Académique Documents
Professionnel Documents
Culture Documents
Phase Diagram
Transféré par
Ting TC0 évaluation0% ont trouvé ce document utile (0 vote)
134 vues3 pagesThe document discusses phase diagrams for pure substances and how they can be used to determine the state of a substance under different temperature and pressure conditions. It asks a series of questions about interpreting points on sample phase diagrams and explaining phenomena such as how melting point and boiling point are affected by pressure changes. Specific substances discussed include water and carbon dioxide.
Description originale:
is about the concept of phase diagram.
Titre original
phase diagram
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentThe document discusses phase diagrams for pure substances and how they can be used to determine the state of a substance under different temperature and pressure conditions. It asks a series of questions about interpreting points on sample phase diagrams and explaining phenomena such as how melting point and boiling point are affected by pressure changes. Specific substances discussed include water and carbon dioxide.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
134 vues3 pagesPhase Diagram
Transféré par
Ting TCThe document discusses phase diagrams for pure substances and how they can be used to determine the state of a substance under different temperature and pressure conditions. It asks a series of questions about interpreting points on sample phase diagrams and explaining phenomena such as how melting point and boiling point are affected by pressure changes. Specific substances discussed include water and carbon dioxide.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 3
Ch e mg u i d e q u e s t i o n s
PHASE DIAGRAMS FOR PURE SUBSTANCES
1. Suppose you had the following phase diagram for a simple pure substance:
a) What physical state would the substance be in under the conditions at point 1?
b) Suppose the temperature was then increased on this substance at constant pressure. What state(s)
would the substance be in at each of points 2 to 5?
c) Suppose the constant pressure was 1 atmosphere. What useful information can you get from the
diagram about the simple physical properties of the substance?
d) Suppose at a much lower pressure, the substance was under the conditions at point 6. Describe
what happens to the substance as you increase the temperature from point 6 to point 8.
In a similar phase diagram:
www.chemguide.co.uk
pressure
temperature
.
. T
C
x x x x x
x x x
1 2 3 4 5
6 7 8
pressure
temperature
.
. T
C
x x x
9
10
11
Ch e mg u i d e q u e s t i o n s
e) What would be the state of the substance at point 9?
f) If the temperature was then increased at constant pressure, what state(s) would the substance be
in at points 10, and 11?
g) Name, and explain the significance of, the point labelled T on these diagrams?
2. The next phase diagram looks at the effect of changing pressure at constant temperature.
a) Give the state(s) at each of the points 1 to 3.
b) Give the state(s) at each of the points 4 to 8.
c) Give the state(s) at points 9 and 10.
d) Name, and explain the significance of, the point C.
3. a) Curve 1 can be thought of as showing
the effect of pressure on melting point.
Explain why most solids have a very
steep curve, sloping slightly forwards as
pressure increases.
b) Curve 2 can be thought of as showing
the effect of pressure on boiling point.
Explain why boiling point increases with
pressure.
www.chemguide.co.uk
pressure
temperature
.
. T
C
x
x
x
x
x
x
x
x
x
x
1
2
3
4
5
6
7
8
9
10
pressure
temperature
.
. T
C
curve 1
curve 2
Ch e mg u i d e q u e s t i o n s
4. The next phase diagram is for water:
a) Mark on the diagram the areas corresponding to ice, liquid water, and water vapour.
b) Explain what curve 1 represents, and why it slopes backwards unlike the corresponding curve 1
in the last diagram.
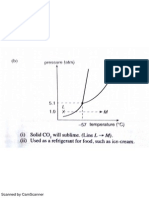
5. This is the phase diagram for carbon dioxide with some values marked on it:
Solid carbon dioxide is commonly known as dry ice. Use the diagram to explain how that term
arises.
www.chemguide.co.uk
pressure
temperature
.
.
curve 1
pressure
temperature
.
