Académique Documents
Professionnel Documents
Culture Documents
Tutorial - c2 - Rev
Transféré par
DanialAzimTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Tutorial - c2 - Rev
Transféré par
DanialAzimDroits d'auteur :
Formats disponibles
TUTORIAL 2
Chapter 2
Organohalides
2.1 Naming alkyl halides
2.2 Structure of alkyl halides
2.3 Preparing alkyl halides from alkanes and alkenes
2.4 Reaction of alkyl halides
________________________________________________________________________________
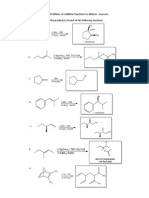
1. Gie the name for each of the follo!ing alkyl halides.
"#$3
"#2"l2
2. %ra! structures corresponding to these names.
3&iodopropene
trans&1&chloro&3&sec&'utylcyclohe(ane
1)2&dichloro&1)1)2)2&tetrafluoroethane
3. *o ans!er the follo!ing +uestions consider the reaction 'elo!,
*he reaction of propyl'en-ene !ith tert&'utylhypochlorite proceeds 'y a radical su'stitution
path!ay. %ra! the structure of the radical intermediate leading to each product.
H C
Cl
C
Cl
Cl
H
H
F C
F
C
F
Cl
H
Br
Cl
Cl
Cl
(CH
3
)
3
COCl
40
0
C
+ +
65% 25% 10%
4. "onsider the reaction 'elo! to ans!er the follo!ing +uestions.
%ra! all the monochlorination products of methylcyclopentane .ignore stereoisomers/.
0. "onsider the reaction 'elo! to ans!er the follo!ing +uestions.
Place asterisks.1/ at all allylic positions in compound A.
%ra! the resonance forms of the allylic radical intermediate that accounts
for the formation of B and C.
2. "hoose the best reagent or se+uence of reagents from the list proided 'elo! for carrying out the
follo!ing transformations. Place the letter of your response to the left of the reaction.
CH
3
Cl
2
light
OH Cl
CH
3
CHCH CHCH
2
CH
3
Br
CH
3
CH CHCHCH
2
CH
3
Br
CH
3
CH CHCH
2
CH
2
CH
3
NBS
C B A
+
hv
CH
3
C H
3
C
CH
3
Br
CH
3
C H
3
C
CH
3
OH
3. Predict the best reagent or se+uence of reagents for carrying out the follo!ing transformations.
4. Propose a synthesis of each of the follo!ing compounds from the gien starting material and any
inorganic reagents necessary.
5. $dentify the reagents a and b in the follo!ing scheme.
NBS
CCl
4
+
HCl
ether
Cl
1. NBS, CCl
4
2. KOH, ethanl
!Br
3
ether
Br
CH
3
CHCH CH
2
CH
3
CH
3
CHCH
2
CH
2
OH
CH
3
"r#
16. "ircle your response in each set 'elo!.
i. *he least reactie compound in an SN2 reaction.
ii. *he least reactie compound in a SN1 reaction.
iii. *he best solent for an SN2 reaction.
11. Cn$i%er the rea&tin 'el( t an$(er the "ll(ing )*e$tin$.
+rite the &#,lete $te,(i$e #e&hani$# "r thi$ rea&tin. Clearl- $h( the "r#atin " 'th ,r%*&t$. Sh(
all ele&trn "l( (ith arr($ an% %ra( all inter#e%iate $tr*&t*re$.
Br
a b
CH
2
.
H .
