Académique Documents
Professionnel Documents
Culture Documents
Collour Metallography of White Cast Iron II
Transféré par
fdcarazoCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Collour Metallography of White Cast Iron II
Transféré par
fdcarazoDroits d'auteur :
Formats disponibles
447
November 2011
Serial Report
Colour Metallography of Cast Iron
By Zhou Jiyang, Professor, Dalian University of Technology, China
Translated by Ph.D Liu Jincheng, Fellow of Institute of Cast Metal Engineers, UK
*
Note: This book consists of fve sections: Chapter 1 Introduction, Chapter 2 Grey Iron, Chapter 3 Spheroidal Graphite
Cast Iron, Chapter 4 Vermicular Cast Iron, and Chapter 5 White Cast Iron. CHINA FOUNDRY publishes this book in
several parts serially, starting from the frst issue of 2009
Chapter 5
White Cast Iron
()
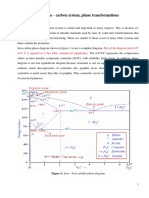
5.5 Eutectic crystallisation of white iron
When undercooled below the eutectic line ECF in the Fe-C
phase diagram, liquid iron will start eutectic transformation
(crystallization): eutectic liquid cementite + austenite.
Eutectic crystallisation is an important stage during the
crystallization of white iron. At this stage, the nucleation and
growth of eutectic cells (consisting of carbide or cementite +
austenite) occur. The carbide in eutectic cells (or eutectic carbide)
is the main hard and brittle phase structure which has an important
effect on the properties of white iron. If there is no primary carbide
in the structure, the effect of eutectic carbide is more prominent.
5.5.1 Thermodynamics and kinetics of eutectic
crystallisation
Whether a eutectic melt follows the meta-stable system to
crystallise as carbide + austenite, or follows the stable system to
crystallise as graphite + austenite eutectic, is dependent on the
nucleation and growth of the two high carbon phases (carbide
and graphite), namely, on thermodynamic and kinetic conditions.
Figure 5-23 shows the comparison of thermodynamic driving
forces of the two eutectics. The two lines in the lower section of the
fgure represent the free energy of the two eutectics respectively
and G
L
is the free energy of the undercooled iron melt. It is easy to
see that the iron melt has the highest free energy and the graphite-
austenite has the lowest free energy; so, following a stable system,
the thermodynamic condition favours the crystallisation of
graphite-austenite eutectic from the iron melt.
However, compared to forming graphite, forming carbide needs
much less carbon atom diffusion; thus, when the cooling rate is
fast, forming carbide austenite has faster growth velocity and the
kinetic conditions favour a meta-stable system of crystallisation.
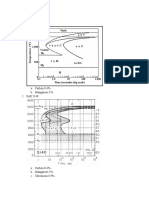
Figure 5-24 shows the relationship between growth velocity
and temperature for the two eutectics
12]
. When an iron melt is
undercooled between 1,148-1,153C, only graphite can nucleate
and grow; if rapidly undercooled below 1,148C, both (Fe
3
C +
austenite) eutectic and (graphite + austenite) eutectic are possible
G
L
Free energy of undercooled liquid iron, G
cm
Free energy of
cementite, G
r
Free energy of austenite, G
gr
Free energy of graphite
Fig. 5-23: Comparison of free energy of formation of the
two eutectics
1 (Fe
3
C + austenite) eutectic, 2 (graphite + austenite) eutectic
T
1
The region spontaneously transferring from the solidifcation
mode of white iron to that of grey iron
T
2
The region spontaneously transferring from the solidification
mode of grey iron to that of white iron
Fig. 5-24: Relationship between (graphite + austenite) eutectic,
(Fe
3
C + austenite) eutectic and temperature
[12]
448
CHINA FOUNDRY
Vol.8 No.4
(a) Transverse section
(b) Longitudinal section
Fig. 5-25: Two-dimensional structure of honeycomb ledeburite
(1) Honeycomb eutectic (often called ledeburite)
Honeycomb ledeburite consists of eutectic cells in which many
austenite rods, along [001], are embedded into cementite plates (or
blocks) based on (001) plane. The eutectic cells show a lamellar
or plate shape; their length and width are far greater than their
thickness. Therefore, under microscope, only the transverse sections
of the austenite rods are observed; after etching, they show as dark
round spots on a white substrate (cementite). The two-dimensional
image of honeycomb ledeburite is illustrated in Fig. 5-25.
The formation process of ledeburite is shown in Fig. 5-26
[20]
:
(a) At the beginning of the eutectic, cementite nucleates first
as the leading phase. For a hypoeutectic white iron, the nucleus
substrates of eutectic cementite are very small and diffcult to fnd
[5]
.
For a hypereutectic white iron, the debris or fragments of primary
cementite or their extent sections are good nuclei for eutectic
cementite. The Fe
3
C embryos have obvious anisotropy, thus Fe
3
C
plates grow easily along the heat fow direction
[21]
, and the base
planes grow quickly into a flat, plate-shape. Because of the
orientation relationships, which exist between certain crystal
planes of cementite and austenite
[22]
: (104)
Fe
3
C
//(101)
and (010)
Fe
3
C
//(310)
, austenite is in close contact with plate-like Fe
3
C and is
easy to grow following a plate crystal growth mode, thus forming
the earliest plate structure.
(a) Growth process
Fig. 5-26: Formation process of honeycomb ledeburite
[20]
to nucleate, but the growth velocity of (Fe
3
C + austenite) eutectic
exceeds that of (graphite + austenite) eutectic, which causes the
iron melt to crystallise totally as white iron. It is known from this
that a rapid cooling rate (or undercooling) is an important factor in
determining whether crystallisation follows a meta-stable system
or not.
Addition of carbide-forming elements will increase the tendency
of an iron melt to form (Fe
3
C + austenite) eutectic and promote
chill formation from thermodynamics and kinetics. Therefore,
the alloyed irons containing medium or high contents of Cr, Mn,
V, W and Nb all solidify as white irons, even under slow cooling
conditions.
5.5.2 Eutectic growth of normal white iron
The eutectic crystallisation of a white iron is a crystallisation
process in which cementite + austenite eutectic (eutectic cells)
form the main structure. According to the principle of eutectic
crystallisation, the formation of a second phase on the leading
phase and their restricted cooperative growth is the key to eutectic
cell formation.
Based on the morphology of cementite and austenite in eutectic,
Richard divided the eutectic structure of normal white iron into
two types
[19]
: honeycomb ledeburite and plate cementite.
a-axis edge growth
b-axis edge growth
c-axis lateral growth
Fe
3
C leading phase edge growth
Lateral growth of Fe
3
C
c-axis growth of Fe
3
C
(b) Change of growth direction
449
November 2011
Serial Report
(b) Due to non-uniform adsorption, constitutional undercooling
and carbon enrichment of the liquid iron between lamellar
austenite plates, the Fe
3
C crystals situated in this region become
unstable
[20]
. This provides an opportunity for austenite dendrites to
change their growth direction from previous a-axis to c-axis and to
grow laterally.
(c) The fat plates of austenite dendrites also branch and grow
laterally, perpendicular to the a-axis. They extend into the gaps
between laterally branched cementite plates, because the carbon
content there is lower. Thus, laterally branched austenite and
laterally grown cementite grow together co-operatively.
At the beginning, laterally growing austenite branches according
to the plate mode, then gradually grows in a rod-shaped form.
Bunin
[5]
considered that the transmission mechanism from plate-
branching to rod is related to accumulated impurities in front of
the eutectic; the formed undercooling also promotes further growth
of the protruding section on austenite. According to research by
Park
[23]
, laterally growing austenite which shows a rod-shape is
the result of the effect of silicon. Si promotes dendrites to branch
and offers more interdendritic grooves. When the silicon content
exceeds 1%, austenite changes from plate-branching to rod shape.
The mass content of silicon in normal white irons usually exceeds
1%, therefore eutectic structures are mainly honeycomb ledeburite.
The edge growth of Fe
3
C and austenite and the lateral growth
of austenite rods perpendicular to Fe
3
C, form the eutectic with
pseudo-regular structure
[22]
. Since the edge growth is faster than
the lateral one, the shape of ledeburite eutectic cells is different
from that of graphite-austenite eutectic cells in grey iron (or in
VG iron); it is not spherical, but a plate-like shaped structure
with length and width both larger than thickness. Only under the
conditions of very severe undercooling (for example, a cooling
rate of 10
4
-10
5
K/s) can the iron form spherical ledeburite
eutectic cells
[5]
. The degree of branching of the two phases in
ledeburite becomes serious with increasing cooling rate, see Fig.
5-27. Among them, the increased austenite branches result in
the formation of many densely distributed small austenite rods;
while the increase of the leading phase, cementite reduces the size
of eutectic cells. Treatment with ferro-boron can refine primary
austenite dendrites; this increases branching of the eutectic
austenite, therefore, decreases the diameter of honeycomb cells
(the transverse section of austenite rods), and also increases the
eutectic cell count.
Fig. 5-27: Effect of cooling rate on the size of ledeburite
(b) Test bar of 30 mm (slow cooling) (a) Test bar of 10 mm (fast cooling)
If primary austenite is well developed and the interdendritic
interval is very narrow, the eutectic liquid in the interdendritic
region is prone to crystallise in the form of a divorced eutectic. At
this time, eutectic cementite precipitates as thin plate-shapes, see
Fig. 5-28. Therefore, a honeycomb eutectic structure cannot be
seen in the interdendritic region.
