Académique Documents
Professionnel Documents
Culture Documents
Proof Paper Depressing Efffect of Eletroacupuncture Quiroz Et Al
Transféré par
Awurela Esubi Fakayode0 évaluation0% ont trouvé ce document utile (0 vote)
20 vues13 pagesCorrections can be made online, via e-mail or by fax. Use a fine black pen and write the correction in the margin, not too close to the edge of the page. Check that the text is complete and that all figures, tables and their legends are included. Your article will be published Online First approximately one week after receipt of your corrected proofs.
Description originale:
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentCorrections can be made online, via e-mail or by fax. Use a fine black pen and write the correction in the margin, not too close to the edge of the page. Check that the text is complete and that all figures, tables and their legends are included. Your article will be published Online First approximately one week after receipt of your corrected proofs.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
20 vues13 pagesProof Paper Depressing Efffect of Eletroacupuncture Quiroz Et Al
Transféré par
Awurela Esubi FakayodeCorrections can be made online, via e-mail or by fax. Use a fine black pen and write the correction in the margin, not too close to the edge of the page. Check that the text is complete and that all figures, tables and their legends are included. Your article will be published Online First approximately one week after receipt of your corrected proofs.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 13
Dear Author,
Here are the proofs of your article.
You can submit your corrections online, via e-mail or by fax.
For online submission please insert your corrections in the online correction form. Always
indicate the line number to which the correction refers.
You can also insert your corrections in the proof PDF and email the annotated PDF.
For fax submission, please ensure that your corrections are clearly legible. Use a fine black
pen and write the correction in the margin, not too close to the edge of the page.
Remember to note the journal title, article number, and your name when sending your
response via e-mail or fax.
Check the metadata sheet to make sure that the header information, especially author names
and the corresponding affiliations are correctly shown.
Check the questions that may have arisen during copy editing and insert your answers/
corrections.
Check that the text is complete and that all figures, tables and their legends are included. Also
check the accuracy of special characters, equations, and electronic supplementary material if
applicable. If necessary refer to the Edited manuscript.
The publication of inaccurate data such as dosages and units can have serious consequences.
Please take particular care that all such details are correct.
Please do not make changes that involve only matters of style. We have generally introduced
forms that follow the journals style.
Substantial changes in content, e.g., new results, corrected values, title and authorship are not
allowed without the approval of the responsible editor. In such a case, please contact the
Editorial Office and return his/her consent together with the proof.
If we do not receive your corrections within 48 hours, we will send you a reminder.
Your article will be published Online First approximately one week after receipt of your
corrected proofs. This is the official first publication citable with the DOI. Further changes
are, therefore, not possible.
The printed version will follow in a forthcoming issue.
Please note
After online publication, subscribers (personal/institutional) to this journal will have access to the
complete article via the DOI using the URL: http://dx.doi.org/[DOI].
If you would like to know when your article has been published online, take advantage of our free
alert service. For registration and further information go to: http://www.link.springer.com.
Due to the electronic nature of the procedure, the manuscript and the original figures will only be
returned to you on special request. When you return your corrections, please inform us if you would
like to have these documents returned.
Metadata of the article that will be visualized in OnlineFirst
ArticleTitle Depressing effect of electroacupuncture on the spinal non-painful sensory input of the rat
Article Sub-Title
Article CopyRight Springer-Verlag Berlin Heidelberg
(This will be the copyright line in the final PDF)
Journal Name Experimental Brain Research
Corresponding Author Family Name Quiroz-Gonzlez
Particle
Given Name Salvador
Suffix
Division Departamento de Acupuntura y Rehabilitacin
Organization Universidad Estatal del Valle de Ecatepec
Address Av. Central s/n, Esq. Leona Vicario, Col. Valle de Anhuac, Secc. A,
Ecatepec, Estado de Mxico, CP 55210, Mexico
Email sqg20@yahoo.com.mx
Author Family Name Segura-Alegra
Particle
Given Name Bertha
Suffix
Division Facultad de Estudios Superiores, FES Iztacala
Organization UNAM
Address Mexico, CP 54090, Mexico
Email bsegura@campus.iztacala.unam.mx
Author Family Name Jimnez-Estrada
Particle
Given Name Ismael
Suffix
Division Departamento de Fisiologa, Biofsica y Neurociencias
Organization Centro de Investigacin y, Estudios Avanzados del IPN
Address Av. Instituto Politcnico Nacional 2508, Col. San Pedro Zacatenco, AP.
14-740, Mexico, DF, CP 07000, Mexico
Email ijimenez@fisio.cinvestav.mx
Schedule
Received 13 February 2014
Revised
Accepted 12 April 2014
Abstract The aim of this study was to explore the effect of electroacupuncture (EA) applied in the Zusanli (ST36) and
Sanyinjiao (SP6) points on the N1 component of the cord dorsum potential (CDP) evoked by electrical
stimulation of the sural nerve (SU) in the rat. The experiments were performed in 44 Wistar rats (250300 g)
anesthetized with ketamine (100 mg/kg) and xylazine (2 mg/kg). A bilateral laminectomy was performed to
expose the L3 to S2 segments of the spinal cord. The SU nerve was exposed and placed on pairs of hook
electrodes for electrical stimulation. The N1-CDPs were recorded with three silver-ball electrodes located on
the dorsal surface of the L5 to S1 segments. Ipsilateral high and low EA stimulation (100, 2 Hz, 6 mA, 30 min)
induced a considerable reduction in the amplitude (45 5.6, 41 6.2 %) of the N1-CDP recorded at the L6
segmental level. Recovery of the N1-CDP amplitude occurred approximately 13 s after EA. Sectioning of
the saphenous and superficial peroneal nerves reduced the depressing effect provoked by the EA stimulation
(18.7 1.3, 27 3.8 %). Similarly, sectioning of the posterior and anterior tibial, deep peroneal and
gastrocnemius nerves partially reduced the effect provoked by EA (11 1.5, 9.8 1.1, 12.6 1.9 %).
Intravenous picrotoxin (1 mg/kg) also reduced the action of low and high EA (23 4.8, 27 5.2 %). It is
suggested that EA stimulation depresses non-painful sensory pathways through the activation of specific
inhibitory pathways that receive modulatory actions from other sensory and muscle afferent inputs in the rat
spinal cord.
