Académique Documents
Professionnel Documents
Culture Documents
Correlation of Ideal Gas Enthalpy, Heat Capacity, and Entropy
Transféré par
José Luís Díaz Vargas0 évaluation0% ont trouvé ce document utile (0 vote)
211 vues4 pagesThe ideal gas enthalpy, heat capacity, and entropy of 89 compounds has been represented by a set of three thermodynamically consistent polynomials. The constants for these equations are derived by minimizing the deviations in all three properties simultaneously. The overall error analysis indicates that thermodynamic consistency can be maintained while yielding excellent predictions for the three properties.
Description originale:
Titre original
Correlation of Ideal Gas Enthalpy, Heat Capacity, And Entropy
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentThe ideal gas enthalpy, heat capacity, and entropy of 89 compounds has been represented by a set of three thermodynamically consistent polynomials. The constants for these equations are derived by minimizing the deviations in all three properties simultaneously. The overall error analysis indicates that thermodynamic consistency can be maintained while yielding excellent predictions for the three properties.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
211 vues4 pagesCorrelation of Ideal Gas Enthalpy, Heat Capacity, and Entropy
Transféré par
José Luís Díaz VargasThe ideal gas enthalpy, heat capacity, and entropy of 89 compounds has been represented by a set of three thermodynamically consistent polynomials. The constants for these equations are derived by minimizing the deviations in all three properties simultaneously. The overall error analysis indicates that thermodynamic consistency can be maintained while yielding excellent predictions for the three properties.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 4
Correlation of Ideal Gas Enthalpy,
Heat Capacity, and Entropy
Charles A. Passut and Ronald P. Dannerl
Department of Chemical Engineering, The Pennsylvania State University, University Park, Pa, 16802
The accepted values of the ideal gas enthalpy, heat capacity, and entropy of 89 compounds, mostly hydro-
carbons, have been represented by a set of three thermodynamically consistent polynomials. The constants
for these equations, derived by minimizing the deviations in all three properties simultaneously, are tabu-
lated together with the average and maximum percent deviation for each compound. The overall error
analysis indicates that thermodynamic consistency can be maintained while yielding excellent predictions
for the three properties.
I deal gas properties are frequently required in correlations
of enthalpy, heat capacity, and entropy. In general, these
properties are correlated in terms of their deviations from
ideality and, thus to obtain reliable absolute values, accurate
predictions of the ideal gas values are required.
The American Petroleum I nstit'ute, Research Project 44
publishes ideal gas enthalpy, heat capacity, and entropy
values in tabular form. These values were corrected for the
molecular weight change to the carbon-12 basis. Frequently
it is more convenient t'o have these values represented by
analytical equations for comput,er applications.
Several methods have been used to obtain analytical ex-
pressions. I n the M I "Technical Dat'a Book-Petroleum
Refining" (1971), a set of three independent polynomial
equations for ideal gas enthalpy, heat capacity, and entropy
are given. Xn independent set of constants is provided for
each polynomial and thus one cannot go from one equation
to the other by the standard t'hermodynamic formula-Le.,
AH* =JCp*dT and AS* =J ( Cp * / T) d T. This method pro-
vides greater accuracy in fitting of the individual properties
at the expense of being thermodynamically inconsistent.
I n a recent publication, Thinh et' al. (19i1) reviewed the
published equations for predicting ideal gas heat capacity and
presented a listing of constants for the ideal gas heat capacity
of numerous hydrocarbons and relat'ed compounds in t'he
form:
Cp* =a +bT +cT2 +dT3
(1)
Values for the entropy and ent'halpy can be predicted from
this equat.ion by the appropriate thermodynamic relations.
However, some loss in accuracy for enthalpy and entropy
values is inevitable by t'his procedure, since t'he actual heat
capacit,y behavior is not theoretically constrained to follow
any particular polynomial. The method described below
yields thermodynamically consistent equations with good
accuracy for all three properties.
