Académique Documents
Professionnel Documents
Culture Documents
ÀPI Gravity Lab
Transféré par
Levi MatthewDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
ÀPI Gravity Lab
Transféré par
Levi MatthewDroits d'auteur :
Formats disponibles
1 | P a g e
CERTIFICATE IN PROCESS OPERATIONS
CHEMISTRY/CORROSION CHEM 1003 SF 18
LABORATORY EXPERIMENT 1: A.P.I. GRAVITY
HYDROMETER METHOD (MODIFIED A.S.T.M. D287)
DATE SUBMITTED: 06/02/14
DONE BY:
DENNIS NANDLAL
ANDERSON RAMSROOP
HANSRAJ SAMLAL
NIKOLAI JOHNSON
2 | P a g e
INTRODUCTION
AIM:
To determine the A.P.I. Gravity of petroleum liquids using the hydrometer method.
THEORY/BACKGROUND INFORMATION:
Density is defined as the ratio of a bodys mass per unit of its volume. It is represented as:
=
Relative density of a material is the ratio of the materials density to the density of some
substance normally used as a reference. This reference material is usually water.
In the Engineering field, Specific Gravity is also used to refer to relative density.
The American Petroleum Institute proposed the API Gravity scale for comparing the densities
of components of petroleum products to themselves or to water. API Gravity gives an indication
of how heavy or light a component is when compared to water. Those components having
values higher than 10 are less dense than water and will float on it, those with values less than
10 are more dense and will sink. A petroleum component will float on another if it has a higher
API value but will sink if it has a lower value.
API Gravity like relative density or specific gravity generally has no units, however its value is
referred to as being in degrees.
3 | P a g e
MEASUREMENT
Measuring the API value is done directly with the use of a hydrometer. Hydrometers are also
calibrated so as to measure specific gravity directly. This instrument is a closed cylindrical glass
tube weighted at the lower end to cause it to float in a perfectly vertical position. The upper
part or stem is graduated so that the gravity of the liquid under investigation is directly read in
degrees API.
The hydrometer is placed in the liquid and allowed to come to rest or equilibrium. At this point,
the line of the flotation level on the stem can be read. In some heavy oils it may be necessary
for the hydrometer to stand in the how overnight to allow gas bubbles to rise before taking a
reading as the presence of air in the liquid will affect the API gravity measurement.
It is important to ensure that the level of the liquid whose specific gravity is being measured is
well above the bulb of the hydrometer so that a reading can be taken at eye level with the
liquid surface against the stem of the hydrometer. For transparent liquids, the reading can be
4 | P a g e
taken easily, however for opaque liquids like crude oil, only the line made at the top of the oil
can be seen. The oil wets the stem of the hydrometer stem causing an upward curve of the
liquid surface at the stem. This wetting effect is compensated for by subtracting 0.1
o
API from
the reading or in the case of specific gravity, 0.001 is added to the result.
Crude oil properties vary at different temperatures. When heated, the crude is thinner and has
a higher gravity than when it is cooled. The oil industry therefore adopted 15.5
o
C or 60
o
F as the
standard at which all gravity readings should be taken. Readings taken at any other
temperature has to be corrected to 15.5
o
C with the aid of gravity correction table.
5 | P a g e
APPARATUS:
Relative density glass hydrometers
Glass thermometers, general purpose in
o
C
250 ml graduated cylinders
Paper towels
PROCEDURE:
1. Samples of diesel, kerosene, gasoline and crude oil were obtained and poured into
separate 250 ml graduated cylinders to that they were about 80% full.
2. Glass hydrometers were gently lowered into the fluid in each cylinder, away from the
cylinder walls so that they floated perfectly upright.
3. The hydrometers were read to the nearest scale division and the correct readings taken
at eye level. In the case of the crude oil which was opaque, the appropriate correction
factor was applied.
4. The temperatures were measured before and after insertion of the hydrometers and in
cases where changes occurred, the average was used
5. When the readings were taken, the thermometers and hydrometers were removed and
dried thoroughly with paper towels and then stored horizontally.