Keywords (separated by '-') Electroacupuncture - Cord dorsum potentials - Sural nerve - Spinal cord
Footnote Information
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
1 3
Exp Brain Res
DOI 10.1007/s00221-014-3965-2
RESEARCH ARTICLE
Depressing effect of electroacupuncture on the spinal non-painful
sensory input of the rat
Salvador Quiroz-Gonzlez Bertha Segura-Alegra
Ismael Jimnez-Estrada
Received: 13 February 2014 / Accepted: 12 April 2014
Springer-Verlag Berlin Heidelberg 2014
stimulation (18.7 1.3, 27 3.8 %). Similarly, sectioning
of the posterior and anterior tibial, deep peroneal and gas-
trocnemius nerves partially reduced the effect provoked by
EA (11 1.5, 9.8 1.1, 12.6 1.9 %). Intravenous picro-
toxin (1 mg/kg) also reduced the action of low and high EA
(23 4.8, 27 5.2 %). It is suggested that EA stimulation
depresses non-painful sensory pathways through the activa-
tion of specic inhibitory pathways that receive modulatory
actions from other sensory and muscle afferent inputs in
the rat spinal cord.
Keywords Electroacupuncture Cord dorsum potentials
Sural nerve Spinal cord
Introduction
Acupuncture is a therapeutic modality that emerged from
Traditional Chinese Medicine. The World Health Organi-
zation recommends the use of acupuncture for the treat-
ment of a wide variety of diseases (Zhang et al. 2014; Yin
et al. 2010; Barnes et al. 2008). A novel contemporary
form of acupuncture, electrical stimulation of acupuncture
points (APs), also known as electroacupuncture (EA), has
been widely used in both clinical and experimental stud-
ies (Vickers et al. 2012; Zhao 2008). Several studies have
demonstrated that EA exerts an analgesic effect on neuro-
pathic pain in rat models (Lau et al. 2008; Kim et al. 2004;
Huang et al. 2004; Hwang et al. 2002) and relieves acute or
chronic inammatory pain (Kim et al. 2006; Zhang et al.
2004). There has been particular interest in determining the
mechanisms involved in the antinociceptive effect of EA
(Zhao 2008; Leung 2012). Acupuncture analgesia is a clear
manifestation of modulatory processes occurring at differ-
ent levels of the central nervous system (Zhang et al. 2014;
Abstract The aim of this study was to explore the effect
of electroacupuncture (EA) applied in the Zusanli (ST36)
and Sanyinjiao (SP6) points on the N1 component of the
cord dorsum potential (CDP) evoked by electrical stimu-
lation of the sural nerve (SU) in the rat. The experiments
were performed in 44 Wistar rats (250300 g) anesthe-
tized with ketamine (100 mg/kg) and xylazine (2 mg/kg).
A bilateral laminectomy was performed to expose the
L3 to S2 segments of the spinal cord. The SU nerve was
exposed and placed on pairs of hook electrodes for elec-
trical stimulation. The N1-CDPs were recorded with three
silver-ball electrodes located on the dorsal surface of the
L5 to S1 segments. Ipsilateral high and low EA stimu-
lation (100, 2 Hz, 6 mA, 30 min) induced a considerable
reduction in the amplitude (45 5.6, 41 6.2 %) of the
N1-CDP recorded at the L6 segmental level. Recovery of
the N1-CDP amplitude occurred approximately 13 s after
EA. Sectioning of the saphenous and supercial peroneal
nerves reduced the depressing effect provoked by the EA
S. Quiroz-Gonzlez (*)
Departamento de Acupuntura y Rehabilitacin, Universidad
Estatal del Valle de Ecatepec, Av. Central s/n, Esq. Leona Vicario,
Col. Valle de Anhuac, Secc. A, CP 55210 Ecatepec,
Estado de Mxico, Mexico
e-mail: sqg20@yahoo.com.mx
B. Segura-Alegra
Facultad de Estudios Superiores, FES Iztacala, UNAM,
CP 54090 Mexico, Mexico
e-mail: bsegura@campus.iztacala.unam.mx
I. Jimnez-Estrada
Departamento de Fisiologa, Biofsica y Neurociencias, Centro
de Investigacin y, Estudios Avanzados del IPN, Av. Instituto
Politcnico Nacional 2508, Col. San Pedro Zacatenco,
AP. 14-740, CP 07000 Mexico, DF, Mexico
e-mail: ijimenez@sio.cinvestav.mx
AQ1
AQ2
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
56
57
A1
A2
A3
A4
A5
A6
A7
A8
A9
A10
A11
A12
A13
A14
A15
A16
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
Quiroz-Gonzlez et al. 2014; Zhao 2008). The rst conver-
gence of impulses originating from pain sites and acupoints
occurs in the spinal dorsal horn, where the neuronal noci-
ceptive responses appear to be depressed by both pre- and
post-synaptic inhibition during EA stimulation (Zhao 2008;
Li et al. 1993). Although the effectiveness of EA for pain
control has been demonstrated experimentally in animal
models, little is known about the possible effects exerted by
EA on the cutaneous non-painful sensory input in the spi-
nal cord. Electrical stimulation of cutaneous nerves induces
the activation of sensory neurons located in the dorsal
horn of the spinal cord (Bernhard 1953; Willis et al. 1973;
Coombs et al. 1956), which produces cord dorsum poten-
tials (CDPs) consisting of clearly dened components. The
rst observed component in the CDPs is the afferent volley
(AV), which is caused by the electrical activation of low-
threshold afferent bers (Coombs et al. 1956). This is fol-
lowed by one negative component (named N1-CDP), which
is generated by the monosynaptic activation of the dorsal
horn neurons via the A afferents or non-painful bers in
the cutaneous nerves (Bernhard 1953; Willis et al. 1973;
Coombs et al. 1956). Subsequently, a long-lasting positive
component occurs, named the P wave, which is ascribed to
the current ows generated during the presynaptic depolari-
zation of afferent bers (PAD) and presynaptic inhibition
(Rudomin and Schmidt 1999). The present study aimed
to analyze the effect of EA stimulation on the non-painful
sensory neurons at the level of the spinal cord. To achieve
this goal, we recorded the N1-CDP evoked by cutaneous
SU nerve stimulation and during EA stimulation. In a sec-
ond series of experiments, we evaluated how this effect is
modied by sectioning cutaneous and muscular nerves.
Because it is amply recognize that GABAergic mechanism
is involved in the modulation of primary afferent depolari-
zation (PAD) of cutaneous and muscular nerves, we ana-
lyzed the possible effect of picrotoxin (PTX) on the actions
of the EA. Some of these observations have been published
previously in abstract form (Quiroz-Gonzalez et al. 2013).
Methods
Animals
Male Wistar rats (n = 44) weighing 250300 g (8
10 weeks old) that were obtained from the animal house
of our institution were used. All animals had free access to
water and were housed under identical environmental con-
ditions of light and dark cycles (12:12 h) and temperature
(2224 C). All experiments were carried out in accordance
with the guidelines of the Mexican Ofcial Norm (NOM-
062-ZOO-1999) and in accordance with the National Insti-
tutes of Health Guide for the Care and Use of Laboratory
Animals (NIH Publication No. 8023, revised in 1978). The
study protocol was approved by the institutional bioethical
committee for Care and Handling of Laboratory Animals
(Protocol 0267-05, CINVESTAV).