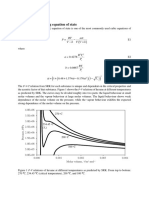
The forms of the equations used were as follows. For the
enthalpy:
H* =A +BT +CT2 +DT3 -+ET4 +FT5
(2)
where 9, B, C, D! E, and F are derived coefficient's, with the
enthalpy in Btu/lb and the temperature in "R.
To whom correspondence should be addressed.
For the heat. capacit'y, ( Cp* =dH*/dT):
Cp* =B +2 CT +3 CT2 +4 ET3 +5 F T 4
( 3)
and for the entropy, [S* =J( Cp*/ T) dT]:
S* =B In - +2 C(T - TB) +"2 D( T2 - TB2) +
T
TB
"3 E( T3 - T B ~ ) +'/4 F(T' - TB') +G'
(4)
where TB is t'he base temperat'ure for the ent'ropy equation
(l "R), and G' is the constant of int'egration. Equation 4 may
be further simplified by combining the constant base tem-
perature terms with the consbant G' t'o give Equation 5 :
S* =B 111T +2 CT +' 1 2 DT2 +
4/ 3ET3 +5/4FT4 +G
( 5)
where t'he constant G now contains the base correction.
The data bases used were 0 Utu/lb at 0"R for the enthalpy
and 0 I3tu/lb "R at 0"R and 1 atm pressure for the entropy-
the same as those used for tmhe XPI Research Project 44 tables.
(I t should be noted that these bases cannot be used for a
system in which a chemical react,ion has occurred.) The en-
thalpy, heat capacity, and entropy data used in these corre-
lations were taken from API Research Project 44 rvith the
exception of SO2, H2S, and XI-1; which were obtained
from AIcBride et al. (1963). The entire temperature range
available from t'he API -RP-44 data was included. The degree
of the polynomial in Equation 2 was chosen by balancing
t'he improved accuracy obtained with additional terms against
the increased complexity of the equations.
Cor r el at i onal Met hod
Since the constants in the equation are linear, a linear
method of solution xas indicated. The three different data
t'ypes (enthalpy, heat capacity, and entropy) were arranged
in one matrix [VI . The seven coefficients t'o the three equa-
t'ions rvere arranged in the coefficient matrix [A]. A tempera-
ture matrix was prepared to sat.isfy Equation 6:
[ T I nx7[ ~15X l =[VI nYJ (6)
The subscripts denote rows and columns, respectively.
To solve for the values in the [ A] matrix, the [TI matrix is
first transformed into a square matrix by multiplying Equa-
tion 6 by the t'ranspose of the [TI matrix,
Ind. Eng. Chem. Process Des. Devel op., Vol. 1 1 , No. 4, 1972 543
0
0
0.1
o.1
t
3
0.1
0
9
5
0
m
a
0
t-
Q,
di
I
Q,
3
0
N
-4
0
I
m
N
N
7
d:
m
Lo
w
M
w
9
I
25
2
- -
h
M
*
G
M
o.1
'3
m
M
0
3
I
0.1
c,
G
0
3
0
'3
m
r-
2
e
- -
I
o1
a
r-
X m
m Lo
0
0
0.1
01
t
0
s
a
2
0
m
r-
0
-4
o.1
m
I
2
0
00
m
0
I
i
r-
CD
0
0
?
3 n
w
0
C
I
c,
G
N
c:
-3
0
d
Lo
m
c,
m
m
0
3
I
i
3
N
m
0
3
N
-+
as
w
2
G
I
m
Lo
-4
M
Lo
3
?
'
Ind. Eng. Chem. Process Des. Develop., Vol. 1 1 , No. 4, 1972
0
0
01
0.1
t
0
e-
0
9
2
0
m
r-
0
8
m
I
2
0
m
m
0
I
-
t .