(2) Plate-like cementite eutectic
Rickard et al
[19]
first observed plate-like cementite eutectic in
hypoeutectic white iron with low silicon (w(Si) = 0.1%). The
features of this eutectic are: cementite, as thick plates, alternatively
distributed in the intervals of primary dendrites, and eutectic
austenite often grows on primary austenite, thus it is diffcult to
distinguish from each other; the austenite and cementite look like a
divorced eutectic structure. Different from honeycomb ledeburite,
this eutectic has no rod-like branch on the side and cementite grows
mainly along the a-axis. However, the a-axis growth velocity is
signifcantly decreased and a lateral growth thickening of the plate
Fig. 5-28: Divorced eutectic structure between austenite
dendrites
occurs. The comparison of the two eutectic structures is illustrated
in Fig. 5-29.
450
CHINA FOUNDRY
Vol.8 No.4
Although the exact reasons for the formation of plate-like
cementite are not yet totally clear, it was recognized that their
formation is related to melt undercooling and composition.
Rickard found that for a low carbon equivalent iron melt with
additions of Te and Sb, under superheating condition, that this
type of eutectic structure could be obtained. The silicon content
has an important effect on the growth of the two phases of the
eutectic; lower silicon can reduce the tendency of lateral rod-
like branching of cementite
[23]
. Phosphorous causes austenite
and cementite to transform in a divorced eutectic mode and makes
cementite grow to network-like blocks. Phosphorous has very low
solubility in solid iron and is easily enriched in the growth front of
cementite, thus retarding the diffusion velocity of Fe and C atoms
towards cementite, and decreasing the growth velocity of cementite.
In addition, the surface activity of phosphorous reduces the interface
tension between cementite and liquid, retards co-operative growth of
eutectic austenite and cementite, thus benefting the crystallisation of
plate-like cementite eutectic
[24, 25]
. When the mass percent of Mn
reaches 5%, it was found that the amount of honeycomb ledeburite
decreases and plate-like cementite increases.
Research on the influence of various types of eutectic on the
mechanical properties of white iron have shown the following:
because the austenite in plate-like cementite eutectic is connected
and the cementite has a non-continuous, isolated distribution, a
white iron with plate-like cementite eutectic has better strength
than a white iron with honeycomb cementite eutectic
[19]
.
Nevertheless, a white iron with honeycomb cementite has better
feeding properties than an iron with plate-like cementite, thus it is
not prone to produce shrinkage-induced crack/tear defects
[26]
.
5.5.3 Solidifcation and eutectic crystallisation of high
Cr white iron
(1) Solidifcation analysis of Fe-Cr-C alloy system
(a) Liquidus surface projection and solidifcation structure: Fe-
Cr-C phase diagram is a tool to analyse the solidifcation process
of high Cr system white iron. Using thermal analysis, Jackson
[27]
obtained the liquidus points of Fe-Cr-C system alloys with various
compositions and drew a liquidus surface projection, see Fig. 5-30.
The diagram is composed of 5 faces: ferrite, austenite, (Cr, Fe)
23
C
6
,
(Cr, Fe)
7
C
3
and (Cr, Fe)
3
C type carbides. Point B is the intersect
of three liquid phases, but not the lowest temperature point; along
the BC groove down to Fe-C eutectic point, that is the lowest point
of the alloy. Under equilibrium conditions, according to C and Cr
content, the types of primary phases can be determined:
For medium to high C and high Cr, the primary phase is K
2
, (Cr,
Fe)
7
C
3
carbide;
For low C and low Cr, the primary phase is or ;
For low C and high Cr, the primary phase is ;
For high C and low Cr, the primary phase is K
c
, that is (Cr,
Fe)
3
C carbide;
For medium C and medium Cr, the primary phase is ;
For medium C and high Cr, the primary phase is K
1
, (Cr,
Fe)
23
C
6
carbide.
(a) Honeycomb eutectic (ledeburite) (b) Plate-like cementite eutectic
Fig. 5-29: Comparison of honeycomb eutectic and plate-like cementite eutectic
[19]
Warren
[28]
re-measured the liquidus surface projection for the
meta-stable Fe-Cr-C system, the comparison of the two phase
diagrams is shown in Fig. 5-31; various data in the diagrams
are listed in Table 5-8. At critical point u
1
, the difference of
Fig. 5-30: Liquidus surface projection of Fe-Cr-C alloy
[27]
451
November 2011
Serial Report
temperature in the two diagrams
is 3, but the difference of C
and Cr content is larger; at u
2
the
temperature difference is 1, C
and Cr contents also have certain
differences.
According to the position of
line u
1
u
2
e
1
(eutectic composition)
in Fig. 5-31, the relationship of
Cr and C contents to the locations
of hypoeutectic, eutectic and
hypereutectic regions is illustrated
i n Fi g. 5-32. Di fferent f rom
binary Fe-C alloy, for ternary
Fe-C-Cr syst em, t he car bon
saturation is not only dependent
on C, but also on Cr.
1970
[27]
1985
[28]
Fig. 5-31: Comparison of the two
meta-stable Fe-Cr-C system
liquidus surfaces
Table 5-8: Comparison of parameters in the liquidus surfaces of two meta-stable Fe-Cr-C systems
[28]
Critical point Reaction Literature
Parameter
Temp. () w (C) (%) w (Cr)(%)
u
1
L + -Fe = -Fe + M
7
C
3
[27] 1,292 2.3 3.5
[28] 1,289 2.81 31.7
u
2
L + M
7
C
3
= -Fe + M
3
C
[27] 1,184 4.1 5
[28] 1,183 4.22 9.73
Fig.5-32: Relationship of Cr and C contents
to the locations of hypoeutectic,
eutectic and hypereutectic regions
in high Cr white iron
The higher the C and Cr, the higher the C saturation degree.
The solidifcation structure of hypoeutectic high Cr white iron (the
lower left in Fig. 5-32) is austenite dendrites + eutectic structure.
The farer away from eutectic line, the more the primary austenite
dendrites and the less the eutectic structure are remained between
austenite dendrites. The eutectic structure of high Cr eutectic
white iron consists of (M
7
C
3
+ austenite) eutectic cells, in which
the carbides display a two-dimensional rose fower pattern. In this
microstructure, neither austenite, nor carbide is the predominant
structure. The structure in hypereutectic high Cr white iron
consists of primary M
7
C
3
(rod-like) + eutectic structure. The
solidifcation structures of hypoeutectic, eutectic and hypereutectic
high Cr irons are illustrated in Fig. 5-33.
(b) Influence of C, Cr, Si, Ni and Mo on solidification
behaviours: The differential thermal analysis (DTA) curve of an
alloy can refect the precipitation of phases during solidifcation.
Figure 5-34 shows a DTA curve of a Fe-Cr-C alloy.
When w(C)3.18%, the primary dendrites precipitate first;
while, for a high Cr white iron of w(C)3.5%, the primary phase
M
7
C
3
precipitates first. If Cr content is reduced to 7%, a third
exothermal peak (C piont) on the DTA curve will appear for the
iron with high C content, see Fig. 5-34(b); a reaction similar to
peritectoid reaction occurs during solidification. The C and Cr
contents have important infuence on the solidifcation behaviours
of high Cr white iron; the influence of C content on liquid
phase temperature (T
L
), eutectic starting temperature (T
EN
) and
solidifcation ending temperature of eutectic (T
ES
) is shown in Fig.
5-35. With increasing carbon content, T
L
, T
EN
and T
ES
decease; for
a constant carbon content, the higher the Cr, the higher the T
EN
and
T
ES
, but T
L
does not change much.
Si, Ni and Mo all decrease T
L
, T
EN
and T
ES
; among them, Si has
the strongest infuence on these critical temperatures. It seems that
Ni has no effect on T
L
and T
EN
. Based on the DTA measurements,
it is obtained following equations of T
L
, T
EN
and T
ES
with C, Cr, Si,
Ni and Mo:
T
L
=1594.5w(C)92.6%w(Cr)0.54%w(Si)29%w(Ni)1.53%
w(Mo)3.4%
T
EN
= 1225. 8w( C) 22. 2%+w( Cr ) 6. 1%w( Si ) 15. 3%
w(Ni)1.35%w(Mo)4.1%
T
S
= 1203w(C)28.8%+w(Cr)6.3%w(Si)10.4%w(Ni)1.4%w
(Mo)17.6%
According to recent research results
[29]
, the effect of Mo on T
L
is larger than those above equations; when the mass fraction of Mo
is increased from 0% to 2.3%, T
L
is decreased from 1,246.8 to
1,231.3.
(2) Precipitation of eutectic carbides
The contents of C and Cr have important effect on the amount of
eutectic carbides; the infuential relationship is shown in Fig. 5-36.
For constant C and Cr, with increasing the content of Si and Mo,
the amount of eutectic carbides is increased slightly. Ni content
1-Hyper eutectic structure (primary carbide + eutectic)
2-Eutectic
3-Hypoeutectic structure (primary austenite + eutectic)
452
CHINA FOUNDRY
Vol.8 No.4
has no infuence on the eutectic carbide amount.
Depending on the contents of C, Cr and other alloying elements,
the carbides in eutectic structure of high Cr iron can be M
3
C,
M
7
C
3
, or duplex M
7
C
3
+ M
3
C, even multiple phase carbides M
7
C
3
+ M
3
C + M
2
C, as illustrated in Fig. 5-37. The type, morphology
and amount of eutectic carbides have important effect on the wear
resistance and mechanical properties, especially the toughness.