Surgical procedures
Initially, the animals were anesthetized with an intraperito-
neal injection of a mixed solution of ketamine (100 mg/kg)
and xylazine (2 mg/kg). This injection was supplemented
every hour by additional doses of ketamine (50 mg/kg),
applied in the same low abdominal region to ensure that an
adequate level of anesthesia, dened as the absence of with-
drawal reexes. We used ketamine as an analgesic because
it has been demonstrated that it did not signicantly reduce
the response of spinal dorsal horn neurons to low-threshold
afferent inputs in the intact animal, but in contrast suppress
noxiously evoked activity of wide dynamic range neurons
(Collins 1986). Subsequently, the femoral vein was exposed
and cannulated for the administration of a solution of pic-
rotoxin (PTX, 1 mg/kg of body weight; Quirz-Gonzalez
et al. 2012). A bilateral laminectomy was performed in the
lumbosacral enlargement (from the L4 to S1 segments) of
the spinal cord. Several nerves of the right hind leg were
carefully exposed and prepared for stimulation and/or
sectioning: the main branch of the sural (SU), supercial
peroneus (SP), tibial (TA), saphenous (SA) deep peroneus
(DP) and gastrocnemius (GS) nerves and the rats were then
secured in a stereotaxic frame. The surgical procedure for
sural nerve dissection on the hind leg was carefully made
to avoid the possible damage of the Zusanli (ST36) and
Sanyinjiao (SP6) acupoints. The animals body temperature
was monitored using a thermal probe located in the back
muscles and connected to an automatic feedback control
unit and a heating blanket to maintain the animals body
temperature at 37 C. The skin aps and muscle around the
exposed tissues were raised and tied to a metal stereotaxic
frame to form a pool, which was lled with warm mineral
oil to prevent the tissues from drying.
SU nerve stimulation
The central end of the sural (SU) nerve in the left hind
limb was mounted on a pair of stimulating silver hooks
connected to an isolated-current-pulse generator (Isoex
D 4030), and the CDPs were produced in all experiments
by single square-current pulses (0.05 ms duration; at 1 Hz)
of graded intensity. The pulses were monitored by record-
ing the voltage drops across a resistor (1,000 ) that was
placed in the current return path. The electrical threshold
(1 T) was established as the minimum stimulus strength
(usually between 0.1 and 0.13 mA) necessary to cause a
discernible CDP response on the surface of the spinal cord.
AQ3
AQ4
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
128
129
130
131
132
133
134
135
136
137
138
139
140
141
142
143
144
145
146
147
148
149
150
151
152
153
154
155
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
Electroacupuncture stimulation
Pairs of stainless-steel acupuncture needles (0.25 mm in
diameter and 5 mm in length) were inserted perpendicu-
larly at a depth of 3 mm at the Zusanli (ST36, 5 mm lateral
to the anterior tubercle of the tibia) and Sanyinjiao acu-
points (SP6, 3 mm proximal to the medial malleolus, at the
posterior border of the tibia). These acupoints are tradition-
ally used in acupuncture-induced analgesia for the treat-
ment of several pain syndromes (Zhang et al. 2003; Huang
et al. 2002). To disclose the region-specic effect of EA,
we stimulate two non-acupoints (NAP) nearby to ST36 and
SP6, the rst located 2 mm lateral to ST36 and the second
3 mm posterior to SP6. The transpositional method (Yin
et al. 2008) for rats was used to determine the acupoint
locations. The needles were connected to an isolated-cur-
rent- pulse generator (Isoex D 4030), and square trains of
high or low frequency (100 or 2 Hz, respectively) and vari-
able strength current pulses (16 mA, 0.1 ms) were applied
in the acupoints located both contralateral and ipsilateral of
the sural nerve stimulation for a total of 30 min.
CDP recording
Chlorinated silver-ball electrodes were placed on the dorsal
surface of several segments in the lumbosacral spinal cord
(L3S2) to record the CDPs (Fig. 1), and the corresponding
reference silver electrodes were inserted into the adjacent
paravertebral musculature. Each recording pair of elec-
trodes was connected to an individual low-noise, high-gain
differential amplier (Grass, model P511; band-pass lters
were set at 0.310 kHz). The resulting recordings were dig-
itized, averaged (n = 40 samples at 1 Hz), and stored in a
digital computer using a specially designed software (built
in the Lab-VIEW environment). The peak amplitude of the
N1-CDPs was measured and subsequently analyzed.
Data analysis
All statistical analyses were performed using the Graph-
Pad Prism (version 4) software. Data were expressed as the
mean standard deviation. A two-way analysis of variance
test for multiple comparisons followed by a Bonferroni cor-
rection was used to determine the differences in the ampli-
tudes of the N1-CDPs produced by SU nerve stimulation
and those produced by EA stimulation on ST36 and SP6.
Repeated measure ANOVA followed by Newman-Keuls
posthoc test was used to determine the differences between
the amplitude of control SU N1-CDPS and the amplitude
of the conditioned SU nerve responses evoked during EA
stimulation. The differences were considered signicant at
p < 0.05.
Results
CDPs provoked by SU nerve and EA stimulation
Single electrical pulses applied to the SU nerve (34 T,
at 1 Hz) produced N1-CDPs of a relatively large amplitude
(Fig. 1a). The largest N1-CDP occurred at the L6-segmen-
tal level and gradually decreased in amplitude in the adja-
cent rostro-caudal spinal segments (L5, L4 and S1, respec-
tively, in Figs. 1a, 2a, 3a). Similar recordings were obtained
in 6 other experiments. This rostro-caudal distribution of
the CDPs agreed well with previous reports (Gonzlez et al.
2011). Single electrical current pulses applied to the ST36
and SP6 acupoints (34 T, at 1 Hz) produced N1-CDPs
with largest amplitude at the L5 and L6 segmental levels,
decreasing in amplitude in L4 and S1 spinal segments. The
N1-CDPs produced by SU nerve stimulation showed a sim-
ilar rostro-caudal distribution than those produced by EA
applied to ST36 and SP6, but they are smaller in amplitude
(p < 0.05; Fig. 1a, b).