0
0
0
e-
+
0
s
W
0
9
I
Q,
0 0.1
0
w
0
n
m
m
Q,
m
M
0
-
I
i
ri
hl
m
0
3
01
*
3
2
0
I
M
m
e
s
M
m
0 0 0 0 0
0 3 3 3 0
m0. 1mC. I m
0. 1mmm0. 1
t t t t t
0 0 0 0 c
e- mMmM
o o c 0 0
0 0 0 c 0
M d mc s X
i 0 C C C
0 0 0 0 0
I
mmm- w
I - mmccm
c o 0 0 3
d r i m m d
Dl mmr - m
U3Me-fii0.1
I l l
0 M m* e -
i c o 0 0
0 0 0 0 3
X m X a a
m c 0 0 0
o c 0 0 3
I I
Hi d00
d o c o o
I
o o o o o
I I 1
o o o o o
I l l 1
o o o o o
o o o c c
I I I I I
0
0
0.1
01
t
C
m
0
m
0
3
?
m
w
C
m
01
M
I
9
Y
m
0
0,
C
M
E
2
9
-
+
0
w
w
0
0
1
0
m
3
-
0.1
0
I
5?
m
M
M
C
0
I
01
m
cc
m
d
d
C
r-
m
0.1
m
z
c
I
m
0
co
M
01
M
?
t t t t t t t t t t t t t t t t t t t t t t t t t t t t t t t t t
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 ~ 0 0 c 0 0 0 0 0 0 0 0 0
. . . , , . . . . .
o o o o o e o o 0 c ~ o o o ~ o o o o c : o d ~ ~ ~ - - - 0 0 0 c 2 c ~ 0
I I I I I I I I I I I I I I I I I I I I I I
Ind. Eng. Chem. Process Des. Develop., Vol. 11, No. 4, 1972 545
The result of this operation is:
( 7)
where [SI and [C] are the resultant matrices.
The [A] matrix may be evaluated by inverting the [SI
matri x The matrix [SI is equivalent to a simultaneous least-
squares analysis for the three sets of ideal equations. A proof
of this procedure is given by Zeleznik and Gordon (1961).
The [SI matrix in Equation 8 can be inverted by standard
reduction techniques to find the values in the coefficient ma-
trix [A]. However, this matrix possesses certain special prop-
erties which can simplify the inversion. The [SI matrix is
positive, symmetric, and nonsingular. Therefore, the method
of Cholesky can be used to invert the matrix (Lapidus, 1962).
This method minimizes round-off errors in the inversion.
The Cholesky method will not be described here, but vas
built into the computer program developed to solve for the
ideal gas property coefficients.
I nitial work with the equations showed that forcing the
enthalpy equation through 0 at 0R was an unnecessary and
undesirable restriction even though the base for the values is
0 at 0R. Since none of the data are in the range of 0R and
since no empirical equation for the enthalpy can be ex-
trapolated outside the range of the available data, there is
no apparent loss from the addition of a constant, temper-
ature-independent term, to the enthalpy equation. I n the
same way the heat capacity equation has a constant term
when the enthalpy equation is differentiated. Again the heat
capacity is not predicted to be zero at absolute zero but the
equation for Cp* is not applicable in this range. The constant
term in Equation 4 (G) is the predicted entropy of the ideal
gas at the chosen base temperature of 1R and unit pressure.
This value comes out of the regression analysis, and once
again, since there is no data in this region, the predicted
value has no real significance. The temperature limitations
of the data are shown in Table I.
Resul ts
The constants obtained for the enthalpy, heat capacity, and
entropy equations of the 89 compounds are given in Table I.
The overall average percent error and maximum error ob-
tained in predicting the absolute values of the enthalpy,
heat capacity, and entropy are also given.
A comparison of the results of this work, those of the API
Technical Data Book (1971) and Thinh et al. (1971) are
shown in Table 11. The equations of Thinh have been com-
pared only for the 7 5 hydrocarbons common to both studies.