RESULTS:
Description of
Sample
Measured Specific
Gravity at ambient
Temperature
Calculated
Specific Gravity at
15.6 degrees C(
consult relative
density tables)
Calculated API
Gravity at 15.6
degrees C
S.G TEMP(
o
C)
S.G TEMP
(
o
C)
Kerosene 0.81 26 0.8172 15.6 41.7
Gasoline 0.75 22 0.7550 15.6 55.9
Diesel/Lube Oil 0.86 26 0.8669 15.6 31.7
Crude Oil 0.88 22.5 0.8845 15.6 28.5
6 | P a g e
SAMPLE CALCULATIONS
1. Correction of specific gravity value of gasoline to 60
o
F:
0.7548 +0.6(0.7552 0.7548) = 0.7550
2. = (
141.5
) 131.5 (for the gasoline)
= (
141.5
0.7550
) 131.5
= 55.9
ANSWERS TO QUESTIONS:
1. A high API gravity will give a low specific gravity value
2. =
141.5
131.5+ 63.5
= 0.7256
3. If a sample had an API gravity of 63.5 at 15.6
o
C, then this sample would be a sample of
heptane or hexane.
7 | P a g e
DISCUSSIONS:
For this experiment, ambient temperature was used as the standard temperature for each
sample so as to obtain consistent specific gravity values.
The hydrometers were rotated to centre them and also to eliminate any bubbles from the
respective liquids.
To minimize parallax errors when reading the hydrometers, each student in the group read the
hydrometers at eye level and an average value was taken. The hydrometers were allowed to
come to stable equilibrium before any readings were taken. The thermometers were thoroughly
wiped clean before use in each sample. Temperature readings were taken at eye level by all
students in the group and an average taken. The thermometers were placed close to the middle
of the measuring cylinders when taking temperature readings since the outer layer of liquid
close to the cylinder wall may have had a slightly different temperature from that closer to the
middle.
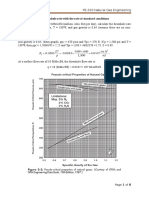
The required document, ASTM Publication D1250-80 was not readily available in its entirety and
therefore an alternative, The National Standard Petroleum Oil Tables, which is approved by API
and ASTM was used to correct specific gravity values.
Specific gravity and API values which were measured and calculated respectively were
consistent with that expected for crude oil fractions, i.e. the lighter fractions have higher API
value and lower specific gravity. The heavier fractions have lower API values but higher specific
gravity values.
CONCLUSION:
The experiment was successful as far as minimizing errors, the measured specific gravity values
and calculated API values were consistent with those for the liquids under investigation.
8 | P a g e
REFERENCES
1. National Bureau of Standards, U.S. Department of Commerce National Standard
Petroleum Oil Tables, Circular C410, Issued: March 4, 1936.
2. U.S. Department of Commerce, National Standard Petroleum Oil Tables, 89-125
3. API gravity, Last modified, Jan 6, 2014, http://en.wikipedia.org/wiki/API_gravity
4. API Gravity, 2013, http://www.petroleum.co.uk/api
5. API gravity
6. API Gravity
U.S. Department of Commerce, National Bureau of Standards, National Standard Petroleum Oil
Tables, Ciruclar C410, Issued March 4, 1936, 89-125
Wikipedia, API gravity, last modified, Jan 6, 2014, http://en.wikipedia.org/wiki/API_gravity
Petroleum.co.uk, API Gravity, 2013, http://www.petroleum.co.uk/api
Vous aimerez peut-être aussi
- API GravityDocument4 pagesAPI GravitySridaar KanaiyaPas encore d'évaluation
- Determination of API Gravity of Crude OilDocument9 pagesDetermination of API Gravity of Crude OilOghale B. E. OmuaborPas encore d'évaluation