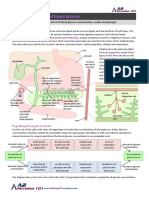
Fig. 1 Experimental arrangement for the N1-CDP recording: a aver-
age N1-CDP produced by SU nerve stimulation and EA stimulation
recorded at L4 to S1 spinal cord segments. b Graphs illustrating the
longitudinal distribution on the spinal segments of the N1-CDP aver-
ages amplitudes (n = 7) produced by SU nerve and EA stimulation at
ST36 and SP6. (*p < 0.05)
156
157
158
159
160
161
162
163
164
165
166
167
168
169
170
171
172
173
174
175
176
177
178
179
180
181
182
183
184
185
186
187
188
189
190
191
192
193
194
195
196
197
198
199
200
201
202
203
204
205
206
207
208
209
210
211
212
213
214
215
216
217
218
219
220
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
Effect of high frequency EA stimulation on the amplitude
of the N1-CDP
The N1-CDP recorded on the surface of the L5 to S1 seg-
ments gradually decreased in amplitude when the high
frequency (100 Hz) EA stimulation strength was progres-
sively increased (from 1 to 6 mA; Fig. 2a, b). The maximal
depression of the N1-CDP (45 5.6 %) was observed at
the L6 segment (Figs. 2a, b, 3a, b) and occurred immedi-
ately upon and during EA stimulation (30 min). In most
Fig. 2 Inhibition of the
N1-CDP by high (100 Hz) EA
stimulation on ST36 and SP6:
a average N1-CDP produced
by SU nerve stimulation and
recorded in several spinal
segments (L5S1) before EA
(control) and during ipsilateral
or contralateral EA stimulation
at different stimulus intensities
(16 and 6 mA, respectively).
b Graphs illustrating the mean
reduction (n = 11) in the
N1-CDP component recorded
in the S1L5 spinal segments
during a 100 Hz ipsilateral EA
stimulation. Asterisks indicate
signicant differences between
N1-CDP responses produced by
SU nerve stimulation before EA
and during high EA stimulation
(*p < 0.05 and **p < 0.01)
Fig. 3 Time course of inhibi-
tory effect of EA on N1-CDP
component: a CDP recording
showing that after EA, there
was a recovery of the N1-CDP
component produced by SU
nerve stimulation in several
spinal segments (S1L5).
b Graphs illustrating the
changes in the percent ampli-
tude of the N1-CDPs produced
before (control), during and
after EA. Asterisks indicate
signicant differences between
N1-CDP responses produced
by SU nerve stimulation before
EA and during posthigh EA
stimulation (*p < 0.05 and
**p < 0.01)
221
222
223
224
225
226
227
228
229
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
cases, the increment in intensity of EA stimulation (above
6 mA) did not increase the magnitude of the depression at
the different segments analyzed. Contralateral EA stimu-
lation (6 mA) and NAP stimulation had no effect on the
N1-CDP (Fig. 2a, b). After removal of the EA stimulus,
recovery of depressive actions on the N1-CDP occurred
within approximately 12 s (Fig. 3a, b).
Effect of low frequency EA stimulation on the amplitude
of the N1-CDP
30 min of low frequency EA stimulation (2 Hz) induce the
reduction in amplitude of the N1-CDP (41 6.2 %), but
only when the N1-CDP caused by EA stimulation occurred
at 100 ms before the N1-CDP produced by SU nerve stimu-
lation (Fig. 4B, C, D). No statistical differences were found
when the N1-CDP caused by EA stimulation occurred after
N1-CDP produced by SU nerve stimulation (Fig 4E, F).
Effect of nerve sectioning on the depressive action of EA
Sectioning the sensory saphenous and supercial pero-
neal nerves (Fig. 5a, b) induced a signicant reduc-
tion in the depressing effect provoked by EA stimulation
on the SU-evoked N1-CDP (18.7 1.3 %, n = 7 and
27 3.8 %, n = 7, respectively). Meanwhile, section-
ing of the tibial, deep peroneal and gastrocnemius nerves
(Fig. 5a, b) reduced the depressing effect provoked by EA
on the N1-CDPs but to a lesser extent (11 1.5 %, n = 7;
9.8 1.1 %, n = 7; and 12.6 1.9 %, n = 7, respectively).
The effect of PTX on the depressive action of EA
In order to analyze the possible GABAergic mechanism on
the depressive actions of EA on the SU-evoked N1-CDP,
systemic injection of a GABA
A
antagonist, picrotoxin
(PTX, 1 mg/kg) was delivered. As shown in Fig. 6D, F, the
application of PTX reduces the depressive actions of low
frequency EA (23 4.8 %, n = 7), as compared with con-
trol recording (Fig. 6A, F). Similar reductions were found
(27 5.2 %, n = 7) on the depressive actions of high fre-
quency EA stimulation (Fig. 6E, F). Intravenous saline
vehicle does not produced any effect on the EA conditioned
depression of the SU-evoked N1-CDP.
Discussion
It is well established that the N1-CDP generated by the
electrical stimulation of cutaneous nerves is produced by
the monosynaptic activation of dorsal horn neurons located
in the Rexeds laminae III to VI via A afferent bers or
low-threshold cutaneous afferent bers (Bernhard 1953;
Willis et al. 1973; Coombs et al. 1956). In our study, stimu-
lation of the sensory SU nerve and EA at the ST36 and SP6
acupoints provoked N1-CDPs that were simultaneously
Fig. 4 Inhibition of the SU N1-CDP by low (2 Hz) EA stimula-
tion applied on the ST36 and SP6 acupoints: a averaged N1-CDP
(n = 16 recordings) produced by SU nerve stimulation and recorded
in L6 spinal segment before EA stimulation, b during EA (EA-CDP)
applied 70 ms previously to the stimulus of the SU nerve, c 40 ms, d
20 ms, and e 30 ms after SU-evoked N1-CDP (ASU-CDP). f Graphs
illustrate averaged (SD) percent reduction values of the N1-CDP
component recorded on the L6 spinal segment in nine animals, during
a 2 Hz ipsilateral EA stimulation. Asterisks indicate signicant differ-
ences between N1-CDP responses produced by SU nerve stimulation
before EA and during low EA stimulation (*p < 0.05 and **p < 0.01)
230
231
232
233
234
235
236
237
238
239
240
241
242
243
244
245
246
247
248
249
250
251
252
253
254
255
256
257
258
259
260
261
262
263
264
265
266
267
268
269
270
271
272
273
274
275
276
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
Fig. 5 Reduction in the
effect of EA at 100 Hz on the
N1-CDP component by section-
ing nerves: a CDPs recording
showing the effect of nerve sec-
tioning on the depressive action
evoked in N1-CDP by high EA
stimulation (100 Hz).