Furthermore since Thinhs equations have been developed
only for the temperature range of 298-1500K for the com-
parison given in Table 11, the equations of this work were
evaluated in this same temperature range. The values of
enthalpy and entropy for comparison with the correlations
of Thinh et al. had to be calculated as the property change
Table II. Comparison of Results wi t h Previous Work
Av % er r or a
capaci t y Ent hal py Entropy
Heat
Complete temperature range,
89 compounds
This work 0 436 0 064 0 040
XPI TechnicalDataBook 0 877 0 388 0 036
This work 0 249 0 14gb 0 721b
Thinh et al. (1971) 0 194 0 2746 0 721b
a ( l OO/ S ) L: [(predicted value - tabulated value)/tabulated
These values are based on predicted property change
298-1500K, 75 compounds
value].
from 298K.
from 298K since Thinhs equations cannot be used to obtain
absolute values.
The results given in Table I1 show marked improvement
over the independent set of three equations reported in the
API Technical Data Book. I n comparison to the correlations
of Thinh et al. for the 75 common hydrocarbons, there is a
loss in accuracy in the prediction of heat capacity, but a
decided improvement was obtained in the enthalpy predic-
tions. Since the enthalpy differences are, in general, the most
useful quantities, this improvement is significant. Further-
more the equations presented in this work have the advantage
of extending the temperature range. On the other hand, the
newly developed equations have the disadvantage of being
one degree higher in their powers of T compared to the equa-
tions of Thinh et al., but this added complexity is a necessity
when the equations are applied over the larger temperature
range available for many compounds.
In conclusion, Equations 2, 3, and 5, together with the
coefficients in Table I, are recommended for predicting the
ideal gas properties of the compound listed. They represent a
thermodynamically consistent set of equations with minimum
overall predictive errors.
l i t er at ur e Cited
American Petroleum Institute, Technical Data Book-Petro-
leuin Refining, 2nd ed., Chap. 7, Washington, D.C., 1971.
American Petroleum Institute Research Project 44, Selected
Values of Physical and Thermodynamic Properties of Hydro-
carbons and Related Compounds, Thermodynamics Research
Center, Texas -4&M University, College Station, Tex. (loose-
leaf data sheets extant 1972).
Lapidus, L., Digital Computation for Chemical Engineers, pp
NcBride, B. J ., Heimel, S., Ehlers, J . G., Gordon, S., SASA
Thinh, T. P., Duran, J . L., Ramalho, R. S. , Kaliaguine, S. ,
Zeleznik. F. J .. Gordon. S. . NASA Tech. Kote D-767, Washing-
240-57, McGraw-Hill, Kew York, N.Y., 1962.
Report hTo. SP-3001, 1963.
Hydrocarbon Proems., 50 (l ), 98-104 (1971).
ton, D.C , 1961.
-
RECEI VED for review J anuary 27, 1972
ACCEPTED J une 1, 1972
This work was sponsored by the American Petroleum Institute,
Division of Refining.