- Reservoir Engineering Lab REPORT SESSION/SEM: 20212022/1: Experiment No. Title Section Group No. Group MembersDocument11 pagesReservoir Engineering Lab REPORT SESSION/SEM: 20212022/1: Experiment No. Title Section Group No. Group MembersDHANASEELAN A/L V G PRAGASAM A19ET0053Pas encore d'évaluation
- Api GravityDocument9 pagesApi GravityLawand RaufPas encore d'évaluation
- API Gravity Lab PDFDocument8 pagesAPI Gravity Lab PDFThinesh BoltPas encore d'évaluation
- Specific Gravity, and API Gravity For Petroleum ProductsDocument7 pagesSpecific Gravity, and API Gravity For Petroleum ProductsMUHAMMAD AKRAM50% (2)
- API Gravity - Wikipedia, The Free Encyclopedia PDFDocument3 pagesAPI Gravity - Wikipedia, The Free Encyclopedia PDFRamu NallathambiPas encore d'évaluation
- Flash Point Lab ReportDocument14 pagesFlash Point Lab ReportNesha Arasu67% (3)
- CPB30503 Petrochemicals & Petroleum Refining Technology Experiment 2: Determination of API Gravity Full Lab ReportDocument9 pagesCPB30503 Petrochemicals & Petroleum Refining Technology Experiment 2: Determination of API Gravity Full Lab ReportSiti Hajar Mohamed67% (3)
- Lab Report (Api Gravity)Document9 pagesLab Report (Api Gravity)nisasoberiPas encore d'évaluation
- Refrigeration Unit Lab Report FKKDocument28 pagesRefrigeration Unit Lab Report FKKKicauan KataPas encore d'évaluation
- CPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab ReportDocument11 pagesCPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab ReportSiti Hajar Mohamed100% (2)
- Reservoir Fluids Properties BookDocument219 pagesReservoir Fluids Properties Bookkexadex2100% (5)
- Redwood ViscometerDocument5 pagesRedwood ViscometerBharath ReddyPas encore d'évaluation
- Gas Hydrates - PCDocument8 pagesGas Hydrates - PCAashish DwivediPas encore d'évaluation
- Chapter 2 PDFDocument39 pagesChapter 2 PDFjeedPas encore d'évaluation
- Petroleum Refinery Lab. Kinematic ViscosityDocument11 pagesPetroleum Refinery Lab. Kinematic ViscositySiyar SaleemPas encore d'évaluation
- Redwood Viscometer ExperimentDocument4 pagesRedwood Viscometer ExperimentSudarshan Ghotekar100% (1)
- Drilling Lab 1 FullDocument14 pagesDrilling Lab 1 FullIdham Arif100% (1)
- Chapter 2 Dry Gas Reservoir: Example 1Document8 pagesChapter 2 Dry Gas Reservoir: Example 1Muhammad Nizam50% (2)
- Experiment 1 Determination of Flash PointDocument7 pagesExperiment 1 Determination of Flash Pointismat irfanPas encore d'évaluation
- Ee - Lab ReportDocument36 pagesEe - Lab ReportNoshaba Noreen75% (4)
- Smoke PointDocument10 pagesSmoke PointMUHAMMAD AKRAMPas encore d'évaluation
- Executive Summary:: Reservoir Engineering Lab SN 02 / Group F Experiment 1 27TH MARCH 2016Document14 pagesExecutive Summary:: Reservoir Engineering Lab SN 02 / Group F Experiment 1 27TH MARCH 2016MelindaPas encore d'évaluation
- Lab Gas FlowmeterDocument7 pagesLab Gas Flowmeterazym94Pas encore d'évaluation
- Residue CarbonDocument7 pagesResidue CarbonAram IbrahimPas encore d'évaluation
- Drilling Engineering Laboratory Report About Marsh Funnel ViscometerDocument10 pagesDrilling Engineering Laboratory Report About Marsh Funnel ViscometerAvericl H n v ejkePas encore d'évaluation
- Lab10 CompleteDocument22 pagesLab10 CompleteMastura Ahmad Termizi100% (1)
- Investigation of Liquid-Solid and Gas-Solid Fluidized BedDocument18 pagesInvestigation of Liquid-Solid and Gas-Solid Fluidized Bedmahbub1332100% (1)
- Ibrahiiiiim Rep. Conradson Carbon ResidueDocument7 pagesIbrahiiiiim Rep. Conradson Carbon ResidueIbrahim Dewali100% (2)