. 5.11 atm
1 atm
-78C
Vous aimerez peut-être aussi
- Chemical Equilibrium: Theodore L. Brown H. Eugene Lemay, Jr. and Bruce E. BurstenDocument40 pagesChemical Equilibrium: Theodore L. Brown H. Eugene Lemay, Jr. and Bruce E. BurstenCaryl Ann C. SernadillaPas encore d'évaluation
- MolarityDocument3 pagesMolarityAaron SamudioPas encore d'évaluation
- Mole Concept Notes 2Document43 pagesMole Concept Notes 2Gupta's StudioPas encore d'évaluation
- SolutionsDocument18 pagesSolutionsShantanu Gontia100% (1)
- Optical ActivityDocument4 pagesOptical ActivityJohn Mark Flores Villena100% (1)
- Ch12 Free Energy and ThermodynamicsDocument8 pagesCh12 Free Energy and ThermodynamicsCitrus_EscapePas encore d'évaluation
- Electrochemical CellsDocument63 pagesElectrochemical CellsHoongPas encore d'évaluation
- (Chapter 24) : Gauss's Law Problems and Quiz QuestionsDocument7 pages(Chapter 24) : Gauss's Law Problems and Quiz Questionsmohammed .muddassirPas encore d'évaluation
- Chapter 3 IsomerismDocument9 pagesChapter 3 IsomerismOchem90Pas encore d'évaluation
- 5b Stereochemistry PostDocument41 pages5b Stereochemistry Postapi-3767370100% (1)
- Chem 340 Answers Concepts 2Document4 pagesChem 340 Answers Concepts 2Ayobami Akindele100% (1)
- Organic Chemistry Worksheet 6Document2 pagesOrganic Chemistry Worksheet 6Daniel WalshPas encore d'évaluation
- Colligative PropertiesDocument28 pagesColligative PropertiesSachin S RanePas encore d'évaluation
- Gen Chem Practice Problems Ch10, 18 & Buffers f08Document6 pagesGen Chem Practice Problems Ch10, 18 & Buffers f08Anonymous rFIshYyPas encore d'évaluation
- MolarityDocument18 pagesMolarityapi-370629050% (2)
- Types of TitrationsDocument23 pagesTypes of TitrationsSURESHPas encore d'évaluation
- Practice Test/Thermochemistry/Ap Chemistry: Combustion F F FDocument3 pagesPractice Test/Thermochemistry/Ap Chemistry: Combustion F F FMaria GinzburgPas encore d'évaluation
- (Chap-5) Physical States of MatterDocument12 pages(Chap-5) Physical States of MatterAafan ShahidPas encore d'évaluation
- Models of Molecular Compounds Lab (Ms. Possible)Document5 pagesModels of Molecular Compounds Lab (Ms. Possible)Steven GomescoelloPas encore d'évaluation
- Acid Base EquilibriaDocument12 pagesAcid Base EquilibriaDoroteo Jose StationPas encore d'évaluation
- Simple Mixtures Colligative Properties: Chapter 7: SlideDocument32 pagesSimple Mixtures Colligative Properties: Chapter 7: SlideputriPas encore d'évaluation
- Chem 2105 Topic 11 Titrations in Analytical ChemistryDocument40 pagesChem 2105 Topic 11 Titrations in Analytical ChemistryDanica Rose ZapanzaPas encore d'évaluation
- Lecture 1 - Fundamentals of Organic ChemistryDocument119 pagesLecture 1 - Fundamentals of Organic ChemistrymjmonfortePas encore d'évaluation
- Simple MixturesDocument37 pagesSimple Mixturesumair35Pas encore d'évaluation
- Gener AL Chemi Stry 1: Week 2Document11 pagesGener AL Chemi Stry 1: Week 2Faith AsdfPas encore d'évaluation
- Percent Yield and Limiting Reactants - 2Document24 pagesPercent Yield and Limiting Reactants - 2Heather WrightPas encore d'évaluation
- Acid-Base Equilibria and ApplicationDocument32 pagesAcid-Base Equilibria and Applicationfechem92100% (1)
- Chapter 11 Introduction To Organic Chemistry: HydrocarbonsDocument14 pagesChapter 11 Introduction To Organic Chemistry: HydrocarbonsKatie-Nicole ChantalPas encore d'évaluation
- Ch2 Hydrocarbon AromaticDocument36 pagesCh2 Hydrocarbon AromaticAlimah Azeli100% (2)
- Introduction To Analytical Chemistry: Rosemarie Ann Cuevas, R.CH., M.Sc. InstructorDocument44 pagesIntroduction To Analytical Chemistry: Rosemarie Ann Cuevas, R.CH., M.Sc. InstructorKurt BiduaPas encore d'évaluation