546 Ind. Eng. Chem. Process Des. Develop., Vol. 11, No. 4, 1972
Vous aimerez peut-être aussi
- 1992 BenderDocument12 pages1992 BenderJohn PACHON MORALESPas encore d'évaluation
- A Comprehensive Comparison of Mixing Rules For Calculation of Phase Equilibria in Complex SystemsDocument8 pagesA Comprehensive Comparison of Mixing Rules For Calculation of Phase Equilibria in Complex Systemsmurdanetap957Pas encore d'évaluation
- Calculation of Vapor-Liquid-Liquid Equilibria For The Fischer-Tropsch Reactor Effluents Using Modified Peng-Robinson Equation of StateDocument31 pagesCalculation of Vapor-Liquid-Liquid Equilibria For The Fischer-Tropsch Reactor Effluents Using Modified Peng-Robinson Equation of StatekenymorenoPas encore d'évaluation
- Acentric Factor EoSDocument10 pagesAcentric Factor EoSdesertflowPas encore d'évaluation
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringD'EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringPas encore d'évaluation
- Thermophysical Properties-Industrial DirectionsDocument15 pagesThermophysical Properties-Industrial DirectionsfabiopdnPas encore d'évaluation
- Mixing RulesDocument15 pagesMixing RulesCremorlabPas encore d'évaluation
- Chemical Engineering Science Volume 1 Issue 4 1952 (Doi 10.1016/0009-2509 (52) 87011-3) D. Annable - Application of The Temkin Kinetic Equation To Ammonia Synthesis in Large-Scale ReactorsDocument10 pagesChemical Engineering Science Volume 1 Issue 4 1952 (Doi 10.1016/0009-2509 (52) 87011-3) D. Annable - Application of The Temkin Kinetic Equation To Ammonia Synthesis in Large-Scale Reactorsmade hPas encore d'évaluation
- Loufllgria: An Improved Correlation For Compressed Liquid Densities of Hydrocarbons. Part 2. MixturesDocument15 pagesLoufllgria: An Improved Correlation For Compressed Liquid Densities of Hydrocarbons. Part 2. MixturesPrasad patgaonkarPas encore d'évaluation
- Riazi Daubert 1980Document6 pagesRiazi Daubert 1980Jeiel FrançaPas encore d'évaluation
- Vapor Pressure PDFDocument9 pagesVapor Pressure PDFArindam BanerjeePas encore d'évaluation
- Tars Siolubility Calculations With PDMS SolventDocument24 pagesTars Siolubility Calculations With PDMS SolventFareeha HasanPas encore d'évaluation
- Vapor Liquid EquilibriaDocument38 pagesVapor Liquid EquilibriadsaiojdoijdsaoPas encore d'évaluation
- A MODIFIED PENG-ROBINSON EQUATION OF STATE FOR - ELV - 20519 - FTP PDFDocument12 pagesA MODIFIED PENG-ROBINSON EQUATION OF STATE FOR - ELV - 20519 - FTP PDFLuiz Roberto TerronPas encore d'évaluation
- Documents - Tips Multicomponent Distillation Column Design A Semi Rigorous ApproachDocument16 pagesDocuments - Tips Multicomponent Distillation Column Design A Semi Rigorous ApproachPriyanshiVadaliaPas encore d'évaluation
- Formula RioDocument13 pagesFormula RioNoeliaPas encore d'évaluation
- Hybrid Type-2 Fuzzy KM Observer For Biogas Production From POME in Anaerobic DigesterDocument9 pagesHybrid Type-2 Fuzzy KM Observer For Biogas Production From POME in Anaerobic DigesterJavier OoiPas encore d'évaluation
- SRK Equation of State ExplainedDocument5 pagesSRK Equation of State Explainednutnicha suwannangkoonPas encore d'évaluation
- Fugacity CoefficientDocument4 pagesFugacity Coefficientsigit1058Pas encore d'évaluation
- Thermodynamics and Its ApplicationsDocument30 pagesThermodynamics and Its ApplicationspoletoPas encore d'évaluation