- Tutorial 2Document3 pagesTutorial 2علي حسين جميلPas encore d'évaluation
- Redwood I UpdatedDocument7 pagesRedwood I UpdatedVitalram RayankulaPas encore d'évaluation
- Refrigerant Experiment (Apparatus, Procedure, Discussion)Document6 pagesRefrigerant Experiment (Apparatus, Procedure, Discussion)Hakimi HarisPas encore d'évaluation
- Mud Densities Exp NewDocument8 pagesMud Densities Exp NewHafizszul FeyzulPas encore d'évaluation
- Natural Gas Physical PropertiesDocument20 pagesNatural Gas Physical PropertieseimrehPas encore d'évaluation
- CPB30503 Petrochemicals & Petroleum Refining Technology - Experiment 1: Determination of Flash Point of Petroleum Products Full Lab ReportDocument17 pagesCPB30503 Petrochemicals & Petroleum Refining Technology - Experiment 1: Determination of Flash Point of Petroleum Products Full Lab ReportSiti Hajar Mohamed100% (2)
- Salt ContentDocument8 pagesSalt Contentf3byzPas encore d'évaluation
- Lab 2 Full Report PDFDocument20 pagesLab 2 Full Report PDFmuhammad ilyas100% (1)
- Distillation Characteristics of Petroleum DieselDocument30 pagesDistillation Characteristics of Petroleum DieselT Richie100% (2)
- Petroleum Product Testing Lab ManualDocument40 pagesPetroleum Product Testing Lab ManualVishesh Sharma100% (1)
- Surface FacilitiesDocument20 pagesSurface Facilitiesheriku_mulyaPas encore d'évaluation
- Differential Liberation - LabDocument12 pagesDifferential Liberation - LabAhmed AmirPas encore d'évaluation
- Calibration of A Pressure Gauge (GRP 7)Document7 pagesCalibration of A Pressure Gauge (GRP 7)Nene Kojo AddicoPas encore d'évaluation
- Assignment 2 Q2Document2 pagesAssignment 2 Q2Johan Aliff0% (1)
- Example 3 Relating Downhole Rate With The Rate at Standard ConditionsDocument8 pagesExample 3 Relating Downhole Rate With The Rate at Standard ConditionsMaisam AbbasPas encore d'évaluation
- Able's ApparatusDocument2 pagesAble's ApparatusRaghu RamPas encore d'évaluation
- Lab Report CMT 450 Tray DryerDocument3 pagesLab Report CMT 450 Tray DryerJohanPas encore d'évaluation
- MT4 Lab FinalDocument19 pagesMT4 Lab FinalAmelia MaharajPas encore d'évaluation
- Aniline Point (Astm D-611) & Diesel Index & Cetane Number (Astm D-613 10A) ObjectDocument3 pagesAniline Point (Astm D-611) & Diesel Index & Cetane Number (Astm D-613 10A) ObjectFAH MAN100% (1)
- CH - 6 Saturated Oil ReservoirsDocument37 pagesCH - 6 Saturated Oil ReservoirszazoPas encore d'évaluation
- Lab Gas Flow (Afif)Document17 pagesLab Gas Flow (Afif)Nurshazwani syuhada bt al- badri100% (1)
- Depletion Drive CalculationsDocument17 pagesDepletion Drive CalculationsFlorian Ananias Byarugaba100% (4)
- Bioproduct Facility Design Lab: Faculty of Engineering Technology Department of Chemical Engineering TechnologyDocument18 pagesBioproduct Facility Design Lab: Faculty of Engineering Technology Department of Chemical Engineering TechnologyAswini Purushothanan0% (1)
- Gas Absorption LabDocument8 pagesGas Absorption Labsolehah misni100% (1)
- DistillationDocument25 pagesDistillationMohammadAslam100% (1)
- University of Tripoli Faculty of Engineering Petroleum EngineeringDocument10 pagesUniversity of Tripoli Faculty of Engineering Petroleum EngineeringesraPas encore d'évaluation
- API Gravity Lab 1Document4 pagesAPI Gravity Lab 1JustinPas encore d'évaluation
- Exp1 Specific and API Gravity of Crude OilDocument12 pagesExp1 Specific and API Gravity of Crude OilMelih Efe ŞirinPas encore d'évaluation