(b) Eutectic (c) Hypereutectic
Fig. 5-33: Solidifcation structure of hypoeutectic, eutectic and hypereutectic high Cr irons
(a) w(Cr) = 20% (b) w(Cr) = 7%
Fig. 5-34: DTA curves of Fe-Cr-C alloys
[15]
The types of eutectic carbides are classifed by the amount of C
and Cr. When w(Cr) = 7%, w(C)2% or w(Cr) = 10%, w(C)
3%, eutectic carbides are type M
7
C
3
and type M
3
C; for the alloy
with w(Cr) =10%, 15%, 20% and w(C)3% (commercial alloy
composition), all the eutectic carbides are M
7
C
3
type; when the
alloy has w(Mo)1%, there exists Mo
2
C eutectic.
(a) Hypoeutectic
Note: the fgure in the table is mass %
Fig. 5-35: Infuence of C and Cr
contents on temperatures
during solidifcation process
of Cr white iron
[15]
453
November 2011
Serial Report
Eutectic M
7
C
3
and primary M
7
C
3
have different growth
behaviour. Under undercooling condition, eutectic M
7
C
3
has twin
defects and is easy to branch and quickly grows to curve-shaped
plate. When undercooling is higher, M
7
C
3
grows to fne rod fbers
with irregular arrangement and the cross section shows small
blocks of multi-grain structure
[30,31]
; each rod is not independent but
grows along [1011] direction connecting one another/each other.
Figure 5-38 shows the thermal analysis curves of hypoeutectic and
hypereutectic high Cr irons. It is seen from the curves in Fig. 5-38
that for hypereutectic high Cr iron, the recalescence for forming
eutectic M
7
C
3
is far more signifcant than that for forming primary
M
7
C
3
, indicating that eutectic M
7
C
3
has faster growth rate than
primary M
7
C
3
.
Note: the fgure in the table is mass %
Fig. 5-36: Effect of C and Cr on the volume
fraction of eutectic carbides
[29]
Note: the fgure in the table is mass %
Fig. 5-37: Types of eutectic carbides in high
Cr white iron
[15]
1 Hypoeutectic, 2Hypereutectic
Fig. 5-38: Thermal analysis curves of hypoeutectic
and hypereutectic high Cr irons
[30]
1 Hypoeutectic, 2Hypereutectic
Eutectic solidifcation velocity, v = 5-20 cm/min
K = diameter of large grain carbides/diameter of small grain carbides
Fig.5-39: Infuence of Cr content on non-uniformity of
eutectic carbides
[35]
The shape of eutectic carbides is closely related to chemical
composition; according to different compositions, following
shaped carbides can be formed: rod, plate, coral or spheroid.
(a) Carbon saturation: For a high Cr iron with eutectic or near
to eutectic composition, the eutectic M
7
C
3
is rod shaped and has
finest size
[9,32]
. The carbides in hypereutectic high Cr iron are
mainly fake plate shape and grow surrounding primary carbides.
The eutectic carbides in hypoeutectic high Cr iron exist in the
regions between primary austenite dendrites and solidify last; the
formed carbides distributed discontinuously and almost all are
flake plate shape. Laird considered
[33]
that the two morphology
carbides can exist in the white irons with three carbon saturations;
the only difference is the ratio of the two types of carbides. The
experiments by Durman confirmed that with increasing carbon
from w(C) = 0.78% to w(C) = 3.40%, at beginning, eutectic
carbides distribute between austenite grain boundaries as thin
film; then following by forming a discontinuous network, and
developed to a continuous network surrounding dendrites. In the
end a eutectic cell forms with carbides growing radially from
the nucleus; at this time, eutectic carbides show rod shape with
hexagonal cross section
[34]
.
(b) Chromium: No matter for hypoeutectic, eutectic or
hypereutectic high Cr iron, when w(Cr) = 10%, the two types
carbides, M
3
C and M
7
C
3
are observed and they are fake plate and
rod like. And, the faster the cooling rate, the more the M
3
C. When
the mass fraction of Cr exceeds 10%, all the carbides become M
7
C.
When w(Cr) 20%, the amount of plate flake eutectic carbides
are gradually decreased; when Cr is increased to w(Cr) = 30% and
w(Cr) = 40%, the carbides almost show rod like with blocky grain
cross section. The infuence of Cr content on the non-uniformity
of eutectic carbide particles is shown in Fig. 5-39. It is seen that
the white iron with w(Cr) = 30% has the most uniform and fnest
eutectic carbide particles.
454
CHINA FOUNDRY
Vol.8 No.4
(c) Vanadium: V can refine primary austenite and separate
the liquid between dendrites into finer spaces, thus narrow the
spaces where carbides grow. In addition, the C-N compounds of
vanadium may become nuclei, thus accelerate the refnement of
vanadium on carbides.
(d) Ni ckel : Ni ckel can di ssol ve i nt o aust eni t e wi t hout
limit, coarsen primary austenite, supply spaces for carbides
crystallisation, and thus make them coarser.
(e) Molybdenum: In high Cr iron when w(Mo)1%, it is
easy to form Mo
2
C, which comprises eutectic ( + Mo
2
C ) with
austenite, and exists in between the coarse chromium carbides.
Figure 5-40 shows that for a white iron with w(Mo) = 4.74%, there
exists a recalescence around 1,150, which shows that eutectic (
+ Mo
2
C) forms. When w(Mo) = 0.96%, no new phase forms
[15]
.
(3) Formation of eutectic grains (cells)
In high Cr iron, following primary phase (austenite or carbide)
precipitates, a ternary equilibrium transformation happens/occurs:
L-F e + M
7
C
3
The products of transformation are eutectic grains consisting of
eutectic austenite (-Fe) and eutectic carbide, that is, eutectic cells.
The forming mechanism of eutectic cells in high Cr white iron can
be described as follows
[9,35,36]
:
The eutectic cells in hypoeutectic high Cr iron are precipitated in
the gaps of austenite dendrites, and their morphology is illustrated
in Fig. 5-41. In the centre region of each eutectic cell there are
fne carbide fbers with fne grain shaped cross section; the fbers
extend from the centre radially as curved flake plates (cross
section shows long string/strip shape). When extending to the
edge of eutectic cells, the released latent heat is increased, leading
to coarse carbides. Within the eutectic cell, eutectic austenite is
full between the intervals of carbides. The morphology is similar
to that of type B graphite eutectic cell in grey iron; however, the
M
7
C
3
carbide in the eutectic cell grows slow radially but fast
longitudinally along [0001] direction in hexagonal crystal system,
forming bunch/sheaf of plates or rods of carbides
[37]
. In the end,
the long rod or long plate type of carbides together with eutectic
austenite form columnar shaped eutectic cells with spherical crown
which are non-uniformly mounted/inlaid/distributed in the pre-
eutectic austenite matrix. The structure pattern of the eutectic cells
is illustrated in Fig. 5-42.
(b) Fine eutectic cells
Fig. 5-41: Morphology of eutectic cells (M
7
C
3
+austenite)
of hypoeutectic high Cr white iron
Fig. 5-42: Structure pattern of the eutectic cell of
hypoeutectic high Cr white iron
[3]
Note: the fgure in the table is mass %
Fig. 5-40: Infuence of Mo on the DTA curve of high
Cr iron with w (Cr) = 20%
(a) Coarse eutectic cells
The eutectic cells in eutectic, high-Cr white iron are formed
randomly. This is because under equilibrium conditions, there
are no pre-precipitated austenite dendrites to act as a substrate for
eutectic cell precipitation. However, the structure and morphology
of eutectic cells (see Fig. 5-43) are similar to that of hypoeutectic
high-Cr white iron.
455
November 2011
Serial Report
The outline of the eutectic cell in hypereutectic high-Cr white
iron is similar to that of eutectic and hypoeutectic high-Cr white
irons; the difference is that in the centre of the eutectic cells,
hexagonal shaped, primary carbide is often observed, see Fig. 5-44.
Eutectic M
7
C
3
grows around primary carbides and there exists
a certain connection between them. However, in some sections,
eutectic carbide has no relationship with primary M
7
C
3
[9]
.
Fig. 5-43: Structure and morphology of eutectic cells
in eutectic high-Cr white iron
Fig. 5-44: Structure and morphology of eutectic cells in a
hypereutectic high-Cr white iron
The growth relationship between primary and eutectic carbides in
the eutectic cells, in the high-Cr white iron is different from that in
the normal white iron. In the eutectic cells in the normal white iron,
cementite (Fe
3
C) is the leading phase, so Fe
3
C grows continuously
and is interconnected within the eutectic cell. For the high-Cr white
iron, it is considered that austenite precipitates frst and then M
7
C
3
is formed in the space between austenite dendrites, where C and
Cr are enriched. Since austenite precipitates before carbide, an
austenite bridge is easily formed at the tip of carbides, which results
in eutectic carbides showing a discontinuous distribution. Powell
confirmed
[30]
that under certain undercooling condition, eutectic
M
7
C
3
easily forms twins, which cause carbide crystals to have
angled grooves (cracks); this promotes eutectic carbides to branch
in a fibrous manner and accelerates austenite nucleation. Powell
also proposed that under the premise of twins existing, eutectic
M
7
C
3
, as a facet phase, can act as nuclei for eutectic growth (M
7
C
3
-
austenite).
The size of eutectic cells directly refects the fneness of eutectic
carbides and infuences the size of carbide grains in the boundary
area of eutectic cells. When the diameter of eutectic cells
increases, the released latent heat of crystallisation is increased;
resulting in coarsening of carbides in the boundary area and this
infuences toughness and wear resistance of a high-Cr white iron.