- Improvement in Patel Teja Eqn of StatesDocument10 pagesImprovement in Patel Teja Eqn of StatesSumukh VermaPas encore d'évaluation
- 37 - 4 - Washington DC - 08-92 - 1855 PDFDocument9 pages37 - 4 - Washington DC - 08-92 - 1855 PDFMohamadMostafaviPas encore d'évaluation
- Fluid Phase Equilibria Volume 95 Issue None 1994 (Doi 10.1016/0378-3812 (94) 80070-7) Monika Johannsen Gerd Brunner - Solubilities of The Xanthines Caffeine, Theophylline and Theobromine in SupeDocument12 pagesFluid Phase Equilibria Volume 95 Issue None 1994 (Doi 10.1016/0378-3812 (94) 80070-7) Monika Johannsen Gerd Brunner - Solubilities of The Xanthines Caffeine, Theophylline and Theobromine in Supenurul latifahPas encore d'évaluation
- NIST-JANAF Thermochemical TablesDocument39 pagesNIST-JANAF Thermochemical TablesVelzaeroPas encore d'évaluation
- Solubility of Solids in Sub - and Supercritical Fluids A ReviewDocument26 pagesSolubility of Solids in Sub - and Supercritical Fluids A ReviewJonatas LopesPas encore d'évaluation
- E4 Batch Distillation 2016Document5 pagesE4 Batch Distillation 2016jayaprinaPas encore d'évaluation
- EOSDocument9 pagesEOSgoicoxPas encore d'évaluation
- Boltzmann constant defines energy-temperature relationshipDocument6 pagesBoltzmann constant defines energy-temperature relationshipuyenphuongdangPas encore d'évaluation
- Ammonia ReactorDocument11 pagesAmmonia ReactorRh GladysPas encore d'évaluation
- Simulation of The Aromatic Recovery Process by Extractive DistillationDocument5 pagesSimulation of The Aromatic Recovery Process by Extractive DistillationaegosmithPas encore d'évaluation
- A New Generalized Alpha Function For A Cubic Equation of StateDocument11 pagesA New Generalized Alpha Function For A Cubic Equation of StateJenn QuintoPas encore d'évaluation
- Modelling The Extraction of Essential Oils With Compressed Carbon DioxideDocument14 pagesModelling The Extraction of Essential Oils With Compressed Carbon DioxideYusiana DewiPas encore d'évaluation
- PETERS, M. S. (1991) - Plant Design and Economics For Chemical Engineers (4th Ed.)Document4 pagesPETERS, M. S. (1991) - Plant Design and Economics For Chemical Engineers (4th Ed.)Isabel Rincon100% (1)
- Calculation of Densities From Cubic Equations ofDocument5 pagesCalculation of Densities From Cubic Equations ofgermangsilvaPas encore d'évaluation
- Supercritical Carbon Dioxide Extraction of Pigments From Bixa Orellana Seeds (Experiments and Modelling)Document8 pagesSupercritical Carbon Dioxide Extraction of Pigments From Bixa Orellana Seeds (Experiments and Modelling)Jacob SteelePas encore d'évaluation
- MSU Chemical Engineering Heat Exchanger DesignDocument15 pagesMSU Chemical Engineering Heat Exchanger DesignjsudbangPas encore d'évaluation
- Propylene-Propane - Howat Swift - McCabe ThieleDocument19 pagesPropylene-Propane - Howat Swift - McCabe ThieleFred FaberPas encore d'évaluation
- Experimental Optimization and Mathematical Modeling of The Supercritical - SADocument12 pagesExperimental Optimization and Mathematical Modeling of The Supercritical - SAJonatas LopesPas encore d'évaluation
- Computation of Phase and Chemical Equilibrium IDocument9 pagesComputation of Phase and Chemical Equilibrium IThou KanshiePas encore d'évaluation
- A Method To Estimate The Patel-Teja Equation of State ConstantsDocument7 pagesA Method To Estimate The Patel-Teja Equation of State Constants1940LaSallePas encore d'évaluation
- Costald 06-82Document6 pagesCostald 06-82boyd.george@bp.com100% (1)
