Académique Documents
Professionnel Documents
Culture Documents
TLS Terry Leach and Greg Corbett
Transféré par
Lizbeth Avena0 évaluation0% ont trouvé ce document utile (0 vote)
106 vues44 pagesFluid mixing as a mechanism for bonanza grade epithermal gold formation Terry Leach & Greg corbett. Fluid sources include Boiling, Cooling, Rapid cooling, Sulphidation reactions, bicarbonate waters and low pH waters. More efficient Mechanisms of Au deposition provide higher Au grades.
Description originale:
Titre original
TLS Terry Leach and Greg Corbett (1)
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentFluid mixing as a mechanism for bonanza grade epithermal gold formation Terry Leach & Greg corbett. Fluid sources include Boiling, Cooling, Rapid cooling, Sulphidation reactions, bicarbonate waters and low pH waters. More efficient Mechanisms of Au deposition provide higher Au grades.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
106 vues44 pagesTLS Terry Leach and Greg Corbett
Transféré par
Lizbeth AvenaFluid mixing as a mechanism for bonanza grade epithermal gold formation Terry Leach & Greg corbett. Fluid sources include Boiling, Cooling, Rapid cooling, Sulphidation reactions, bicarbonate waters and low pH waters. More efficient Mechanisms of Au deposition provide higher Au grades.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 44
Fluid mixing
as a mechanism for bonanza grade
epithermal gold formation
Terry Leach*
&
Greg Corbett
Boiling
Lihir Island
1984
Boiling textures
Porgera Zone VII
Magmatic arc geothermal systems
Character sampling
Cracow early 1990s
Vera Nancy late 1990s
Banded epithermal veins
& Multiple fluid sources
A
B
C
Au deposition and
epithermal vein formation
from different
hydrothermal fluids
Mechanisms of Au deposition
More efficient mechanisms of Au deposition
provide higher Au grades
Several mechanisms to consider
Boiling
Cooling
Rapid cooling
Sulphidation reactions
Carbon reactions
Mixing with oxygenated groundwaters
Mixing with bicarbonate waters
Mixing with low pH waters
Have a minor effect on Ag:Au ratios
Kupol, Russia
chalcedony, ginguro & adularia
58 g/t Au, 1184 g/t Ag
Banded chalcedony-ginguro Au-Ag vein
Banded quartz vein -
Golden Cross
Adularia
-El Peon..
Quartz pseudomorphing platy
carbonate
Vera Nancy Hishikari
Cracow
Ginguro bands
Visible Au
Asacha, Kamchatka
Hishikari, J apan
Vera Nancy, Aust
Midas, Nevada
Patagonia
Bilimoia, PNG
Slow cooling
- low Au grades,
good metallurgy
in quartz-
sulphide Au
Cowal
Rapid Cooling
Arsenian Pyrite, Lihir Island, Papua New Guinea
123 g/t Au
15-30 g/t Au
Rapid cooling
opal in contact with sulphides
Fresnillo
Mariana vein
Arcata, Peru
Chatree, Thailand
Huevos Verde,
Patagonia
Epithermal vein formation
from different
hydrothermal fluids
Sulphidation
Lihir, arsenian pyrite 13.1 g/t
CARLIN TREND
Meikel 15 g/t Au
Goldstrike 2-3 g/t Au
Epithermal vein formation
from different
hydrothermal fluids
Gold solubility
Fresnillo,
Mexico
Mixing with
oxygenated waters
hypogene haematite
Palmarejo Mexico
Kupol, Russia
86 g/t Au 1370 g/t Ag
Kupol polymetallic Ag-Ag
40 cm @ about 1,500 g/t Au, 15,000 g/t Ag
Bicarbonate waters
Carbonate-base metal Au
Leach and Corbett, 1993, 1994, 1995; Corbett and Leach, 1998
Porgera
Kelian
Mixing with bicarbonate waters in
polymetallic Ag-Ag vein systems
Santa Ana, Peru
Bicarbonate waters
Mixing with with bicarbonate waters
Rhodochrosite
69 g/t Au, 42 g/t Ag
Dolomite
7.93 gt/ Au, 19 g/t Ag
Calcite 2.8 g/t Au 11 g/t Ag
Manganese wad
Mt Kare
Papua New Guinea
Mixing with low pH waters
derived from acid sulphate
caps
Arcata
Acid cap
Ares -
Kaolin
Kupol - kaolin
45 g/t Au, 117 g/t Ag
55 g/t Au, 355 g/t Ag
602 g/t Au, 2082 g/t Ag
Kaolin intergrown with ginguro
Sleeper Gold