The main parameters (see Fig. 5-45) of describing eutectic cell
characteristics include: the diameter of eutectic cells, the spacing
distance of the boundary areas where eutectic carbides exist and
the spacing distance of the area where carbides occupy the centre
of a eutectic cell. Factors influencing the diameter of eutectic
cells include solidification velocity, Cr content, other alloying
elements and the modifcation agent; among these, Cr content has
the closest relationship with the diameter
[35]
. The lower the Cr
content, the larger the diameter of eutectic cells and conversely,
with increasing Cr content, the diameter of eutectic cells becomes
smaller. When w(Cr) = 30%, the diameter of eutectic cells is the
smallest and the eutectic structure is the finest. When w(Cr) >
30%, the eutectic cell size becomes coarser again, as illustrated
in Fig. 5-46. It can be seen from Fig. 5-46 that with increasing
solidifcation velocity, the effect of Cr content on the diameter of
eutectic cells is gradually weakened.
A-A Diameter of eutectic cells;
B-B Spacing distance of the cell boundary areas where eutectic
carbides exist;
C-C Spacing distance of the area where carbides occupy the centre of
eutectic cells.
Fig. 5-45: Characteristic parameters of eutectic cell size
(transverse section)
[35]
The influencing rule of Cr content on eutectic cell size
corresponds to its infuence on the eutectic solidifcation interval
[36]
. Figure 5-47 shows the measured solidification interval for
w(Cr) 6%-50%; when w(Cr) = 15%, the solidifcation interval
is the largest, i.e. 65, while w(Cr) = 30%, the solidification
interval is the smallest at only 21. For an alloy with a large
eutectic solidifcation interval, the growth time is long and thus the
fnal size of eutectic cells is large. For the same reason, eutectic
456
CHINA FOUNDRY
Vol.8 No.4
solidifcation interval infuences B-B, but has no effect on C-C.
This is because the central area of eutectic cells precipitates at
the earliest stage and thus has no relationship with the eutectic
solidifcation interval.
Molybdenum increases the diameter of eutectic cells. Tenorio
found from experiments
[29]
that for an iron of w(Cr) = 15%, when
the mass fraction of Mo increases from 0 to 2.3%, the diameter of
eutectic cells increases from 38 m to 46 m.
5.5.4 Eutectic crystallisation of a W system white iron
Regardless of whether it is a hypoeutectic or a hypereutectic
iron, in a W system white iron, for most cases, when entering a
eutectic reaction, M
6
C is precipitated frst because eutectic liquid
contains more W atoms, thus creating better kinetic conditions for
M
6
C to form. In addition, below the eutectic temperature the free
energy of formation for M
6
C is far below that of austenite, which
causes M
6
C to be more stable. If primary M
6
C exists, it is easier to
(a) Solidifcation velocity 0.5 cm/min (b) Solidifcation velocity 1 cm/min (c) Solidifcation velocity 2 cm/min.
hypoeutectic eutectic hypereutectic
Fig. 5-46: Infuence of Cr content on the diameter of eutectic cells
[35]
-, -K
2
eutectic start -, -K
2
eutectic completion --K
c
eutectic start --K
c
eutectic completion -, -K
2
[27]
Fig. 5-47: Infuence of Cr content on eutectic
solidifcation interval
[36]
Fig. 5-48: Schematic diagram of M
6
C austenite
fshbone-like binary eutectic growth
[14]
For a hypoeutectic W system white iron, with lower W/C, M
3
C
type carbides appear in the divorced form in the eutectic structure
and are distributed between the austenite dendrites, thus showing
a net-work style. If W/C > 6, M
23
C
6
type carbide is formed, which
exists as austenite + M
6
C + M
23
C
6
ternary eutectic and grows in a
divorced manner.
nucleate M
6
C again using M
6
C as the substrate. After precipitation
of M
6
C, the liquid in front of the solidifcation interface is depleted
in W and C, but enriched in Fe, which creates conditions for
austenite precipitation. Due to crystal lattice correspondence,
austenite is easy to nucleate on the (111) face of M
6
C, which
accelerates M
6
C branching and growth along the <100> direction.
When eutectic crystallises, austenite and M
6
C show an alternative
and co-operative growth mode, but still maintain the crystal
characteristics of M
6
C type carbide structure and grow to a
fishbone-like eutectic; the growth relationship is shown in Fig
5-48.
Secondary axis
Primary axis
457
November 2011
Serial Report
In W-Cr system white irons with different Cr content, two types
of ternary eutectic can exist
[38]
; for a W system white iron of w(Cr)
< 7%, the ternary eutectic is austenite + M
3
C + M
6
C; when w(Cr)
> 7%, M
7
C
3
is formed and the ternary eutectic becomes austenite
+ M
7
C
3
+ M
6
C.
5.5.5 Modifcation of the eutectic structure of white iron
For improving the toughness and service life of white iron, in
addition to heat treatment to change the matrix structure and
morphology of carbides, the alternative method is to control the
solidifcation process, to modify the eutectic structure.
The measures to improve eutectic structure include:
Refne eutectic cells
Disconnect eutectic carbides
Change the morphology of carbides (Platerodspherical
shape)
The purpose of refining eutectic cells is to refine eutectic
carbides and thus improve the service performance of white iron.
For an as-cast white iron for making malleable iron, refining
the eutectic cells is to shorten the graphitisation annealing
time because fine eutectic carbides significantly increase the
interfaces between carbides and austenite and thus accelerate the
decomposition process of cementite. The measures of refining
eutectic cells is to increase the nuclei number of eutectic cells;
therefore, increasing the cooling rate during solidification,
adjusting chemical composition, changing the nucleus status and
adding heterogeneous nuclei (inoculation) are effective measures
of refning eutectic cells.
Breaking the continuity of eutectic carbides is an important
measure to improve the toughness of white iron. In normal white
iron, the cementite in ledeburite eutectic cells is connected each
other and formed net-work between primary austenite dendrites,
seriously reducing the toughness of the white iron.
Changing carbide morphology in the white iron is an effective
measure to improve the toughness and strength because like the
coarse flake graphite in a grey iron, the plate carbides are very
brittle and have no strength; serious stress concentration occurs
on the tip of carbide plates, and crack is easy to propagate quickly
along the sharp tip.
The process measures for improving the eutectic structure of
white iron are:
(a) Adjust chemical composition
Increasing certain alloying elements by a large amount can
change the carbide type, leading to a change in morphology. For
a Cr system white iron, the carbide type has a close relationship
with Cr content. When w(Cr) 10% and w(Cr)/w(C)4, eutectic
carbides change from plate-like Fe(Cr)
3
C to rod-like Cr
7
C
3
.
In a low Cr white iron, vanadium can change the morphology of
carbides. When w(V) = 4%, carbides are disconnected signifcantly;
when w(V)=6%, spherical carbides begin to form and when
w(V)=8%, most of the carbides are spherical in shape
[39-41]
.
Tungsten has a similar effect on carbides as chromium and can
change the morphology and distribution of carbides. When w(W)
6%, the carbides are M
3
C type, but maintain a net-work or
disconnected net-work. When w(W) = 13%-15%, the carbides
show a disconnected and isolated distribution, and are mainly
M
6
C type
[42]
; when w(W) is around 20%, the carbides are
M
6
C type and show compacted and isolated blocks (rhombus,
polygon, rod, etc.)
[13, 14]
.
In normal white iron, the eutectic structure is ledeburite with
a honeycomb structure. When w(Si)<0.5% and pronounced
undercooling occurs, the honeycomb carbide network becomes
gradually disconnected
[23]
. However, the effect of silicon on
the structure of high chromium white iron is the opposite;
appropriately increased silicon can increase the degree of
disconnection and signifcantly improve fracture toughness. This is
because when the mass fraction of silicon increases from 0.4% to
1.4%, the crystallisation orientation of eutectic carbide is changed
and a scattered structure occurs, thus disconnecting the network.
Practical experience has confrmed that for white iron of w(Cr)
= 11%-13.5%, when w(Si) is increased to within the range 1.0%-
2.2%, the impact fatigue spalling resistance and impact abrasive
wear resistance are better than that of high Cr white iron of w(Cr)
= 15%
[44, 45]
.
The morphology of carbides in a low-alloyed white iron is
influenced by carbon content; controlling the carbon content
to a low level of w(C) = 2.2%-2.6% is benefcial for carbide
spheroidisation after treatment by a compound rare-earth
modifer
[46]
.
(b) Increase cooling rate
With increased cooling rate, eutectic cells become smaller and
carbides are refned; for example, extremely fne rod-like M
7
C
3
can
be obtained when using a metal mould for casting or at the surface
of a weld deposit
[30]
. However, solidifcation rate cannot change
the carbide types, thus it is diffcult to change the morphology of
the carbides.
(c) Inoculation
According to the type of alloy and application, the purposes of
inoculation of white iron include improving toughness and wear
resistance, and decreasing annealing temperature and/or shortening
the annealing time for malleable iron.
Unlike the inoculation of grey iron, in white iron, carbide
refinement is achieved by refinement of primary austenite and
making the inter-dendritic area smaller. The inoculant elements
used for white iron are V, Ti, B, RE and Al. Among these, the
formed inclusions of rare earth with S and O in the liquid iron
can act as substrates for primary austenite nucleation, resulting
in increased nuclei and refined austenite dendrites. Ti and C
form excellent heterogeneous nuclei for austenite, increasing the
nucleation rate of austenite. Both V and B carbides are favourable
for austenite nucleation.