CH
3
CH
2
O/$
H Br
(CH
3
)
3
C . !hCHCH
3
Br
CH
3
CHCH
3
F
Cl
H0!1
CHCl
3
H
2
O CH
3
CH
2
OH
H
3
C Cl
H
3
C OH
HO CH
3
40%
60%
H
2
O
ethanl
+
Vous aimerez peut-être aussi
- Year 12 Chem 2021 Task 5 Acids Bases Redox TestDocument17 pagesYear 12 Chem 2021 Task 5 Acids Bases Redox TestSamruddhi MohitePas encore d'évaluation
- CHE 332 Final Review NameDocument11 pagesCHE 332 Final Review Nametlyons1188Pas encore d'évaluation
- Understanding Alkyl Halides, Aromatic Compounds, and Functional Group ReactionsDocument5 pagesUnderstanding Alkyl Halides, Aromatic Compounds, and Functional Group ReactionsSchool BackupPas encore d'évaluation
- Petency Based QuestionsDocument1 pagePetency Based QuestionsKRISH PATELPas encore d'évaluation
- Alcohol Phenol Ether OldDocument1 pageAlcohol Phenol Ether OldAdvanced AcademyPas encore d'évaluation
- Chapter Two Functional Groups in Organic ChemistryDocument64 pagesChapter Two Functional Groups in Organic ChemistrykidanePas encore d'évaluation
- Chemical Equations & ReactionsDocument78 pagesChemical Equations & ReactionsDelsie FalculanPas encore d'évaluation
- Chemistry Revision Test - No. 5 (Haloalkanes & Haloalkanes & Alcohols, Phenols & Ethers)Document9 pagesChemistry Revision Test - No. 5 (Haloalkanes & Haloalkanes & Alcohols, Phenols & Ethers)Charles Murgasan Charles MurgasanPas encore d'évaluation
- Chapter 4 - ElectrochemistryDocument66 pagesChapter 4 - ElectrochemistryNa Ru ToPas encore d'évaluation
- ALDEHYDES & KETONES GUIDEDocument40 pagesALDEHYDES & KETONES GUIDEMGoyalPas encore d'évaluation
- Physical Science Balancing EquationDocument44 pagesPhysical Science Balancing EquationRobertson LaguindayPas encore d'évaluation
- Homework 1 2302271 Organic Chemistry IDocument2 pagesHomework 1 2302271 Organic Chemistry IVee Worabhorn0% (1)
- Aldehydes and KetonsDocument8 pagesAldehydes and KetonsnishaninishaPas encore d'évaluation
- SN1 SN2 MECHANISMS HALOALKANES HALOARENESDocument3 pagesSN1 SN2 MECHANISMS HALOALKANES HALOARENESAryan SharmaPas encore d'évaluation
- Chem 331Document11 pagesChem 331Satya KamPas encore d'évaluation
- Reaction MechanismDocument41 pagesReaction MechanismVarsha Dange88% (8)
- Aloalkanes and Haloarenes - 4 Marks Questions: C-X Bond C-X BondDocument50 pagesAloalkanes and Haloarenes - 4 Marks Questions: C-X Bond C-X Bondilias1973Pas encore d'évaluation
- Objectives That Need To Be Met For Topic 10Document8 pagesObjectives That Need To Be Met For Topic 10sara bdeirPas encore d'évaluation
- Organic Chemistry Question PaperDocument2 pagesOrganic Chemistry Question PaperMOHAMED HISHAMPas encore d'évaluation
- Grace Fafel - Unit 6 Chemistry Test Study GuideDocument3 pagesGrace Fafel - Unit 6 Chemistry Test Study GuideGrace FafelPas encore d'évaluation
- Alkenes and AlkynesDocument2 pagesAlkenes and AlkynesLewis AlfonsoPas encore d'évaluation
- Reduction Reactions Mechanisms and SelectivityDocument34 pagesReduction Reactions Mechanisms and SelectivityFaTin AziEyatiPas encore d'évaluation
- Cubane SynthesisDocument5 pagesCubane SynthesissquarainePas encore d'évaluation
- Structure of Alkanes: Different Representa4ons of Organic MoleculesDocument11 pagesStructure of Alkanes: Different Representa4ons of Organic MoleculesJanice ChuaPas encore d'évaluation
- Chapter 6 Properties of HaloalkaneDocument5 pagesChapter 6 Properties of HaloalkaneRen Liew Jia QingPas encore d'évaluation