b Graphs illustrating the
changes in the percent ampli-
tude of the N1-CDPs produced
by EA and abolished by nerve
sectioning (n = 7 animals per
experimental procedure). SP
supercial peroneous; SA saphe-
nous; TA tibial; GS gastrocne-
mius soleus nerves. Asterisks
indicate signicant differences
between control N1-CDP
responses evoked by SU nerve
stimulation and during EA
stimulation before and after the
sectioning of cutaneous and/or
muscular nerves (*p < 0.05 and
**p < 0.01)
Fig. 6 Effect of picrotoxin (PTX; 1 mg/kg) on the depressive effect
of EA on the N1-CDP: a averaged N1-CDP produced by SU nerve
stimulation and recorded in L6 spinal segment before EA stimula-
tion, b during low frequency EA (2 Hz) applied 40 ms previously to
the stimulus of the SU nerve, c with high frequency EA (100 Hz),
d The effect of intravenous injection of PTX on the low frequency
EA conditioned depression of the SU-evoked N1-CDP, e PTX under
high frequency EA conditioned depression, f, g low or high frequency
EA+ vehicle intravenous administration, h graphs illustrate averaged
(SD) percent reduction values of the N1-CDP component recorded
on the L6 spinal segment, during a 2 Hz EA stimulation (7 animals),
100 Hz EA stimulation (7 animals) intravenous PTX (7 animals),
and saline vehicle control (3 animals). Asterisks indicate signi-
cant differences between N1-CDP responses produced by SU nerve
stimulation before EA, during EA stimulation and intravenous PTX
(*p < 0.05 and **p < 0.01)
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
recorded on several segments of the lumbosacral enlarge-
ment (L4 to S1). The SU nerve evoked N1-CDP with the
largest was recorded on the L6 segment, and it is consist-
ent with those reported in other studies (Willis et al. 1973;
Gonzlez et al. 2011). Meanwhile, the largest N1-CDPs
evoked by EA were recorded at the L5L6 spinal segments.
These observations may suggest that the sensory inputs
activated by sural nerve and EA acupoint stimulation prob-
ably share spinal pathways which probably interact synap-
tically at the spinal cord level. According to the later, we
also found that conditioning low and high frequency EA
stimulation depressed the N1-CDPs produced by SU nerve
stimulation. In contrast, EA stimulation on non-acupoint
sites does not evoke signicant changes in the SU nerve
evoked N1-CDP, suggesting a specic acupoint effect of
EA. It is proposed that EA reduces the activation of dorsal
horn neurons provoked by low-threshold cutaneous afferent
bers by the activation of specic sensory pathways in the
spinal dorsal horn of the rat.
Transmission of non-nociceptive and nociceptive infor-
mation via primary afferents is modulated at the rst spinal
relay by highly complex processes (Rudomin and Schmidt
1999; Le Bars 2002; Besson and Chaouch 1987). It has
been accepted that electrical stimulation of primary affer-
ent bers effectively modulates the synaptic efcacy of
the same and/or other afferent bers in the spinal cord (De
LaTorre et al. 2009; Rudomin and Hernandez 2008). Elec-
trophysiological evidences have shown that electrical stim-
ulation of A bers may depress nociceptive activation of
spinal dorsal horn neurons for short periods of time (Chung
et al. 1984a, b). Moreover, both brief electrical stimulation
of afferent C-bers and prolonged high frequency burst
stimulation of the sciatic nerve at A ber strength produce
long-term depression (LTD) of C-ber-evoked eld poten-
tials (Liu et al. 1998). In addition, LTD of synaptic trans-
mission in substantia gelatinosa neurons can be induced
by low frequency stimulation of primary A-afferent bers
(Sandkuhler et al. 1997).
Electroacupuncture stimulation also provokes consider-
able changes in the neuronal activity evoked by peripheral
nerve stimulation. Kim et al. (2011) showed that EA pro-
duced a signicant reversal of enhanced evoked responses
of the deep dorsal horn (lamina IVVII) neurons as well as
after discharges developed in ankle-sprained rats. The EA-
induced inhibition lasted for at least 30 min after the ter-
mination of EA. In other study, it was found that 2 Hz EA
induce LTD in the C-ber-evoked eld potentials recorded
within the spinal dorsal horn of rats with neuropathic pain.
In contrast, 100 Hz EA-induced long-term potentiation
(LTP) but LTD in control rats (Xing et al. 2007).
In the present study, we found a signicant reduction in
the amplitude of the N1-CDPs evoked by SU nerve stimu-
lation during ipsilateral EA stimulation (46 mA) in several
spinal segments, particularly at the L6 segment of the rat
spinal cord. The ST36 acupoint receives sensory innerva-
tion from saphenous, supercial peroneal, and lateral sural
cutaneous nerves and motor innervation from the deep per-
oneal and anterior tibialis nerves (Zhou et al. 2010), while
the SP6 acupoint receives sensory innervation from the
saphenous, sural and medial crural nerves and motor inner-
vation from the tibial nerve (Zhou et al. 2010). The spinal
projection of these nerves showed a considerable overlap,
particularly at the L5L6 segmental level, even though they
innervate different hind limb areas (Maslany et al. 1992;
Panneton et al. 2005). It thus seems reasonable to expect
that during EA stimulation, the sensory and motor inner-
vation of the acupoints are activated and that the highest
effect of EA is observed at the L6 segment.
It is known that the acupuncture effect may occur in a
gradual manner and last for a long period of time (Zhao
2008; Leung 2012). Several lines of evidence suggest that
neurotransmitters and endogenous opioids are involved in
the depressive effect of EA on spinal nociceptive neurons
and that they participate in the analgesic effect of acupunc-
ture (Zhao 2008; Leung 2012). In the present study, the
depressive effect of EA on the N1-CDP component showed
a fast onset (<1 min) and continued during EA stimulation
and after the removal of the stimulation for a relative short
period of time (12 s). According to the latter, it could be
proposed that the effect of EA on nociceptive and non-
nociceptive pathways may be mediated by different spinal
mechanisms.
It is well established that the N1-CDP could be
depressed by the conditioning stimulation of sensory
peripheral nerves and such effect is mainly attributed
to PAD and presynaptic inhibition through GABAergic
mechanism (Lidierth 2006; Quirz-Gonzalez et al. 2012).
This kind of inhibition had its highest effect at 2030 ms
and last until 100 ms (Rudomin and Schmidt 1999; Lidi-
erth 2006; Quirz-Gonzalez et al. 2012). We found in
this study that the depressive effect of low frequency EA
occurred when the N1-CDP produced by SU nerve stim-
ulation appear between 5 and 90 ms after the occurrence
of the N1-CDP evoked by EA stimulation, with the high-
est effect between 20 and 40 ms, this interval of depression
and the time of the highest effect are quite similar to the
time course of the presynaptic depolarization of afferent
bers and presynaptic inhibition. This observation is rein-
forced by the antagonist effect of Picrotoxin (PTX) on the
depression exerted by EA on cutaneous spinal responses.