(d) Modifcation
This is a treatment, achieved by the addition of a small amount
of substance into a liquid iron, to modify the morphology of
carbides.
Similar to grey iron, the eutectic crystallisation in white iron also
belongs to non-facet-facet (metallic-non-metallic) eutectic. The
high carbon phases in the two irons have a similar growth mode:
graphite and cementite both have a lamellar structure, and have
458
CHINA FOUNDRY
Vol.8 No.4
strong anisotropy and preferred growth orientation. In addition,
during the growth of eutectics, both graphite and cementite are in
front of austenite, thus leading the progress of eutectic. Therefore,
some of the modifcation mechanisms and processes used for grey
iron can be useful for white iron. For example, the mechanism, in
which Mg and rare earth Ce increase undercooling of grey iron and
change graphite from fake to spheroidal, also exists in white iron;
a rare earth containing modifer can also change the morphology
of M
3
C carbides. Because, for white iron, rare earth metals are
also surface active elements which are enriched in the liquid in
front of a solidifcation interface, and these increase undercooling
and enhance austenite growth velocity. When the leading phase
changes to austenite, it is possible for austenite to bridge at the
tip of carbides; the austenite dendrites connect with each other,
disconnect the carbide and change carbide from plate to plate-
strip or rod shaped. Besides, preferred absorption of rare elements
on carbide crystals inhibits the growth of M
3
C crystal along the
a-direction.
However, in an M
3
C crystal, atoms are combined by a strong
Fe-C covalent bond within each atom layer, but connected by
a weak Fe-Fe metallic bond between layers; the anisotropy of
interatomic bonds in carbide is far stronger than that in graphite.
This results in the growth velocity in a-direction being much
faster than that in the c-direction, meaning that the above rare-
earth elements cannot play their modification role easily. This
is the reason why the modification of normal white iron cannot
be as successful as in grey iron. Nevertheless, changing carbide
morphology through modification to improve strength and
toughness is very signifcant and research work on this aspect has
been persistently performed by many scholars
[47-53]
.
The eutectic carbides in high Cr white iron show a disconnected,
curved-plate shape (or fine fibrous rod shape under high
undercooling) and toughness is signifcantly improved compared
with the continuous plate M
3
C in normal white iron. However,
for M
7
C
3
, because of the inherent preferred growth characteristics
along the [0001] orientation, it is difficult to change the crystal
morphology characteristics in which the length is larger than
radial size, thus its toughness is still low. For this reason,
the spheroidisation of M
7
C carbide has been focused on by
researchers. Rare earth metals have a certain inhibiting effect on
the growth of M
7
C
[49]
. For example, the addition of a small amount
of Al and rare earth element(s) can make carbides in white iron
with w(Cr) = 4%, become isolated, and when w(Al)8%, carbides
can become fully spheroidised
[41]
. The treatment of high Cr white
iron using a modifer containing K and Na, can change the form of
M
7
C
3
to dispersed fne particles, which form irregular spheroids
[53,
54]
. This is because as surface active elements, alkali metals K and
Na are absorbed onto the interface between carbide and austenite,
and cause constitutional undercooling, which is favourable for
forming a divorced eutectic. In addition, the strong de-oxidation
effect of K and Na purifies the liquid iron, thus carbides grow
under a diffusion restriction condition, therefore decreasing the
growth velocity of eutectic carbides along the preferred [010]
orientation.
5.6 Crystallisation of liquid in the
LTF regions during the last
stage of eutectic solidifcation
of white iron
During the last stage of eutectic crystallisation of white iron, the
eutectic regions grow gradually and contact each other; in the end,
the remaining liquid in the LTF (last to freeze) regions, between
eutectic cells, solidifes.
5.6.1 Crystallisation of LTF regions in normal white iron
The eutectic cells (ledeburite) in normal white iron show a long,
plate-like, block shape and intersect primary austenite dendrites
randomly, thus resulting in a diffculty of identifying the outline
and location of LTF regions. In addition, the austenite in ledeburite
is prone to connect with austenite in the LTF regions, making it
more difficult to identify these regions. Wolf etc. considered
[55]
that not all nucleus locations of the leading phase in ledeburite,
(Fe
3
C), occur preferentially between primary austenite dendrites;
it is possible for ledeburite eutectic cells to nucleate at different
locations and after these eutectic cells grow, LTF regions exist
between primary austenite dendrites, as illustrated in Fig. 5-49.
The observations by the author also confrm this, see Fig. 5-50.
Fig. 5-49: LTF regions form between austenite
dendrites in a normal white iron
[55]
Because the distribution law of elements in LTF regions in a
white iron is different from that in a grey iron, carbide-forming
elements are not enriched in the LTF regions, thus the remaining
liquid does not necessarily have a tendency to form carbides.
5.6.2 Crystallisation of LTF regions in high-alloyed
white iron
Although the eutectic cells in a high-alloyed white iron do
not present a spheroidal shape, they show their own specific
morphology; the eutectic cells in high Cr-Mo system white iron
show a morphology of a cylinder with a spherical crown, while
the eutectic cells in high-W system white iron show a fshbone-
like shape, therefore it is possible to identify the location of LTF
regions from their special morphology.
Different from grey iron, the alloying elements in high-
alloyed white iron, such as Cr, Mn, Mo, W and V, are not frst
concentrated in LTF regions, but exist in various primary or
eutectic carbides. Nevertheless, when forming low melting point
459
November 2011
Serial Report
where W
C
[M] mass fraction of M in carbide (%)
W
-Fe
[M] mass fraction of M in austenite (%)
Similar to the definition K
s
in grey iron, the ratio of M mass
fraction of an element in the centre of a phase to that at the edge of
a phase is named as segregation coeffcient K
s
.
According to their distribution characteristics in carbides
and austenite, alloying elements can be divided into two types:
elements which increase carbon activity and elements which are
prone to form carbides.
The elements that enhance carbon activity, such as Si, Al, Cu,
Ni and Co (known as negative segregation elements in grey iron)
have the same distribution within the structure of white iron as in
grey iron and are concentrated mainly in austenite. These elements
frst dissolve in primary austenite and then distribute in eutectic
austenite.Thus, their content in primary austenite is the highest
and that in eutectic austenite is lower, but the content in austenite
is always higher than the average in the iron. However, the
segregation behaviour of these elements (except Al) in austenite
is different from that presented in grey iron during solidifcation,
showing positive segregation characteristics, i.e. lower in the
centre and higher at the edge of austenite. Because Si content at
the edge is higher than that in the centre of primary austenite,
this causes ferrite to form first at the edge of a dendrite during
annealing of white iron for making malleable iron, see Fig. 5-51.
eutectics, these alloying elements may remain in LTF regions and
precipitate last. For example, for high-Cr white iron with Mo, (when
w(Mo) 2%), low melting point, lamellar eutectics consisting of
Mo
2
C and austenite, (Mo
2
C + ), solidify last in the LTF regions.
5.7 Segregation of white iron
During the crystallisation of white iron, the non-uniform
distribution of elements in various crystallisation phases (that is,
micro segregation) has a stronger infuence on structure formation,
phase transformation and properties after solidifcation, than for a
grey iron. This is because most white irons (except white iron used
for making malleable iron) have various, high content, alloying
elements and the distribution characteristics of these elements can
cause the irons to have complicated structure changes.
5.7.1 Distribution of elements in low-alloyed white iron
The ratio of mass fraction of alloying element M in carbide to that
in austenite is referred to as the distribution coeffcient of element
M: S
C/-Fe
[M]
(a) Longitudinal section
(b) Transverse section
Fig. 5-50: Microstructure of the LTF regions in normal
white iron
S
C
/
-Fe
[M] =
W
C
[M]
W
-Fe
[M]
(b) High magnifcation
Fig. 5-51: Infuence of the positive segregation of Si in
austenite in white iron during the annealing
process (blue ferrite, brown austenite)
(a) Low magnifcation
460
CHINA FOUNDRY
Vol.8 No.4
The content of elements Si, A1, Cu, Ni and Co in cementite is
lower than the average value in the iron; among these, Si and Al
are almost insoluble in cementite. Except for Al, the other four
elements Si, Cu, Ni and Co are positive segregation elements in
cementite.
The elements that increase carbon activity have a low content
in cementite, but are enriched in austenite, thus the distribution
coeffcient of Si, Al, Cu, Ni and Co is S
C/-Fe
[M]1. Among these
S
C/-Fe
[Si] is the smallest and S
C/-Fe
[Co] is the largest.
The elements that have a higher affnity with carbon than with
iron, such as Mn, Cr, W, Mo and V, are distributed mainly in
cementite, and have a lower relative content dissolved in austenite.
Thus S
C/-Fe
[M]1. Bunin found that Cr and V have a negative
segregation in the cementite of ledeburite eutectic cells and the
austenite matrix, so their content is higher in the centre but lower
at the edge
[5]
; while W, Mo and Mn show positive segregation in
both primary cementite and eutectic cementite. Table 5-9 lists the
distribution of elements in white iron eutectic cells.
Table 5-9: Distribution of elements in austenite and cementite in white iron eutectic cells
[5]
(mass%)
Element In iron
In austenite of ledeburite In cementite of ledeburite
Dist. coeff.