- Carbon Compund - Form 5Document26 pagesCarbon Compund - Form 5Novin Noel Roy100% (1)
- Austrian National Chemistry Olympiad 1998Document21 pagesAustrian National Chemistry Olympiad 1998Muhammad GhifariPas encore d'évaluation
- Halides and Arenes Chemistry ProblemsDocument7 pagesHalides and Arenes Chemistry ProblemsVishnuPas encore d'évaluation
- SR Inter CHEMISTRY IMP-New With 70% Syllabus-Converted-1Document6 pagesSR Inter CHEMISTRY IMP-New With 70% Syllabus-Converted-1B. SwapnaPas encore d'évaluation
- Redox Reactions: Half-Reactions and TheoryDocument5 pagesRedox Reactions: Half-Reactions and TheoryTaro PurplePas encore d'évaluation
- AP Summer Review PacketDocument5 pagesAP Summer Review PacketAndreaMarkhamPas encore d'évaluation
- Intro To Chemistry Unit: Exam RevisionDocument17 pagesIntro To Chemistry Unit: Exam RevisioncocoPas encore d'évaluation
- Organic Chemistry ADocument113 pagesOrganic Chemistry AChelsea Kyrell TupasPas encore d'évaluation
- Arul Kumar AnDocument22 pagesArul Kumar Anelainejoi.sauraPas encore d'évaluation
- Alkyl Halides: S5 Chemistry 29/NOV/2021Document31 pagesAlkyl Halides: S5 Chemistry 29/NOV/2021Nelima Stella mercyPas encore d'évaluation
- 1-6 Chemical Reactions of Alkanes and AlkenesDocument2 pages1-6 Chemical Reactions of Alkanes and AlkenesBryan100% (1)
- Reactor ModelDocument12 pagesReactor ModelTanuja ThanuPas encore d'évaluation
- F Naoh: Column - I Column - IiDocument4 pagesF Naoh: Column - I Column - Iipankaj16xissPas encore d'évaluation
- Organic Chemistry - II (1) - Print-OkDocument7 pagesOrganic Chemistry - II (1) - Print-OkB. Srini VasanPas encore d'évaluation
- SO and 0.7 M H PO - (Total 6 Solutions, Three Each)Document2 pagesSO and 0.7 M H PO - (Total 6 Solutions, Three Each)sandalailaPas encore d'évaluation
- CML 100 Questions PDFDocument7 pagesCML 100 Questions PDFDivyansh GuptaPas encore d'évaluation
- CML 100 QuestionsDocument7 pagesCML 100 QuestionsamanPas encore d'évaluation
- Elimination Reactions HandoutDocument21 pagesElimination Reactions HandoutDewi PurnamaPas encore d'évaluation
- Clxichewk 091Document7 pagesClxichewk 091neerajtrvPas encore d'évaluation
- HaloalkanesDocument218 pagesHaloalkanesVidhan PatniPas encore d'évaluation
- Wa0001.Document15 pagesWa0001.Thrivikram ArepalliPas encore d'évaluation
- Ilcpa 12 04 17Document22 pagesIlcpa 12 04 17JuanManuelAmaroLuisPas encore d'évaluation
- PUT QUESTION BANK-Organic ChemistryDocument2 pagesPUT QUESTION BANK-Organic ChemistryDhruv TomarPas encore d'évaluation
- S.NO. Unit VSA SAI Saii LA Total (1 Mark) (2 Marks) (3 Marks) (5 Marks)Document6 pagesS.NO. Unit VSA SAI Saii LA Total (1 Mark) (2 Marks) (3 Marks) (5 Marks)api-243565143Pas encore d'évaluation
- 07 Addition Reactions 343 AnsDocument4 pages07 Addition Reactions 343 AnsPedro Henrique CesarPas encore d'évaluation
- Types of Solids, Crystal Structure, Amorphous SolidsDocument3 pagesTypes of Solids, Crystal Structure, Amorphous SolidsAishwarya RaghavanPas encore d'évaluation
- Organic ChemistryDocument155 pagesOrganic ChemistryHakuna MatataPas encore d'évaluation
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesD'EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesPas encore d'évaluation
- Cbse SR Jee Apex & Neet Wisdom Chemistry Phase-1 QP (10.11.2023)Document6 pagesCbse SR Jee Apex & Neet Wisdom Chemistry Phase-1 QP (10.11.2023)SÁMÃÑ KANNAPas encore d'évaluation