In concordance with this several evidences, we proposed
that presynaptic mechanism could participate in the depres-
sive actions of acupuncture. It is also shown that PTX
not abolish the effect of EA, the remaining PTX-resistant
depression of the N1-CDP may be ascribe to the activation
of non-GABAergic mechanism, most likely glycinergic
AQ5
AQ6
277
278
279
280
281
282
283
284
285
286
287
288
289
290
291
292
293
294
295
296
297
298
299
300
301
302
303
304
305
306
307
308
309
310
311
312
313
314
315
316
317
318
319
320
321
322
323
324
325
326
327
328
329
330
331
332
333
334
335
336
337
338
339
340
341
342
343
344
345
346
347
348
349
350
351
352
353
354
355
356
357
358
359
360
361
362
363
364
365
366
367
368
369
370
371
372
373
374
375
376
377
378
379
380
381
382
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
pathways (Rudomin and Schmidt 1999) or including to the
accumulation of potassium ions in the spinal cord (Kremer
and Lev-Tov 1998).
We also found that the depressive effects evoked by EA
were partially abolished by the sectioning of cutaneous and
muscle nerves that innervated the ST36 and SP6 acupoints.
The changes produced by sectioning of cutaneous nerves
were larger than those produced by the section of muscle
nerves. According to this evidence, it could be suggested
that specic heterosynaptic inhibitory pathways receiving
sensory and muscular inputs could be involved in the effect
of EA on low-threshold sensory pathways.
It is well known that the N1-CDP is produced by a
groups of sensory neurons that receive cutaneous large
A, afferent bers (Bernhard 1953). These spinal neurons
located in the laminae III-VI are responsible to transmit
ne touch, vibration, propiocepcion to supraspinal cent-
ers (Willis et al. 1973). It may be suggest that EA at low
or high frequency affects the transmission of these differ-
ent sensory modalities at the spinal cord level and could
have some implications in the process of the information
in the somatosensory cortex. Several lines of evidence
have hypothesized that large diameter sensory bers play a
major role in the pathogenesis of some types of neuropathic
pain (Devor 2009; Campero et al. 1998). Devor (2009)
has showed that dorsal root ganglion A afferents, which
normally signals touch and vibration, change their electri-
cal and neurotransmitter characteristics when they are sec-
tioned (axotomized). Such condition seems to switch the
sensory input of A afferents from non-painful to painful
signals (phenotypic switching), triggering, and maintain-
ing central sensitization.
Since it has been shown that EA stimulation exerts anal-
gesic and antinociceptive effects by modulating the activ-
ity of spinal dorsal horn neurons and the experimental
evidence obtained in this study indicates that high and low
frequency EA stimulation also affect low-threshold non-
painful sensory pathways at the spinal cord level in the rat,
it could be proposed that the depression of low-threshold
cutaneous pathways is involved in the reduction in neuro-
pathic pain produced by EA stimulation. However, further
studies are necessary to analyze this possibility by analyz-
ing the effect of EA on low-threshold sensory pathways in
an animal model of neuropathic pain.
In conclusion, the present study showed that EA stim-
ulation depressed non-painful sensory spinal pathways
through the activation of specic inhibitory pathways that
receive modulatory actions from sensory and muscle affer-
ent inputs in the rat spinal cord.
Acknowledgments We thank American Journal Experts for edit-
ing the English of this text, Jos Carlos Guadarrama Olmos for tech-
nical assistance and to Enrique Velazquez and Porrio Reyes for
their programming assistance. This work was partially supported
by fellowships granted to I. Jimnez-Estrada and B. Segura-Alegra
from the Sistema Nacional de Investigadores. S. Quiroz-Gonzalez
was partially supported by PROMEP (No. 103.5-13-6729) and
SNI-CONACYT.
References
Barnes PM, Bloom B, Nahin RL (2008) Complementary and alter-
native medicine use among adults and children. Natl Health Stat
Report 10:123
Baron R, Saguer M (1993) Postherpetic neuralgia. Are C-nociceptors
involved in signaling and maintenance of tactile allodynia? Brain
116:14771496
Bernhard CG (1953) The spinal cord potentials in leads form the cord
dorsum in relation to peripheral source of afferent stimulation.
Acta Physiol Scand 29:129
Besson JM, Chaouch A (1987) Peripheral and spinal mechanisms of
nociception. Physiol Rev 67:67186
Campero M, Serra J, Marchettini P, Ochoa JL (1998) Impulse gen-
eration and autoexcitation in single myelinated afferent bers in
patients with peripheral neuropathy and positive sensory symp-
toms. Muscle Nerve 21:16611667
Chung JM, Fang ZR, Hori Y, Lee KH, Endo K, Willis WD (1984a)
Prolongated inhibition of primate spinothalamic tracks cells by
peripheral nerve stimulation. Pain 19:259275
Chung JM, Lee KH, Hori Y, Endo K, Willis WD (1984b) Factors
inuencing peripheral stimulation produced inhibition of primate
spinothalamic tracs cells. Pain 19:277293
Collins JG (1986) Effects of ketamine on low intensity tactile sensory
input are not dependent upon a spinal site of action. Anesth Analg
65(11):11231129
Coombs JS, Curtis DR, Landgren S (1956) Spinal cord potentials
generated by impulses in muscle and cutaneous afferent bers. J
Neurophysiol 19:452467
De LaTorre S, Rojas-Piloni G, Martnez-Lorenzana G, Rodrguez-
Jimnez J, Villanueva L, Conds-Lara M (2009) Paraventricular
oxytocinergic hypothalamic prevention or interruption of long-
term potentiation in dorsal horn nociceptive neurons: electro-
physiological and behavioral evidence. Pain 144:320328
Devor M (2009) Ectopic discharge in A afferents as a source of neu-
ropathic pain. Exp Brain Res 196:115128
Eccles JC, Schmidt RF, Willis WD (1963) Depolarization of the central
terminals of cutaneous afferent bers. J Neurophysiol 26:646661
Gonzlez SQ, Alegra BS, Olmos JC, Jimnez-Estrada I (2011) Effect
of chronic undernourishment on the cord dorsum potentials and
the primary afferent depolarization evoked by cutaneous nerves
in the rat spinal cord. Brain Res Bull 85:6874
Huang C, Wang Y, Han JS, Wan Y (2002) Characteristics of elec-
troacupuncture-induced analgesia in mice: variation with