- EXPT1Document7 pagesEXPT1Arthur Christian SolemnePas encore d'évaluation
- Oil and Gas Property Lab .: Test No: Test NameDocument7 pagesOil and Gas Property Lab .: Test No: Test NameMUHAMMED FUADPas encore d'évaluation
- Marshall Mix DesignDocument27 pagesMarshall Mix DesignSAQAR ALGHAMDI100% (5)
- 077154C - 1813 PDS 1543 001 A Technip India LTD.: Client MRPL Location Mangalore, Karnata Unit New LPG AtuDocument1 page077154C - 1813 PDS 1543 001 A Technip India LTD.: Client MRPL Location Mangalore, Karnata Unit New LPG AtuChakravarthy BharathPas encore d'évaluation
- Ce8311 Civil CML Even Iiise LabmanualDocument54 pagesCe8311 Civil CML Even Iiise LabmanualUthra MohanPas encore d'évaluation
- AASHTO Test ProceduresDocument126 pagesAASHTO Test ProceduresJi Ding100% (5)
- Seal Selection Guide (John Crane)Document50 pagesSeal Selection Guide (John Crane)Jai-Hong Chung100% (9)
- Aggregate Lab ManualDocument24 pagesAggregate Lab ManualnikhilPas encore d'évaluation
- TestDocument104 pagesTestkasvikrajPas encore d'évaluation
- C1407 8174Document2 pagesC1407 8174Andres PortilloPas encore d'évaluation
- Physcial Pharmacy Lec PrelimsDocument12 pagesPhyscial Pharmacy Lec PrelimsAlfie16Pas encore d'évaluation
- Bituminous PDFDocument96 pagesBituminous PDFbsranjhaPas encore d'évaluation
- Reservoir Fluid PropertiesDocument11 pagesReservoir Fluid PropertiesTushar LanjekarPas encore d'évaluation
- Marshall Mix DesignDocument24 pagesMarshall Mix DesignMuhammad Ali Hafeez100% (1)
- Wingtack 95 FlakeDocument1 pageWingtack 95 FlakeeducardxPas encore d'évaluation
- Physical Properties of Cashew Nut Shell LiquidDocument4 pagesPhysical Properties of Cashew Nut Shell LiquidInternational Journal of Application or Innovation in Engineering & ManagementPas encore d'évaluation
- CH 02Document32 pagesCH 02TahaPas encore d'évaluation
- Hydrometer InformationDocument4 pagesHydrometer InformationArun RaoPas encore d'évaluation
- Quality Control of Milk ProcessingDocument235 pagesQuality Control of Milk ProcessingBen Dresim67% (3)
- Ec 2870 2000Document27 pagesEc 2870 2000Aldo EstelaPas encore d'évaluation
- Di Pa FinalllllllllDocument62 pagesDi Pa FinallllllllllouryPas encore d'évaluation
- 2502 LevelTrol ControllerDocument24 pages2502 LevelTrol Controllerhtm64Pas encore d'évaluation
- CAESAR II PIPING Tutorial A Pages 120 To 157 From C2APDocument38 pagesCAESAR II PIPING Tutorial A Pages 120 To 157 From C2APLuis OrtizPas encore d'évaluation
- ASTM D1461 (1994) - Moisture or Volatile Distillates in Bituminous Paving MixturesDocument5 pagesASTM D1461 (1994) - Moisture or Volatile Distillates in Bituminous Paving Mixturesnoto SugiartoPas encore d'évaluation
- Example 3 Relating Downhole Rate With The Rate at Standard ConditionsDocument8 pagesExample 3 Relating Downhole Rate With The Rate at Standard ConditionsMaisam AbbasPas encore d'évaluation
- Engineering Properties of AggregatesDocument4 pagesEngineering Properties of AggregatessuryakantamePas encore d'évaluation
- Paper Subject Oil, Water and Gas) : (SaturationDocument9 pagesPaper Subject Oil, Water and Gas) : (Saturationمحمد أحمد عبداللطيفPas encore d'évaluation
- Aashto r46 - SmaDocument12 pagesAashto r46 - SmaKleberson Ramos100% (1)
- Astm D 792Document7 pagesAstm D 792IngJGM100% (1)
- ESP 9-Step DesignDocument31 pagesESP 9-Step Designeng.osama100% (2)
- 9162 PDFDocument65 pages9162 PDFPawan Kumar100% (1)
- Catalogo Alfa 2016Document275 pagesCatalogo Alfa 2016david marazPas encore d'évaluation