- Alcohols IIDocument38 pagesAlcohols IIRafael G. Garcia SanchezPas encore d'évaluation
- CHAPTER 6 Alkyl Halides and Aryl HalidesDocument150 pagesCHAPTER 6 Alkyl Halides and Aryl HalidesexpertwritersPas encore d'évaluation
- Methods for Oxidation of Organic Compounds V1: Alcohols, Alcohol Derivatives, Alky Halides, Nitroalkanes, Alkyl Azides, Carbonyl Compounds Hydroxyarenes and AminoarenesD'EverandMethods for Oxidation of Organic Compounds V1: Alcohols, Alcohol Derivatives, Alky Halides, Nitroalkanes, Alkyl Azides, Carbonyl Compounds Hydroxyarenes and AminoarenesPas encore d'évaluation
- Critical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsD'EverandCritical Evaluation of Equilibrium Constants Involving 8-Hydroxyquinoline and Its Metal Chelates: Critical Evaluation of Equilibrium Constants in Solution: Part B: Equilibrium Constants of Liquid-Liquid Distribution SystemsPas encore d'évaluation
- Air Pressure Control TheoryDocument2 pagesAir Pressure Control TheoryDanialAzim100% (1)
- Typical Plant AirDocument2 pagesTypical Plant AirOmar EzzatPas encore d'évaluation
- Process Control Systems Precisely Regulate Liquids and GasesDocument3 pagesProcess Control Systems Precisely Regulate Liquids and GasesDanialAzimPas encore d'évaluation
- Dcs IntroDocument1 pageDcs IntroDanialAzimPas encore d'évaluation
- Tutorial CHAPTER 4: Alcohol and CarbonylsDocument2 pagesTutorial CHAPTER 4: Alcohol and CarbonylsDanialAzimPas encore d'évaluation
- Chapter 1Document128 pagesChapter 1Muhammad NursalamPas encore d'évaluation
- Discussion: P ρ g v h=constant P ρ g vDocument3 pagesDiscussion: P ρ g v h=constant P ρ g vDanialAzimPas encore d'évaluation
- Idea of Self Confidence FaisalDocument1 pageIdea of Self Confidence FaisalDanialAzimPas encore d'évaluation
- AbsorptionDocument20 pagesAbsorptionRininta Triananda NoorPas encore d'évaluation
- Tutorial - c3 - RevDocument3 pagesTutorial - c3 - RevDanialAzimPas encore d'évaluation
- Tutor Chapter 6 (Che225)Document1 pageTutor Chapter 6 (Che225)DanialAzimPas encore d'évaluation
- JUN2013 - Assignment 1 and 2Document2 pagesJUN2013 - Assignment 1 and 2DanialAzimPas encore d'évaluation
- CHE225 Course Outline (Jun-Sep2013)Document3 pagesCHE225 Course Outline (Jun-Sep2013)DanialAzimPas encore d'évaluation
- 2nd Semester All Courses-100Document194 pages2nd Semester All Courses-100Ejiade PeterPas encore d'évaluation
- CHM Analysis and Comparisons of CRUS MULTHULLS2013Document60 pagesCHM Analysis and Comparisons of CRUS MULTHULLS2013kkd108Pas encore d'évaluation
- Numerical Simulation of Screw Displacement Pile Interaction With Non-Cohesive SoilDocument12 pagesNumerical Simulation of Screw Displacement Pile Interaction With Non-Cohesive Soilmohamed magdyPas encore d'évaluation
- Contemporary Philippine Arts From The Regions: Quarter 3Document15 pagesContemporary Philippine Arts From The Regions: Quarter 3Ackie Inacay RosarioPas encore d'évaluation
- Mastercam 8.1 Beta 4: New Verification Engine in Beta 4! Sub-Programs Post ChangesDocument48 pagesMastercam 8.1 Beta 4: New Verification Engine in Beta 4! Sub-Programs Post ChangesSaul Saldana LoyaPas encore d'évaluation
- Engineering Aspects of Food Emulsification and HomogenizationDocument325 pagesEngineering Aspects of Food Emulsification and Homogenizationfurkanturker61Pas encore d'évaluation