strain, frequency, intensity and opioid involvement. Brain Res
945(1):2025
Huang C, Li HT, Shi YS, Han JS, Wan Y (2004) Ketamine potentiates
the effect of electroacupuncture on mechanical allodynia in a rat
model of neuropathic pain. Neurosci Lett 368:327331
Hwang BG, Min BI, Kim JH, Na HS, Park DS (2002) Effects of elec-
troacupuncture on the mechanical allodynia in the rat model of
neuropathic pain. Neurosci Lett 320:4952
Jimnez I, Rudomin P, Solodkin M (1987) Mechanisms involved in
the depolarization of cutaneous afferents produced by segmen-
tal and descending inputs in the cat spinal cord. Exp Brain Res
69:195207
Kerr FW, Wilson PR, Nijensohn DE (1978) Acupuncture reduces
the trigeminal evoked response in decerebrate cats. Exp Neurol
61:8495
AQ7
AQ8
383
384
385
386
387
388
389
390
391
392
393
394
395
396
397
398
399
400
401
402
403
404
405
406
407
408
409
410
411
412
413
414
415
416
417
418
419
420
421
422
423
424
425
426
427
428
429
430
431
432
433
434
435
436
437
438
439
440
441
442
443
444
445
446
447
448
449
450
451
452
453
454
455
456
457
458
459
460
461
462
463
464
465
466
467
468
469
470
471
472
473
474
475
476
477
478
479
480
481
482
483
484
485
486
487
488
489
490
491
492
493
494
495
496
497
A
u
t
h
o
r
P
r
o
o
f
U
N
C
O
R
R
E
C
T
E
D
P
R
O
O
F
Journal : Large 221 Dispatch : 19-4-2014 Pages : 9
Article No : 3965 LE TYPESET
MS Code : EBR-14-0106 CP DISK
Exp Brain Res
1 3
Kim JH, Min BI, Na HS, Park DS (2004) Relieving effects of elec-
troacupuncture on mechanical allodynia in neuropathic pain
model of inferior caudal trunk injury in rat: mediation by spinal
opioid receptors. Brain Res 998:230236
Kim HW, Roh DH, Yoon SY, Kang SY, Kwon YB, Han HJ, Lee HJ,
Choi SM, Ryu YH, Beitz AJ, Lee JH (2006) The anti-inamma-
tory effects of low- and high-frequency electroacupuncture are
mediated by peripheral opioids in a mouse air pouch inamma-
tion model. J Altern Complement Med 12:3944
Kremer E, Lev-Tov A (1998) GABA-receptor-independent dorsal root
afferents depolarization in the neonatal rat spinal cord. J Neuro-
physiol 79:25812592
Lau WK, Chan WK, Zhang JL, Yung KK, Zhang HQ (2008) Electroa-
cupuncture inhibits cyclooxygenase-2 up regulation in rat spinal
cord after spinal nerve ligation. Neuroscience 155:463468
Le Bars D (2002) The whole body receptive eld of dorsal horn mul-
tireceptive neurons. Brain Res Rev 40:2944
Leung L (2012) Neurophysiological basis of acupuncture-induced
analgesiaan updated review. J Acupunct Meridian Stud
5:261270
Li CY, Zhu LX, Li WM, Ji CF (1993) Relationship between presyn-
aptic depolarization and effect of acupuncture, -aminobutyric
acid, opioid peptide substance P. Acupunct Res 18:178182
Lidierth M (2006) Local and diffuse mechanisms of primary afferent
depolarization and presynaptic inhibition in the rat spinal cord. J
Physiol 576:309327
Liu XG, Morton CR, Azkue JJ, Zimmermann M, Sandkuhler J (1998)
Long-term depression of C-bre-evoked spinal eld potentials by
stimulation of primary afferent A-bres in the adult rat. Eur J
Neurosci 10:30693075
Maslany S, Crockett DP, Egger MD (1992) Organization of cutane-
ous primary afferent bers projecting to the dorsal horn in the rat:
WGA-HRP versus B-HRP. Brain Res 569:123135
Ochoa JL (1994) Pain mechanisms in neuropathy. Curr Opin Neurol
7:407414
Panneton WM, Gan Q, Juric R (2005) The central termination of sen-
sory bers from nerves to the gastrocnemius muscle of the rat.
Neuroscience 134:175187
Quiroz-Gonzalez S, Guadarrama Olmos J, Segura Alegra B, Jimenez
Estrada I (2013) Depressing effect of electroacupuncture on the
N1 component of the cord dorsum potential in the rat spinal cord.
Abstr Soc Neurosci S-3601
Quiroz-Gonzlez S, Segura-Alegra B, Guadarrama-Olmos JC, Jim-
nez-Estrada I (2014) Cord dorsum potentials evoked by electroa-
cupuncture applied to the hind limbs of rats. J Acupunct Meridian
Stud 7:2532
Quirz-Gonzalez S, Segura-Alegra B, Olmos JC, Jimnez-Estrada I
(2012) The effect of chronic undernourishment on the synaptic
depression of cutaneous pathways in the rat spinal cord. Brain
Res Bull 89:97101
Rudomin P (2007) In search of lost presynaptic inhibition. Exp Brain
Res 196:139151
Rudomin P, Hernandez E (2008) Changes in synaptic effectiveness of
myelinated joint afferents during capsaicin-induced inammation
of the footpad in the anesthetized cat. Exp Brain Res 187:7184
Rudomin P, Schmidt R (1999) Presynaptic inhibition in the vertebrate
spinal cord revisited. Exp Brain Res 129:137
Sandkuhler J, Chen JG, Cheng G, Randic M (1997) Low-Frequency
stimulation of afferent A-Fibers induces long term depression at
primary afferent synapses with substantia gelatinosa neurons in
the rat. J Neurosci 17:64836491
Vickers AJ, Cronin AM, Maschino AC, Lewith G, MacPherson H,
Foster NE et al (2012) Acupuncture for chronic pain: individual
patient data meta-analysis. Arch Intern Med 172:14441453
Willis WD, Weir MA, Skinner RD, Bryan RN (1973) Differen-
tial distribution of spinal cord eld potentials. Exp Brain Res
17:169176
Xing GG, Liu FY, Qu XX, Han JS, Wan Y (2007) Long-term syn-
aptic plasticity in the spinal dorsal horn and its modulation by
electroacupuncture in rats with neuropathic pain. Exp Neurol
208:323332
Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG
(2008) A proposed transpositional acupoint system in a mouse
and rat model. Res Vet Sci 84:159165
Yin CS, Shim BS, Lee H, Choi SH (2010) Acupuncture in accom-
plishing health for all. Neurol Res 32:1821
Zhang YQ, Ji GC, Wu GC, Zhao ZQ (2003) Kynurenic acid enhances
electroacupuncture analgesia in normal and carrageenan-injected
rats. Brain Res 966:300307
Zhang RX, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM
(2004) Involvement of opioid receptors in electroacupuncture-
produced anti-hyperalgesia in rats with peripheral inammation.
Brain Res 1020:1217
Zhang R, Lao L, Ren K, Berman BM (2014) Mechanisms of acu-
puncture-electroacupuncture on persistent pain. Anesthesiology
120:482503
Zhao ZQ (2008) Neural mechanism underlying acupuncture analge-
sia. Prog Neurobiol 85:355375
Zhou F, Huang D, Xia Y (2010) Neuroanatomic basis of acupuncture
points. In: Xia Y, Cao XD, Wu GC, Cheng JS (eds) Acupunc-
ture therapy for neurological diseases: a neurobiological view.