- En Analysis of Thermal Efficiency Limit of Steam Methane Reforming ProcessDocument8 pagesEn Analysis of Thermal Efficiency Limit of Steam Methane Reforming ProcessAgam HanasichulaPas encore d'évaluation
- Extension of Peng-Robinson For Complex MixturesDocument18 pagesExtension of Peng-Robinson For Complex MixturesMandy NelsonPas encore d'évaluation
- Oliveira Et Al 2012Document10 pagesOliveira Et Al 2012Daniela De Araujo SampaioPas encore d'évaluation
- Chemical Equilibrium by Gibbs Energy Minimization on SpreadsheetsDocument5 pagesChemical Equilibrium by Gibbs Energy Minimization on SpreadsheetskaarevalomPas encore d'évaluation
- Phase Equilibrium Properties of The Ternary Mixture Dibutylether + Toluene + Heptane at 313.15 KDocument5 pagesPhase Equilibrium Properties of The Ternary Mixture Dibutylether + Toluene + Heptane at 313.15 Kmurdanetap957Pas encore d'évaluation
- Guide to the Physical and Chemical Properties of MethanolDocument24 pagesGuide to the Physical and Chemical Properties of MethanolKellyCristinaPas encore d'évaluation
- Heat Exchanger Analysis - NTU 3.624, ε 0.9647Document31 pagesHeat Exchanger Analysis - NTU 3.624, ε 0.9647Loken Rizal0% (1)
- Heat Transfer in Packed BedDocument10 pagesHeat Transfer in Packed BedNidhi JainPas encore d'évaluation
- Phase Equilibria InsightsDocument19 pagesPhase Equilibria InsightsjacPas encore d'évaluation
- TEG Water EquilibriumDocument9 pagesTEG Water Equilibriumlakonas_740100% (1)
- Combining Pinch and Exergy Analysis ForDocument13 pagesCombining Pinch and Exergy Analysis Forasad0071Pas encore d'évaluation
- Wong Sandler (1992)Document10 pagesWong Sandler (1992)Anonymous PO7VwbBnPas encore d'évaluation
- Je000301o PDFDocument20 pagesJe000301o PDFRLPas encore d'évaluation
- Thermodynamics Project: TOPIC: Fugacity of Pure SubstancesDocument6 pagesThermodynamics Project: TOPIC: Fugacity of Pure SubstancesRaman K. BediPas encore d'évaluation
- CRE AssignmentDocument5 pagesCRE AssignmentKuldeepChoudharyPas encore d'évaluation
- Binary Interaction Parameters in Cubic e PDFDocument6 pagesBinary Interaction Parameters in Cubic e PDFagarwalashwin32Pas encore d'évaluation
- Phase Diagrams in Chemical EngineeringDocument23 pagesPhase Diagrams in Chemical Engineeringchemsac2Pas encore d'évaluation
- High Pressure Phase Behaviour of Multicomponent Fluid MixturesD'EverandHigh Pressure Phase Behaviour of Multicomponent Fluid MixturesPas encore d'évaluation
- Investigating The Percentage of Energy Loss of Each Bounce of A Rubber BallDocument4 pagesInvestigating The Percentage of Energy Loss of Each Bounce of A Rubber BallKahfiantoro100% (1)
- F. K. Richtmyer - Introduction To Modern Physics-McGraw-Hill Book Company, Inc. (1928)Document628 pagesF. K. Richtmyer - Introduction To Modern Physics-McGraw-Hill Book Company, Inc. (1928)jor marketingPas encore d'évaluation
- DEMODocument16 pagesDEMOEarl CalingacionPas encore d'évaluation
- AP Physics - Chapter 8 Practice Test: Multiple ChoiceDocument7 pagesAP Physics - Chapter 8 Practice Test: Multiple Choicedipankar65Pas encore d'évaluation
- Determination of The Best Models For Reinforced Concrete Struts and TiesDocument9 pagesDetermination of The Best Models For Reinforced Concrete Struts and TiesBadr AmmarPas encore d'évaluation
- Methods of Heat Transfer: Grade 1VDocument4 pagesMethods of Heat Transfer: Grade 1VAnne 닌 야 PiedadPas encore d'évaluation