S
C/-Fe
[M]
In centre In edge Segr. Coeff K
s
In centre In edge Segr. coeff., K
s
Al 0.62 1.09 0.96 1.1 0.041 0.037 1.1 0.04
Si 0.70 1.06 1.20 0.87 0.02 0.03 0.67 0.02
Cu 0.86 1.26 1.41 0.89 0.12 0.14 0.86 0.09
Ni 0.94 1.31 1.46 0.90 0.33 0.36 0.92 0.25
Co 0.93 1.09 1.16 0.94 0.63 0.66 0.95 0.58
Mn 1.06 0.77 0.94 0.82 1.20 1.37 0.88 1.6
Cr 0.94 0.46 0.30 1.50 1.83 1.22 1.5 3.9
W 0.89 0.38 1.03 0.37 0.72 1.36 0.53 1.9
Mo 0.99 0.36 1.26 0.28 0.62 1.78 0.35 1.7
V 0.82 0.35 0.30 1.2 1.59 1.42 1.1 4.5
Note: 1: The carbon saturation is nearly 1; 2: Cooling rate of the samples is 0.5/s, water quenched after solidifed.
5.7.2 Distribution of elements in high Cr white iron
In addition to large amounts of Cr, elements such as Mo, Ni, V
and W often exist in high Cr white iron. Their distribution rules
are different for different elements.
Chromium: In high Cr white iron, Cr is enriched mainly in
carbide, partially dissolved in austenite, and only a very small
amount of Cr occurs in other carbides and inclusions. The
distribution feature of Cr affects the stability of austenite and at
the same time infuences the type and amount of carbides. Figure
5-52 shows the variation of Cr in different phases
[56]
: with an
increase in Cr content, the Cr content in primary austenite, eutectic
austenite and eutectic carbide, increases correspondingly. Cr is
concentrated mainly in the carbide phases, accounting for 40%-
70% (mass) of total Cr; the amount dissolved in austenite in only
10%-20% (mass), therefore the distribution coeffcient S
C/-Fe
[Cr]
= 4-5. Also, the Cr content is higher in primary austenite than
in eutectic austenite. For a constant Cr content, with increase in
carbon, the amount of carbides is increased, but the Cr content in
eutectic carbides is decreased signifcantly and the Cr content in
eutectic austenite is decreased slightly, see Fig. 5-53.
Vanadium: V is enriched in Cr-eutectic carbides and only
partially dissolved in austenite, S
C/-Fe
[V]10.
Nickel: Ni is dissolved in austenite without limit, so with an
increase in Ni content, the amount dissolved in austenite is also
increased. Ni does not exist in carbide, thus S
C/-FFe
[Ni] = 0.
Molybdenum: Mo segregates to the edge of eutectic cells,
increases the Mo content in LTF regions and significantly
decreases the solidification temperature of the metal in LTF
regions. Thus, carbide size around the edge of eutectic cells
becomes coarse
[29]
. Mo is distributed in austenite, carbides and
high Mo phases. Among these, Mo content is the highest in high-
Mo phases, next in carbides and least in austenite, S
C/-Fe
[Mo] =
1-2.
Tungsten: Similar to Mo, W exists in the above three phases
respectively. However, the W content in austenite and carbide is
low, and is mainly enriched in the high-W phase (M
6
C).
The content of other alloying elements in high-Cr white iron is
low and therefore these have less effect on the distribution of Cr.
The data of distribution examples of V, Ni, Mo and W in high-Cr
white iron are listed in Table 5-10
[56]
; these distributions have a
certain infuence on the microstructure and properties of high-Cr
white iron.
Reference
[1] Su Junyi. Chromium System Wear Resistant White Cast Iron. China
Machine Press, 1990. (in Chinese)
[2] .
. , 1972.
[3] David J R. Cast Irons. ASM Specialty Handbook, ASM International
Materials Park, OH, 1996.
461
November 2011
Serial Report
Fig. 5-52: Infuence of Cr on Cr content in different phases
in a high-Cr iron with w(Cr) = 2.0%
Fig. 5-53: Infuence of C on Cr content in different phases
in a high-Cr iron with w(Cr) = 15%
Table 5-10: Distribution of V, Ni, Mo and W in different phases in high-Cr iron (mass %)
Alloying element Primary austenite Eutectic austenite Eutectic carbide High Mo phase High W phase
V
0.48 0.36 0.27 2.83
0.94 0.45 0.38 5.26
1.52 0.70 0.72 7.72
1.99 1.02 0.91 9.40
Ni
1.06 1.05 0.96 0.00
1.51 1.60 1.56 0.00
2.02 2.11 1.98 0.00
2.49 2.86 2.66 0.00
Mo
0.92 1.12 3.12 3.44 55.5
1.83 2.40 3.86 6.33 61.5
2.93 3.31 6.35 79.0
4.01 5.29 5.00 9.85 79.0
W
1.03 0.61 0.8 0.78 64.0
2.12 0.80 1.07 2.14 56.5
3.08 1.86 2.39 2.34 51.0
4.42 2.73 3.63 4.86 55.6
Note: Other composition of the sample (mass %) C = 2.0%, Si = 1.0% - 1.2%, Mn = 0.7%, Cr = 16%
and Technology Press, 1964. (in Chinese)
[9] Ohide T, Ohira G. Solidifcation of High Chromium Alloyed Cast
Iron. The British Foundryman, 1983(1): 7-14.
[10] Wu H Q, Sasaguri N, Matsubara Y and Hashimoto M. Solidifcation
of Multi-Alloyed White Cast Iron: Type and Morphology of Carbides.
AFS Transactions, 1996, 104: 103-108.
[11] . Conspectus of Metallography. Translated by Zhou
Huijiu and Wang Xiaotong, Beijing: Higher Education Press, 1958. (in
Chinese)
[12] Hillert M and Subba Rao V V. The Solidifcation of Metals. Iron Steel
Inst. Publ., London, 1968.
[13] Wang Yuzhu, Luo Huasheng, Ge Fengde. Study on carbides and
[4] Yu Chuntian, Ogi Keisaku, Yamamoto Kaoru.Effects of Si Content
and Cooling Rate on the Carbide in Medium Chromium Cast Iron.
Foundry, 2001(5):258-262. (in Chinese)
[5] , et al. The Structure of Cast Iron. Translated by Ren
Shanzhi, Ge Shoude, Wang Yunzhao,Beijing: China Machine Press,
1988. (in Chinese)
[6] Chang HiroshiAkechi Kiyoaki, Hanawa Kenzo. Ductile Graphite Cast
Iron. Translated by Harbin University of Technology, Beijing: China
Machine Press, 1977. (in Chinese)
[7] Wu Chengjian, Chen Guoliang, Qiang Wenjiang. Metal Material
Science, Beijing: Metallurgial Industry Press, 2000. (in Chinese)
[8] Xu Zhuyao. Principles of Metallography. Shanghai: Shanghai Science
462
CHINA FOUNDRY
Vol.8 No.4
their crystal process of W alloyed white cast iron. Journal of Harbin
University of Technology, 1979: 91-106. (in Chinese)
[14] Ren Shanzhi, Wang Mengchun, Wang Xubao, Ran Yisha. Study on
as-cast microstructures and carbides in W alloyed white cast iron.
Journal of Harbin University of Technology, 1982(2): 75-94. (in
Chinese)
[15] Ching Ping Tong, Suzuki T and Umeda T. Eutectic Solidifcation of
High Chromium Cast Iron. The physical Metallurgy of Cast Iron IV.
(Edited by G. Ohira, T. kusakawa and E. Niyama ), MRS Pittsburgh,
Pennsylvania, 1989: 403-410.
[16] Heine R W. Spiking Solidifcation and Defects in White Cast Irons.
AFS Transactions, 1966, 74: 734-741.
[17] Dpp R. Solidifcation and graphite formation in white cast iron. The
Metallurgy of Cast Iron (Proceedings of the Second International
Symposium on the Metallurgy of Cast Iron, Geneva, Switzerland,
May 29-31, 1974), Edited by B. Lux, I. Minkaff, F. Mollara, Georgi
Publishing Company, St Saphorin, Switzerland, 1975.
[18] Dpp R, Moll A, Brackelsberg H, et al. Vergleich von weissem
Temperguss aus Elektro- and Kupolofen-Erstarrungsverhalten,
Gefige and Eigenschaften. Giesserei, 1990(2): 40-48.
[19] Rickard J, Hughes I C. Eutectic Structure in White Cast Iron. BCIRA
Journal, 1961(9): 11-25.
[20] Stefanescu D M. Cast Iron in Metals Handbook. 9th ed. ASM
International, Metals Park, OH, 1988, 15: 168-181.
[21] Elliott R. Eutectic Solidifcation Processing. London: Butterworths
Co., 1983.
[22] Elliott R. Cast Iron Technology. London: ButterworthsCo., Ltd.,
1988.
[23] Park J S, Verhoeven J D. Directional Solidification of White Cast
Iron. Metallurgical and Materials Transactions A, 1996, 27A(8):
2328-2337.
[24] Morrogh H. The Solidification of Iron-phosphorus-carbon Alloys.
Iron Steel Inst., 1954 (4): 382-386.
[25] Yao Shufang, Chang Anguo, Zhong Xueyou. Study on microstructure
and properties of medium phosphorus white cast iron. Foundry,
1977(4): 23-25. (in Chinese)
[26] Heine R W, Barton J E. Eutectic Solidifcation of White iron and its
Effects on Malleable Iron Castings. AFS Transactions, 1977, 85:
379-388.
[27] Jackson R S. The Austenite Liquidus Surface and Constructional
Diagram for the Fe-Cr-C Metastable System. J. Iron Steel Inst., 1970,
208: 163-167.