- CHEM 114 PRELIM Module 1 1st Sem 2020 2021revisedDocument24 pagesCHEM 114 PRELIM Module 1 1st Sem 2020 2021revisedJULLIE MAE SAULON100% (1)
- Chapter 15: Thermochemistry Key Notes: Fundamentals Aspects Thermochemistry Is AnDocument11 pagesChapter 15: Thermochemistry Key Notes: Fundamentals Aspects Thermochemistry Is AnSarthakPas encore d'évaluation
- Group1 Report Phase EquilibriumDocument54 pagesGroup1 Report Phase EquilibriumJobertCastillanes100% (1)
- Principles of Chemical EquilibriumDocument17 pagesPrinciples of Chemical EquilibriumkaditasookdeoPas encore d'évaluation
- Kinetics LabDocument12 pagesKinetics LabJessePas encore d'évaluation
- Methods of PurificationDocument10 pagesMethods of PurificationhadysuciptoPas encore d'évaluation
- The Nature of Analytical ChemistryDocument18 pagesThe Nature of Analytical ChemistryAzalia Tugade PanizalesPas encore d'évaluation
- Notes and Questions: Aqa GcseDocument12 pagesNotes and Questions: Aqa Gcseapi-422428700Pas encore d'évaluation
- ThermochemistryDocument17 pagesThermochemistryBrian Smith100% (9)
- Notes - Mixtures and Pure SubstancesDocument32 pagesNotes - Mixtures and Pure Substancesapi-326942798Pas encore d'évaluation
- Measurements of Surface TensionDocument11 pagesMeasurements of Surface TensionHema ParasuramanPas encore d'évaluation
- 1e Aldehyde & KetoneDocument48 pages1e Aldehyde & KetoneJonathan Wyatt100% (1)
- ACH150X Complexation Reactions and Titrations 2020Document40 pagesACH150X Complexation Reactions and Titrations 2020Kgaugelo TraciaPas encore d'évaluation
- Colligative PropertiesDocument61 pagesColligative PropertiesSubhasish Sau100% (1)
- Quantitative Chemical Analysis: Solution DefinitionDocument8 pagesQuantitative Chemical Analysis: Solution Definitionodubade opeyemiPas encore d'évaluation
- 5 CH241 Stereochemistry 8th EdDocument84 pages5 CH241 Stereochemistry 8th EdEfrain AnayaPas encore d'évaluation
- CH01 Analy ChemDocument29 pagesCH01 Analy Chemαγαπημένη του ΧριστούPas encore d'évaluation
- NH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidDocument12 pagesNH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidKhang TrầnPas encore d'évaluation
- Analytical ChemistryDocument50 pagesAnalytical ChemistryNguyễn Trịnh Anh MinhPas encore d'évaluation
- Atoms Molecules and IonsDocument46 pagesAtoms Molecules and Ionschandro57Pas encore d'évaluation
- Ismail Yusuf: Arts, Science and Commerce College, MumbaiDocument43 pagesIsmail Yusuf: Arts, Science and Commerce College, MumbaiBapu ThoratPas encore d'évaluation
- Gen Chem Chapt.1Document45 pagesGen Chem Chapt.1Dave Cercado BugadorPas encore d'évaluation
- 04 EnergeticsDocument14 pages04 EnergeticsafshinPas encore d'évaluation
- Analaytical Chemistry Module 2Document50 pagesAnalaytical Chemistry Module 2May Joy VasquezPas encore d'évaluation
- Nano and The Environment: Potential Risks, Real Uncertainties & Urgent IssuesDocument12 pagesNano and The Environment: Potential Risks, Real Uncertainties & Urgent IssuesGeorgina Catacora V.Pas encore d'évaluation
- Introduction To Organic ChemistryDocument47 pagesIntroduction To Organic ChemistryDevendran MahendhrenPas encore d'évaluation
- Review On Bioremediation of Heavy MetalsDocument6 pagesReview On Bioremediation of Heavy MetalsNicolas Porras Carvajal100% (1)
- Stoichiometry PDFDocument33 pagesStoichiometry PDFEvernim OmpacanPas encore d'évaluation
- CH102 Lab 5 Aldehydes and Ketones PDFDocument10 pagesCH102 Lab 5 Aldehydes and Ketones PDFAnonymous caERsAPas encore d'évaluation