- D. Bauer - Theory of Intense Laser-Matter InteractionDocument106 pagesD. Bauer - Theory of Intense Laser-Matter InteractionYsam2Pas encore d'évaluation
- ASTM E 139-06 Standard Test Methods For Conducting Creep, Creep-Rupture, and Stress-Rupture Tests of Metallic Materials1Document14 pagesASTM E 139-06 Standard Test Methods For Conducting Creep, Creep-Rupture, and Stress-Rupture Tests of Metallic Materials1Angel RamirezPas encore d'évaluation
- Layer Coefficients For NHDOT Pavement MaterialsDocument52 pagesLayer Coefficients For NHDOT Pavement Materials김원봉Pas encore d'évaluation
- Triaxial Extension and Tension TestsDocument18 pagesTriaxial Extension and Tension TestsSara Reis RodriguesPas encore d'évaluation
- Thi I HD! This Course Is Hard!: Physics 122 September 7, 2010Document33 pagesThi I HD! This Course Is Hard!: Physics 122 September 7, 2010Penny LanePas encore d'évaluation
- Integrals 5 QuestionsDocument3 pagesIntegrals 5 QuestionsJoseph Neil TacataniPas encore d'évaluation
- Grinda Cadru Transversal - Stalp: 2.1. Data of ColumnDocument17 pagesGrinda Cadru Transversal - Stalp: 2.1. Data of ColumnGanea Marius BogdanPas encore d'évaluation
- Optistruct For Linear Dynamics: Modal, FRF, and Transient AnalysisDocument103 pagesOptistruct For Linear Dynamics: Modal, FRF, and Transient Analysisvinod reddy mPas encore d'évaluation
- Identification and Prediction of Piping System NoiseDocument7 pagesIdentification and Prediction of Piping System NoisejjirwinPas encore d'évaluation
- Fundamental Physics Experiments 2-FixDocument15 pagesFundamental Physics Experiments 2-FixDavid HadidPas encore d'évaluation
- Beam to Beam Connection PropertiesDocument22 pagesBeam to Beam Connection PropertieskalpanaadhiPas encore d'évaluation
- A 3D Constitutive Wood Model Using The Concepts of Continuum Damage MechanicsDocument19 pagesA 3D Constitutive Wood Model Using The Concepts of Continuum Damage MechanicsAnonymous 7MdZQn1Pas encore d'évaluation
- ElectronicsObj Outcomes12021Document30 pagesElectronicsObj Outcomes12021khaleqPas encore d'évaluation
- Model For Record Report PROBLEM 1Document3 pagesModel For Record Report PROBLEM 1Siddhartha Harsha OmmiPas encore d'évaluation
- Bristol Book 2 PDFDocument89 pagesBristol Book 2 PDFuri johnPas encore d'évaluation
- 3.3.3 Skipping of Loads (Pattern Loading)Document2 pages3.3.3 Skipping of Loads (Pattern Loading)Atul ShrivastavaPas encore d'évaluation
- Magazine - Car Mechanics - 03.2018Document100 pagesMagazine - Car Mechanics - 03.2018Roberto OrtegaPas encore d'évaluation
- Module 4.1 Method of Consistent DeformationDocument9 pagesModule 4.1 Method of Consistent DeformationJayLord Mico PacisPas encore d'évaluation
- Chapter-2 - Free UnDamped VibrationsDocument12 pagesChapter-2 - Free UnDamped VibrationsMuhammad Daud AliPas encore d'évaluation
- Things To Learn Today: 1. Use Energy Method To Derive Equation of Motion 2. Damping ElementsDocument15 pagesThings To Learn Today: 1. Use Energy Method To Derive Equation of Motion 2. Damping ElementsSayantan GhoshPas encore d'évaluation
- Distance Learning Programme: Jee (Main) : Leader Test Series / Joint Package CourseDocument38 pagesDistance Learning Programme: Jee (Main) : Leader Test Series / Joint Package CourseAbuzar AzharPas encore d'évaluation
- Simple Harmonic Motion (QB)Document8 pagesSimple Harmonic Motion (QB)Raju Singh100% (1)
- QP 12345Document2 pagesQP 12345Siva ShankarPas encore d'évaluation