[28] Warren R, Thorpe and Bruno Chicco. The Fe-rich Corner of the
Metastable Fe-Cr-C Liquidus Surface. Metallurgical Transactions A,
1985, 16A(9): 1541-1549.
[29] Tenorio J A S, Albertin E and Espinosa D C R. Effects of Mo
Additions on the Solidifcation of High Chromium Cast Iron. Int. J.
Cast Metals Res., 2000, 13: 99-105.
[30] Powell G L F. Solidifcation of Undercooled Bulk Melts of Fe-Cr-C,
Co-Cr-C and Ag-Ge Alloys of Near-eutectic Composition. Materials
Transactions. JIM, 1990, 31(2): 110-117.
[31] Powell G L F, Carlson R A, Randle V. The Morphology and
Microstructure of M
7
C
3
Carbides in Fe-Cr-C and Fe-Cr-C-Si Alloys
of Near Eutectic Composition. Journal of Materials Science, 1994,
29: 4889-4896.
[32] Dogan O N, Hawk J A and Laird II G. Solidifcation Structure and
Abrasion Resistance of High Chrommium White Irons. Metallurgical
and Materials Transactions A, 1997, 28A(6): 1315-1328.
[33] Larid II G, Rawers J C and Adams A. Fractal Analysis of Carbide
Morphology in High-Cr White Cast Irons. Metallurgical Transactions
A, 1992, 23A(10): 2941-2945.
[34] Durman R W. Fractography of High Chromium Alloys. The British
Foundryman, 1981(2): 45-55.
[35] Matsubara Y, Ogi K, Matsuda K. Eutectic Solidification of High
Chromium Cast IronEutectic Structures and Their Quantitative
Analysis. AFS Transactions, 1981, 89: 183-196.
[36] Ogi K, Matsubara Y, Matsuda K. Eutectic Solidification of High
Chromium Cast Iron Mechanism of Eutectic Growth. AFS
Transactions, 1981, 89: 197-204.
[37] Randle V, Laird G. A Microtexture Study of Eutectic Carbides in
White Cast Irons Using Electron Back-scatter Diffraction. Journal of
Materials Science, 1993, 28: 4245-4249.
[38] Ren Shanzhi et al. Formation and Morphology of M
6
C Carbide in
Tungsten-chromium White Cast Irons. The Physical Metallurgy of
Cast Iron .(Edited by G. Ohira, T. Kusakawa and E. Niyama) MRS
Pittsburgh, Pennsylvania, 1989: 381-386.
[39] Ye Yifu, Shang Yuxia, Cui Shenqiu. Spheroidization of carbides and
their value electronic structures. Science Transaction, 1996, 41(4):
381-382.
[40] Ye Yifu. Effect of V on spheroidization behavior of carbides in low
Cr white cast iron. Modern Cast Iron, 1988 (3): 17-20. (in Chinese)
[41] Ye Yifu. Spheroidization of Carbides in White Cast Iron. Modern
Cast Iron, 1995 (3): 28-32. (in Chinese)
[42] Shenyang Research Institute of Foundry. Carbide Phases Containing
W in W-Alloyed White Cast Iron. Foundry, 1997(5): 14-24. (in
Chinese)
[43] Powell G, Randle V. The Effect of Si on the Relationship between
Orientation and Carbide Morphology in High Chromium White Irons.
Journal of Materials Science, 1997, 32: 561-565.
[44] Li Wei, Xu Xiaohui, Su Junyi. Development of High Chromium-
Silicon Wear-Resistant Cast Iron. Modern Cast Iron, 2000 (3): 6-9. (in
Chinese)
[45] Tang Yulin, Sun Guoxiong, Zhang Zhongqiu. Review on the 64
th
World Foundry Congress. Foundry World Report, 2001(5/6): 6-9. (in
Chinese)
[46] Lu Xifang. Infuence of Carbon Content on Morphology of Carbide of
White Cast Iron. Modern Cast Iron, 2001(2): 56-60. (in Chinese)
[47] Zu Fangqiu, Li Shijun, Zhu Youping. Study on Composite Modifcation
of Low Alloy White Cast Iron. Foundry, 1991(1): 12-16. (in
Chinese)
[48] Han Fusheng, Pan Xuefeng, Zhang Rong, et al. Improving the
Structure and the Properties of Low Chromium White Cast Iron by
Modifcation. Foundry, 1990(1): 1-5. (in Chinese)
[49] Liang Gongying, Su Junyi. Effect of RE elements on growth of
eutectic carbides in white cast iron containing Cr. Journal of Xian
Jiao Tong University, 1991 (2): 121-125. (in Chinese)
[50] Wang Zhaochang. RE Modification of low alloy white cast iron.
Journal of Beijing Iron and Steel Institute, 1986(2): 22-30. (in
Chinese)
[51] Piao Dongxue. Studies on Improving Toughness and Widening
Application of High Chromium White Cast Iron. In: Proceedings
of 1986 Beijing International Foundry Conference, Published by
Foundry Institution of Chinese Mechanical Engineering Society,
1986: 716-730. (in Chinese)
[52] Yang Xiangshou, Song Minghan, Huang Zhongbo. Primary
Experiment on Isolation and Spheroidization Behavior of Carbides in
As-Cast White Cast Iron. Ductile Iron, 1978(4): 16-21. (in Chinese)
[53] Xue Qiang, Yang Hua, Bian Xiufang. Effect of SG Modifier on
Properties and Structure of White Cast Iron. Modern Cast Iron, 2001
(2): 25-27. (in Chinese)
[54] Fu Hanguo, Wu Jianzhong, Fan Dingsheng. Research and Application
of Cast Iron containing kalium and Natrium. Modern Cast Iron,
1998(1): 36-38. (in Chinese)
[55] Wolf G, Flender E and Sahm P R. Solidification Behavior of
Technical Metaltable Near Eutectic Iron-Carbon Alloys. (Edited by H.
Fredriksson and M. Hillert). MRS North Holland Amsterdam, 1984,
34: 241-249.
[56] Chen Jingju. Alloyed High Chromium Cast Iron and Their
Application. Beijing: Metallurgial Industry Press, 1999. (in Chinese)
The end of the book
Vous aimerez peut-être aussi
- The Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelD'EverandThe Working of Steel Annealing, Heat Treating and Hardening of Carbon and Alloy SteelÉvaluation : 5 sur 5 étoiles5/5 (4)
- Heat Treatment of CastingDocument15 pagesHeat Treatment of CastingjmmshahPas encore d'évaluation
- TTT DiagramDocument6 pagesTTT DiagramDeepa PujariPas encore d'évaluation
- Abrasion Resistance of MaterialsDocument212 pagesAbrasion Resistance of MaterialsJosé Ramírez100% (1)
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelD'EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelPas encore d'évaluation
- AAR - M 107 - M 208 2009 Wheels, Carbon SteeLDocument36 pagesAAR - M 107 - M 208 2009 Wheels, Carbon SteeLUlises Quintana Carhuancho100% (1)
- Extraction of Nuclear and Non-Ferrous Metals by Sujay Kumar Dutta, DharmeshDocument247 pagesExtraction of Nuclear and Non-Ferrous Metals by Sujay Kumar Dutta, DharmeshUlises Quintana Carhuancho0% (1)
- Iron-IronCarbide Phase DiagramDocument3 pagesIron-IronCarbide Phase Diagramumangmodi32Pas encore d'évaluation
- The Structures of Alloys of Iron: An Elementary IntroductionD'EverandThe Structures of Alloys of Iron: An Elementary IntroductionPas encore d'évaluation
- Microstructural Analyses of Grain Boundary Carbides.Document9 pagesMicrostructural Analyses of Grain Boundary Carbides.Ulises Quintana CarhuanchoPas encore d'évaluation
- An Overview On Bainite Formation in SteelsDocument10 pagesAn Overview On Bainite Formation in SteelssajadPas encore d'évaluation
- 2020 Ductile Mode Cutting of Brittle MaterialsDocument303 pages2020 Ductile Mode Cutting of Brittle MaterialsSasidhar Reddy MuramPas encore d'évaluation
- Gray Cast Iron Metallurgy and InoculationDocument21 pagesGray Cast Iron Metallurgy and InoculationArjyajyoti Goswami100% (1)
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument79 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgPas encore d'évaluation
- Colour Metallography of Cast IronDocument9 pagesColour Metallography of Cast IronJoaquim Pedroso SilvaPas encore d'évaluation
- BS EN - 13979-1-2003+A1-2009 Railway Applications-Wheelset and Bogies-Monoblock Wheels-Technical Approval-ProcedureDocument52 pagesBS EN - 13979-1-2003+A1-2009 Railway Applications-Wheelset and Bogies-Monoblock Wheels-Technical Approval-ProcedureUlises Quintana CarhuanchoPas encore d'évaluation
- Phase Transformation in Metals: Dr. Aneela WakeelDocument29 pagesPhase Transformation in Metals: Dr. Aneela WakeelmazharPas encore d'évaluation
- Introduction To Refractories For Iron - and Steelmaking by Subir BiswasDocument469 pagesIntroduction To Refractories For Iron - and Steelmaking by Subir BiswasUlises Quintana Carhuancho100% (3)
- Extractive Metallurgy 2: Metallurgical Reaction ProcessesD'EverandExtractive Metallurgy 2: Metallurgical Reaction ProcessesÉvaluation : 5 sur 5 étoiles5/5 (1)
- Cast Iron InoculationDocument12 pagesCast Iron InoculationCaio Fazzioli TavaresPas encore d'évaluation
- Cast Iron SolidificationDocument8 pagesCast Iron SolidificationMostafa OthmanPas encore d'évaluation
- 02.Iron-Phase DiagramDocument21 pages02.Iron-Phase Diagrampopular102001Pas encore d'évaluation