Springer, Berlin, pp 3280
AQ9
498
499
500
501
502
503
504
505
506
507
508
509
510
511
512
513
514
515
516
517
518
519
520
521
522
523
524
525
526
527
528
529
530
531
532
533
534
535
536
537
538
539
540
541
542
543
544
545
546
547
548
549
550
551
552
553
554
555
556
557
558
559
560
561
562
563
564
565
566
567
568
569
570
571
572
573
574
575
576
577
578
579
580
581
582
583
584
585
586
587
588
589
590
A
u
t
h
o
r
P
r
o
o
f
Journal: 221
Article: 3965
1 3I
Author Query Form
Please ensure you ll out your response to the queries raised below and return this form along
with your corrections
Dear Author
During the process of typesetting your article, the following queries have arisen. Please check your typeset proof
carefully against the queries listed below and mark the necessary changes either directly on the proof/online grid or in the
Authors response area provided below
Query Details Required Authors Response
AQ1 Please check and conrm the author names and initials are correct. Also, kindly
conrm the details in the metadata are correct.
AQ2 Please check whether the mail ID "quiroz@sio.cinvestav.mx" should appear in the
publication.
AQ3 Please check and conrm the clarity of the sentence This injectionreexes
AQ4 Collins et al. (1986) has been changed to Collins (1986) so that this citation matches
the list.
AQ5 Reference Kim et al. (2011) is cited in text but not provided in the reference list. Please
provide reference in the list or delete these citations.
AQ6 Quiroz et al. (2012) has been changed to Quirz-Gonzalez et al. (2012) so that this
citation matches the list.
AQ7 The term transmition has been changed to transmission in the article. Please check
and approve.
AQ8 References Baron and Saguer (1993), Eccles et al. (1963), Jimnez et al. (1987), Kerr
et al. (1978), Ochoa (1994), Rudomin (2007) are given in list but not cited in text.
Please cite in text or delete from list.
AQ9 Please provide volume number for reference Quiroz-Gonzalez et al. (2013).
A
u
t
h
o
r
P
r
o
o
f
Vous aimerez peut-être aussi
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (120)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Gastrointestinal SystemDocument109 pagesGastrointestinal SystemRaju Mehta100% (1)
- FundusDocument10 pagesFundusKristin NataliaPas encore d'évaluation
- Block Hepatobiliary SystemDocument29 pagesBlock Hepatobiliary SystemKevin KusumanPas encore d'évaluation
- ENT Anatomy and PhysiologyDocument32 pagesENT Anatomy and Physiologyoxford_commaPas encore d'évaluation
- Short Tricks Biology 11thsDocument36 pagesShort Tricks Biology 11thskomatipallis615Pas encore d'évaluation
- Diabetes MellitusDocument18 pagesDiabetes Mellitusrajirajesh100% (1)
- 3 Tissues in The Human Body 22864Document11 pages3 Tissues in The Human Body 22864Musharaf RehmanPas encore d'évaluation
- Hypothalamus Pituitary Glands Adrenal CortexDocument2 pagesHypothalamus Pituitary Glands Adrenal CortexEmPas encore d'évaluation
- 1757 1626 1 333Document5 pages1757 1626 1 333Ade CandraPas encore d'évaluation
- Science5 q2 Mod1 ThePartsOfTheReproductiveSystemAndTheirFunctions v2Document24 pagesScience5 q2 Mod1 ThePartsOfTheReproductiveSystemAndTheirFunctions v2Shiela AsiloPas encore d'évaluation
- General Physiology Control Systems of Homeostasis: By: Dr. Hamza Al-TradDocument22 pagesGeneral Physiology Control Systems of Homeostasis: By: Dr. Hamza Al-TradibrahimPas encore d'évaluation
- Thyroid Status Examination OSCE GuideDocument20 pagesThyroid Status Examination OSCE GuideemmaPas encore d'évaluation
- K10 Kuliah NefrourologiDocument170 pagesK10 Kuliah NefrourologimarinanananaPas encore d'évaluation
- 1 6 Regulation of Blood Glucose PDFDocument3 pages1 6 Regulation of Blood Glucose PDFtiaraPas encore d'évaluation
- Icopim Vol 2Document169 pagesIcopim Vol 2DannKusumaPas encore d'évaluation
- Themenstrualcycle 120606211927 Phpapp02Document16 pagesThemenstrualcycle 120606211927 Phpapp02Ludy LynPas encore d'évaluation
- Clinical Oriented Anatomy of Urinary SystemDocument81 pagesClinical Oriented Anatomy of Urinary SystemRizcky Naldy Eka Putra100% (1)
- Mote - 'Hugh'Document18 pagesMote - 'Hugh'Melissa R.Pas encore d'évaluation
- Medical Surgical Nursing OrthopedicDocument22 pagesMedical Surgical Nursing Orthopedicroger80% (5)
- KOYANO Et Al-2012-Journal of Oral Rehabilitation PDFDocument9 pagesKOYANO Et Al-2012-Journal of Oral Rehabilitation PDFKathy Cuevas HinojosaPas encore d'évaluation
- Radiology in Pediatric Dentistry 2Document44 pagesRadiology in Pediatric Dentistry 2Aima Cuba100% (1)
- Evaluation of Length Intestine Ratio With Long Body of FishDocument10 pagesEvaluation of Length Intestine Ratio With Long Body of FishPuri RahmaPas encore d'évaluation
- Anatomy of Urinary Bladder and UrethraDocument16 pagesAnatomy of Urinary Bladder and UrethraAmalina ZolkefleePas encore d'évaluation
- Science 10 Q3 - M2Document14 pagesScience 10 Q3 - M2Avha CortesPas encore d'évaluation
- Kents New RemediesDocument168 pagesKents New RemediesluckyPas encore d'évaluation
- Human Reproduction PDFDocument35 pagesHuman Reproduction PDFsqhaa100% (4)
- Endocrine System ChartDocument2 pagesEndocrine System ChartValeria Guadalupe Ramírez Moctezuma100% (2)
- Blood Distribution During Rest and ExerciseDocument2 pagesBlood Distribution During Rest and ExercisePannir SelvaPas encore d'évaluation
- (PED) T.10.2 - Pediatric Endocrinology (Part II) PDFDocument7 pages(PED) T.10.2 - Pediatric Endocrinology (Part II) PDFLiberty AgcaoiliPas encore d'évaluation
- The Heart Has Several Pacemakers Known As Autonomic FociDocument1 pageThe Heart Has Several Pacemakers Known As Autonomic FociAnonymous mLYupGyNPas encore d'évaluation