pagesSSRN Id1583304 PDFEdward PittsPas encore d'évaluation
- Chemistry and Biotechnology: A Productive Union Meets New ChallengesDocument3 pagesChemistry and Biotechnology: A Productive Union Meets New ChallengesEdward PittsPas encore d'évaluation
- 170 - 058 - Conservation of Medicinal Plants in BangladeshDocument22 pages170 - 058 - Conservation of Medicinal Plants in BangladeshEdward PittsPas encore d'évaluation
- Stats ch10 PDFDocument14 pagesStats ch10 PDFEdward PittsPas encore d'évaluation
- Hypothesis TestingDocument8 pagesHypothesis TestingPriyanka SinghPas encore d'évaluation
- SpnotesDocument11 pagesSpnotesBfgi MemberPas encore d'évaluation