- 19174the Rise of Industrial Big Data WP Gft834Document6 pages19174the Rise of Industrial Big Data WP Gft834em01803257Pas encore d'évaluation
- 03-Mechanical Seal &seal System Basics-REV01Document39 pages03-Mechanical Seal &seal System Basics-REV01Fayez Al-ahmadiPas encore d'évaluation
- Improving Students' Science Process SkillsDocument9 pagesImproving Students' Science Process SkillsNovia RahmawatiPas encore d'évaluation
- Project Information for 2x660 MW Lube Oil PumpsDocument93 pagesProject Information for 2x660 MW Lube Oil PumpsghostamirPas encore d'évaluation
- LearnEnglish Video Zone How These Women Changed Science ForeverDocument3 pagesLearnEnglish Video Zone How These Women Changed Science ForeverDaniella MensatoPas encore d'évaluation
- Xoro Hrs 8540 HD Sat ReceiverDocument33 pagesXoro Hrs 8540 HD Sat ReceiverPinoPas encore d'évaluation
- Well Serve CingDocument140 pagesWell Serve CingYounes MakPas encore d'évaluation
- AWWA M28 Rehabilitation of Water Mains 3rd Ed 2014Document133 pagesAWWA M28 Rehabilitation of Water Mains 3rd Ed 2014millini67% (3)
- BS 7941-1-2006Document20 pagesBS 7941-1-2006Willy AryansahPas encore d'évaluation
- 2019 Torch and Consumables Catalog: For Mechanized Plasma SystemsDocument64 pages2019 Torch and Consumables Catalog: For Mechanized Plasma SystemsRaj DomadiyaPas encore d'évaluation
- Lisa - Add New Front: Process Matching/Installation and Qualification (IQ)Document62 pagesLisa - Add New Front: Process Matching/Installation and Qualification (IQ)Thanh Vũ NguyễnPas encore d'évaluation
- EAPP w2Document13 pagesEAPP w2Elijah AquinoPas encore d'évaluation
- PCB Table of Contents GuideDocument3 pagesPCB Table of Contents GuidePreet ChahalPas encore d'évaluation
- Sepuran® N Module 4": in NM /H at 7 Barg 25°CDocument2 pagesSepuran® N Module 4": in NM /H at 7 Barg 25°CsanjaigPas encore d'évaluation
- RESISTANCEDocument9 pagesRESISTANCERohit SahuPas encore d'évaluation
- Chapter 31. Current and Resistance Chapter 31. Current and Resistance Current and ResistanceDocument11 pagesChapter 31. Current and Resistance Chapter 31. Current and Resistance Current and ResistanceArwaa AlmaghrabiPas encore d'évaluation
- Prob Stats Module 4 2Document80 pagesProb Stats Module 4 2AMRIT RANJANPas encore d'évaluation
- Installation & Testing of Fire Protection SystemsDocument7 pagesInstallation & Testing of Fire Protection Systemssunny_84tPas encore d'évaluation
- Gas Exchange in Plants and AnimalsDocument7 pagesGas Exchange in Plants and AnimalsMarvin MelisPas encore d'évaluation
- Medicinal Chemistry 1 - Drug MetabolismDocument39 pagesMedicinal Chemistry 1 - Drug MetabolismPark arimaPas encore d'évaluation
- Chocolate - Useful Physical ConstantsDocument2 pagesChocolate - Useful Physical ConstantsJuan CPas encore d'évaluation
- Blower Selection For Wastewater Aeration PDFDocument10 pagesBlower Selection For Wastewater Aeration PDFRobert MontoyaPas encore d'évaluation
- M700-70 Series Programming Manual (M-Type) - IB1500072-F (ENG)Document601 pagesM700-70 Series Programming Manual (M-Type) - IB1500072-F (ENG)Mert SertPas encore d'évaluation
- PENERAPAN ARSITEKTUR TROPIS PADA HOTEL RESORT DI PANTAI KRAKALDocument12 pagesPENERAPAN ARSITEKTUR TROPIS PADA HOTEL RESORT DI PANTAI KRAKALleo adoPas encore d'évaluation