- Vragen Fasediagrammen Zuivere Componenten PDFDocument3 pagesVragen Fasediagrammen Zuivere Componenten PDFbilberPas encore d'évaluation
- TEMPLET ReBeST SEBENAR 2019 PDFDocument1 pageTEMPLET ReBeST SEBENAR 2019 PDFTing TCPas encore d'évaluation
- Kalendar STPM 2018Document2 pagesKalendar STPM 2018Ting TCPas encore d'évaluation
- Answer For Objective QuestionsDocument20 pagesAnswer For Objective QuestionsTing TCPas encore d'évaluation
- Answer, Pre-Exam Practice Chem Sem 3 EssayDocument29 pagesAnswer, Pre-Exam Practice Chem Sem 3 EssayTing TCPas encore d'évaluation
- Analysis Past Year Chemistry SPM Question (2003-2017)Document7 pagesAnalysis Past Year Chemistry SPM Question (2003-2017)Ting TCPas encore d'évaluation
- Periodic TableDocument8 pagesPeriodic TableKhairiyah AbdullahPas encore d'évaluation
- EssayDocument9 pagesEssayTing TCPas encore d'évaluation
- Shape of O3Document2 pagesShape of O3Ting TCPas encore d'évaluation
- Skema Penang Trial SPM 2014Document21 pagesSkema Penang Trial SPM 2014Ting TCPas encore d'évaluation
- Kelantan Kim K1 PDFDocument22 pagesKelantan Kim K1 PDFTing TCPas encore d'évaluation
- New Doc 2017-07-31Document2 pagesNew Doc 2017-07-31Ting TCPas encore d'évaluation
- Important Diagrams LatestDocument5 pagesImportant Diagrams LatestTing TCPas encore d'évaluation
- Skema Penang Trial SPM 2014Document21 pagesSkema Penang Trial SPM 2014Ting TCPas encore d'évaluation
- A4 Sheet Labels TemplateDocument3 pagesA4 Sheet Labels TemplateEk AjnabiPas encore d'évaluation
- Break SheetDocument1 pageBreak SheetTing TCPas encore d'évaluation
- Chapter 2 Chemistry Form 6Document10 pagesChapter 2 Chemistry Form 6Ting TCPas encore d'évaluation
- Answer Scheme Term 2 TrialDocument3 pagesAnswer Scheme Term 2 TrialTing TCPas encore d'évaluation
- Answer Chapter 1Document3 pagesAnswer Chapter 1Ting TCPas encore d'évaluation
- Break SheetDocument1 pageBreak SheetTing TCPas encore d'évaluation
- Answer Scheme Term 2 TrialDocument3 pagesAnswer Scheme Term 2 TrialTing TCPas encore d'évaluation
- New Document ScanDocument6 pagesNew Document ScanTing TCPas encore d'évaluation
- Break ExsheetDocument1 pageBreak ExsheetTing TCPas encore d'évaluation
- SPM Physics Formula List Form 5Document0 pageSPM Physics Formula List Form 5HaziAmiPas encore d'évaluation
- Break SheetDocument1 pageBreak SheetTing TCPas encore d'évaluation
- Exp10 PDFDocument17 pagesExp10 PDFAnurag BajpaiPas encore d'évaluation
- Exp10 PDFDocument17 pagesExp10 PDFAnurag BajpaiPas encore d'évaluation
- Physical Chemistry Lab ManualDocument36 pagesPhysical Chemistry Lab ManualTing TC100% (1)
- Break SheetDocument1 pageBreak SheetTing TCPas encore d'évaluation
- How To Fix Number Formatting in Mail Merge Greenwich - Computer - Help PDFDocument5 pagesHow To Fix Number Formatting in Mail Merge Greenwich - Computer - Help PDFTing TCPas encore d'évaluation
- Electromagnetic Braking SystemDocument16 pagesElectromagnetic Braking SystemGallant Info100% (1)
- Local Buckling Behaviour of Polygonal SectionsDocument13 pagesLocal Buckling Behaviour of Polygonal SectionsMatheus ÍtaloPas encore d'évaluation
- CRSI Manual To Design RC Diaphragms - Part27Document4 pagesCRSI Manual To Design RC Diaphragms - Part27Adam Michael GreenPas encore d'évaluation
- dl300 Shop ManualDocument3 pagesdl300 Shop Manualoscar el carevergaPas encore d'évaluation
- Pipecoater-Iii/900: Internal Pipecoater For Pipe ID 300 MM To 900 MM (12" - 36")Document2 pagesPipecoater-Iii/900: Internal Pipecoater For Pipe ID 300 MM To 900 MM (12" - 36")BepdjPas encore d'évaluation
- Bibby CouplingsDocument25 pagesBibby CouplingsKemoy JohnsonPas encore d'évaluation