- Heat Treatment: Dr. Santosh S. HosmaniDocument7 pagesHeat Treatment: Dr. Santosh S. Hosmaniprakush01975225403Pas encore d'évaluation
- Ferrous AlloysDocument7 pagesFerrous AlloysKeshav DesaiPas encore d'évaluation
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanPas encore d'évaluation
- Tugas Essay Cast IronDocument11 pagesTugas Essay Cast IronGalih SenopatiPas encore d'évaluation
- Niobium in Cast IronDocument13 pagesNiobium in Cast IronTayyab HussainPas encore d'évaluation
- 03 - Iron - Iron CarbideDocument35 pages03 - Iron - Iron CarbidebotobotoakbarPas encore d'évaluation
- Ledeburite: Solidification of Cast IronsDocument14 pagesLedeburite: Solidification of Cast IronsFauzul ImanPas encore d'évaluation
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Trans FasesDocument6 pagesTrans FasesIveth Carmona GonzalezPas encore d'évaluation
- Trans FasesDocument6 pagesTrans FasesIveth Carmona GonzalezPas encore d'évaluation
- The Effects of Alloying Elements On SteelsDocument36 pagesThe Effects of Alloying Elements On SteelsRahul PandeyPas encore d'évaluation
- First Page PDFDocument1 pageFirst Page PDFPradyut TripathyPas encore d'évaluation
- Austenite Perlite TransformationDocument1 pageAustenite Perlite TransformationkapsarcPas encore d'évaluation
- Lec 7 Fe C DiagramDocument45 pagesLec 7 Fe C DiagramAdnan MehmoodPas encore d'évaluation
- 19.09.2019 09h45 What Is An Inoculant and What Does It DoDocument14 pages19.09.2019 09h45 What Is An Inoculant and What Does It DoVairamuthu SiveshPas encore d'évaluation
- Mech 473 Lectures: Professor Rodney HerringDocument40 pagesMech 473 Lectures: Professor Rodney HerringWalid Ben AmirPas encore d'évaluation
- The Iron-Carbon Phase DiagramDocument16 pagesThe Iron-Carbon Phase DiagramMeena SivasubramanianPas encore d'évaluation
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- Phase DiagramsDocument109 pagesPhase DiagramsDimpy KhatriPas encore d'évaluation
- 1996 Bombay Foundry Congress - Inoculation of Grey and Ductile Iron PDFDocument23 pages1996 Bombay Foundry Congress - Inoculation of Grey and Ductile Iron PDFhabibi1328100% (1)
- Iron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Document33 pagesIron-Carbon Phase Diagram: By: Awad Elaraby ID:052022009Mahmoud RefaatPas encore d'évaluation
- Metallurgy of Carbon SteelDocument5 pagesMetallurgy of Carbon SteelMadhavan SoundararajanPas encore d'évaluation
- Seminar Report On Cast IronDocument48 pagesSeminar Report On Cast IronPulkit bajaj100% (3)
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manPas encore d'évaluation
- Tugas Individu 1 - Radifan Abrar Tahrizi - 1806187732Document6 pagesTugas Individu 1 - Radifan Abrar Tahrizi - 1806187732Radifan AbrarPas encore d'évaluation
- Bramhajeet EutectoidDocument4 pagesBramhajeet EutectoidBasab SahaPas encore d'évaluation
- Welding Cast Irons CottrelDocument32 pagesWelding Cast Irons CottrelArvind KumarPas encore d'évaluation
- Metallury of SteelsDocument10 pagesMetallury of SteelsDalitso MwanzaPas encore d'évaluation
- Bhadeshia 2006Document11 pagesBhadeshia 2006prabuPas encore d'évaluation
- Heat Tretment 3Document8 pagesHeat Tretment 3Qasim SaadPas encore d'évaluation
- Cementite: Cementite (Or Iron Carbide) Is A Compound of Iron and Carbon, More Precisely An IntermediateDocument3 pagesCementite: Cementite (Or Iron Carbide) Is A Compound of Iron and Carbon, More Precisely An IntermediateVysakh VasudevanPas encore d'évaluation
- The Effects of Alloying Elements On Steels 1Document36 pagesThe Effects of Alloying Elements On Steels 1Common ManPas encore d'évaluation
- The Mechanism of Acicular Ferrite in Weld DepositsDocument12 pagesThe Mechanism of Acicular Ferrite in Weld DepositsPedro CunhaPas encore d'évaluation
- 2 Iron-Carbon Alloy SystemDocument36 pages2 Iron-Carbon Alloy SystemYour EntertainerPas encore d'évaluation
- Iron Iron-Carbide Equilibrium SystemDocument26 pagesIron Iron-Carbide Equilibrium SystemHiral HiraniPas encore d'évaluation
- Cast IronsDocument9 pagesCast IronsSatria Adi NugrohoPas encore d'évaluation
- Engineering Materials 27-29Document40 pagesEngineering Materials 27-29Sanu SouravPas encore d'évaluation
- Marten SiteDocument2 pagesMarten SiteaqhammamPas encore d'évaluation
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument39 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (15)
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongPas encore d'évaluation
- The Metallurgy of Carbon SteelDocument5 pagesThe Metallurgy of Carbon SteeltsoheilPas encore d'évaluation
- Chapter 10 Powders For Porous Powder Metallurgy TechnologyDocument12 pagesChapter 10 Powders For Porous Powder Metallurgy TechnologyUlises Quintana CarhuanchoPas encore d'évaluation
- Chapter 4 NanopowdersDocument22 pagesChapter 4 NanopowdersUlises Quintana CarhuanchoPas encore d'évaluation
- Chapter 7 Carbonyl Method of Metal Powder ProductionDocument9 pagesChapter 7 Carbonyl Method of Metal Powder ProductionUlises Quintana CarhuanchoPas encore d'évaluation
- Chapter 6 Gas-Phase Method of Metal Powder ProductionDocument11 pagesChapter 6 Gas-Phase Method of Metal Powder ProductionUlises Quintana CarhuanchoPas encore d'évaluation
- Chapter 9 Electrochemical Methods of Metal Powder ProductionDocument31 pagesChapter 9 Electrochemical Methods of Metal Powder ProductionUlises Quintana CarhuanchoPas encore d'évaluation
- The ImportanceDocument178 pagesThe ImportanceMsazryPas encore d'évaluation
- The ImportanceDocument178 pagesThe ImportanceMsazryPas encore d'évaluation
- Fluidity of Aluminum in Green Sand MoldsDocument77 pagesFluidity of Aluminum in Green Sand MoldsUlises Quintana CarhuanchoPas encore d'évaluation
- The ImportanceDocument178 pagesThe ImportanceMsazryPas encore d'évaluation
- The Importance of Metallographic Etching For Failure Analysis of MetalsDocument2 pagesThe Importance of Metallographic Etching For Failure Analysis of MetalsUlises Quintana CarhuanchoPas encore d'évaluation
- A Study of Mixed Manufacturing Methods in Sand Casting Using 3D Sand Printing and FDM Pattern-Making Based On Cost and TimeDocument89 pagesA Study of Mixed Manufacturing Methods in Sand Casting Using 3D Sand Printing and FDM Pattern-Making Based On Cost and TimeJose Ramon Borja JimenezPas encore d'évaluation
- E MS-70CDR PDFDocument36 pagesE MS-70CDR PDFTim MapstonePas encore d'évaluation
- Casting TolerancesDocument2 pagesCasting TolerancesAnonymous shUWODvoPas encore d'évaluation
- A Large-Scale Impact Spalling TestDocument13 pagesA Large-Scale Impact Spalling TestUlises Quintana CarhuanchoPas encore d'évaluation
- Wrought Carbon Steel Wheels: Standard Specification ForDocument8 pagesWrought Carbon Steel Wheels: Standard Specification ForJosé Ramón GutierrezPas encore d'évaluation
- RT-119 ASTM G65 Dry Sand Rubber Wheel Aabrasion TesterDocument2 pagesRT-119 ASTM G65 Dry Sand Rubber Wheel Aabrasion TesterUlises Quintana CarhuanchoPas encore d'évaluation
- Exploring Ore Grindability Tests With The Steel Wheel Abrasion Test SWAT MachineDocument84 pagesExploring Ore Grindability Tests With The Steel Wheel Abrasion Test SWAT MachineMr29RicardoPas encore d'évaluation
- C DOCUME 1 - 8 - LOCALS 1 Temp Plugtmp Plugin-C33jthermodDocument61 pagesC DOCUME 1 - 8 - LOCALS 1 Temp Plugtmp Plugin-C33jthermode1mcPas encore d'évaluation
- Grain Size Analysis in Metals and AlloysDocument9 pagesGrain Size Analysis in Metals and AlloysUlises Quintana CarhuanchoPas encore d'évaluation
- Determination of Charpy Transition Temperature of Ferritic Steels Using Miniaturized SpecimensDocument10 pagesDetermination of Charpy Transition Temperature of Ferritic Steels Using Miniaturized Specimensarkan1976Pas encore d'évaluation
- Acticated Atmosfere Case Hardening of SteelsDocument127 pagesActicated Atmosfere Case Hardening of SteelsUlises Quintana CarhuanchoPas encore d'évaluation
- Growth and Removal of Inclusions During Ladle RefiningDocument188 pagesGrowth and Removal of Inclusions During Ladle RefiningakshukPas encore d'évaluation