- محاضرات داينمك 20082009Document16 pagesمحاضرات داينمك 20082009subhyPas encore d'évaluation
- Scorpio Crde Wiring Manual Rev3 Reduced PDFDocument51 pagesScorpio Crde Wiring Manual Rev3 Reduced PDFhelorcaPas encore d'évaluation
- Allison Transmission 30004000 Series Fault Code List - Download PDF Manual PDFDocument10 pagesAllison Transmission 30004000 Series Fault Code List - Download PDF Manual PDFasdrubal laraPas encore d'évaluation
- Draftsmangrade II PDFDocument3 pagesDraftsmangrade II PDFakhilaPas encore d'évaluation
- Metallurgical Features of NANOHITEN and Application To Warm StampingDocument6 pagesMetallurgical Features of NANOHITEN and Application To Warm Stampingdhafi kecePas encore d'évaluation
- Vigo Elec Wiring DiagramDocument18 pagesVigo Elec Wiring DiagramVinh PhạmPas encore d'évaluation
- General Description: Gr56C-Zid - GQ.CR - 116180/A01 - Portfolio U+Na - Cpro EcblueDocument11 pagesGeneral Description: Gr56C-Zid - GQ.CR - 116180/A01 - Portfolio U+Na - Cpro EcblueviniciusschwabPas encore d'évaluation
- Cosmetics' Quality Control (2012)Document28 pagesCosmetics' Quality Control (2012)Hashim AliPas encore d'évaluation
- Interactive Schematic: This Document Is Best Viewed at A Screen Resolution of 1024 X 768Document18 pagesInteractive Schematic: This Document Is Best Viewed at A Screen Resolution of 1024 X 768Ramiro Pedro Tarazona Hinostroza100% (1)
- HHU 100 ON.1 Steering Units TRAININGDocument13 pagesHHU 100 ON.1 Steering Units TRAININGHuseyin TASKINPas encore d'évaluation
- Supracell Circular Daf: Dissolved Air Flotation ClarifierDocument2 pagesSupracell Circular Daf: Dissolved Air Flotation ClarifierKevin KeanePas encore d'évaluation
- CaracterizacióndeSalpicadurasSMAW Molleda 2007Document5 pagesCaracterizacióndeSalpicadurasSMAW Molleda 2007Tamara Maria Ortiz MendezPas encore d'évaluation
- Fundamentals of Fluid Mechanics Chapter 11 Analysis of Compressible FlowDocument210 pagesFundamentals of Fluid Mechanics Chapter 11 Analysis of Compressible FlowJake OkuyePas encore d'évaluation
- Packing ListDocument28 pagesPacking ListsryPas encore d'évaluation
- SQ LOD200 v01 AllSizesDocument3 pagesSQ LOD200 v01 AllSizesharute gundamPas encore d'évaluation
- 1-Relative Velocity in Special Relativity: Page898 CollegeDocument26 pages1-Relative Velocity in Special Relativity: Page898 CollegeAbdulrhman fPas encore d'évaluation
- CY 6102BG - 36 型柴油机备件目录: Model Cy6102Bg-36 Diesel Engine Spare Parts CatalogueDocument72 pagesCY 6102BG - 36 型柴油机备件目录: Model Cy6102Bg-36 Diesel Engine Spare Parts Cataloguesobirin100% (1)
- Camisas Refrigeracion Flowsleeves Pi-058 GB 2013-06-12Document38 pagesCamisas Refrigeracion Flowsleeves Pi-058 GB 2013-06-12daniel2rialPas encore d'évaluation
- ComparatorsDocument25 pagesComparatorsMayank KhinchiPas encore d'évaluation
- 315B Forest Swing Machine Electrical System: 7RZ284-UPDocument2 pages315B Forest Swing Machine Electrical System: 7RZ284-UPGilvan JuniorPas encore d'évaluation
- FXDQ-NBVE Technical Data PDFDocument13 pagesFXDQ-NBVE Technical Data PDFLuis CarlosPas encore d'évaluation
- Application Note .: Measuring Torsional Operational Deflection Shapes of Rotating ShaftsDocument8 pagesApplication Note .: Measuring Torsional Operational Deflection Shapes of Rotating Shaftsjhon vargasPas encore d'évaluation
- Tolerances For Frame Side Members (RLT) : Accuracy of ShapeDocument8 pagesTolerances For Frame Side Members (RLT) : Accuracy of ShapeAkmal NizametdinovPas encore d'évaluation
- RDSO TrainingDocument20 pagesRDSO Trainingmanish kumarPas encore d'évaluation