Mine, Nevada
Kaolin-pyrite
intergrown
Fine black silica-sulphide
Palmarejo Mexico
186 g/t Au, 3720 g/t Ag
Hishikari J apan
Hishikari J apan
50,000 g/t Au
Active Hanging Wall Splay
Palinpinon Geothermal
Intersection of collapsing acid sulphate
waters and rising mineralised fluids
223 g/t Au & 17,642 g/t Ag
Champagne Pool,
Waitapu, New
Zealand
Orange precipitate in ppm or %
Au 80, Ag 170, 170 Hg, 2% As,
2% Sb
Hypogene oxidation in high sulphidation
epithermal systems
Pierina, Peru
Veladero, Argentina
Conclusion
Several mechanisms may account for the
deposition of Au in low sulphidation epithermal
Au systems
While boiling does deposit Au, this is not always
the case
Several different mixing reactions may account for
elevated Au grades with increased efficiency and
hence higher Au grade involving:
Oxygenated groundwaters
Bicarbonate waters
Low pH acid sulphate waters
Au deposition and
epithermal vein formation
from different
hydrothermal fluids
Vous aimerez peut-être aussi
- Alumina to Zirconia: The History of the CSIRO Division of Mineral ChemistryD'EverandAlumina to Zirconia: The History of the CSIRO Division of Mineral ChemistryÉvaluation : 1 sur 5 étoiles1/5 (1)
- Ojt Evaluation Forms (Supervised Industry Training) SampleDocument5 pagesOjt Evaluation Forms (Supervised Industry Training) SampleJayJay Jimenez100% (3)
- Din 48204Document3 pagesDin 48204Thanh Dang100% (4)
- Depositos Epitermals - EjemplosDocument39 pagesDepositos Epitermals - Ejemplosoops07Pas encore d'évaluation
- Greg Corbett - Lachlan Orogen Magmatic Arc Au-Cu-AgDocument46 pagesGreg Corbett - Lachlan Orogen Magmatic Arc Au-Cu-AgoguzgeoPas encore d'évaluation
- Gold in Minerals and The Composition of Native GoldDocument24 pagesGold in Minerals and The Composition of Native GoldRickyadhitamaPas encore d'évaluation
- Granitic Pegmatites As Refl Ections of Their Sources: Petr Cerný, David London, and Milan NovákDocument6 pagesGranitic Pegmatites As Refl Ections of Their Sources: Petr Cerný, David London, and Milan NovákAna Luz Condorhuaman SuarezPas encore d'évaluation
- Shear Zones and Crustal Blocks of Southern IndiaDocument42 pagesShear Zones and Crustal Blocks of Southern IndiaS.Alec Knowle100% (1)
- GeobabaDocument25 pagesGeobabarabkaliPas encore d'évaluation
- Corbett and Leach 1997 (LIBRO)Document317 pagesCorbett and Leach 1997 (LIBRO)Juan Crisostomo RamosPas encore d'évaluation
- Calderon Porphyry Au-Cu of ColombiaDocument29 pagesCalderon Porphyry Au-Cu of ColombiaRicardo Cesar100% (1)
- 8 Hydroth Breccia Formation WindhoekDocument93 pages8 Hydroth Breccia Formation WindhoekAlberto Lobo-Guerrero Sanz100% (1)
- Geochem PerspectiveDocument136 pagesGeochem Perspectivevalentina robayoPas encore d'évaluation
- 1london Granitic Pegmatites - Scientific Wonders and Economic BonanzasDocument6 pages1london Granitic Pegmatites - Scientific Wonders and Economic BonanzasWendy GilPas encore d'évaluation
- Hammond TexturesDocument37 pagesHammond TexturesMartin Zegarra100% (3)
- 3 Related Deposit TypesDocument35 pages3 Related Deposit Typesaudah100% (2)
- E14 6 PDFDocument80 pagesE14 6 PDFJoãoDeSouzaPas encore d'évaluation
- Central Java Fieldtrip (Instructed by Peter Lunt)Document65 pagesCentral Java Fieldtrip (Instructed by Peter Lunt)aprilana07Pas encore d'évaluation
- Listing of Mining Properties Available For Bidding PDFDocument13 pagesListing of Mining Properties Available For Bidding PDFAba Emmanuel OchePas encore d'évaluation
- Witts - Etal - 2012 Barito Tanjung FM PDFDocument28 pagesWitts - Etal - 2012 Barito Tanjung FM PDFSandy Wahyu PratamaPas encore d'évaluation
- Orogenic Gold DepositDocument17 pagesOrogenic Gold DepositDwi LekatompessyPas encore d'évaluation
- Abstracts Actas Iagod 2019 PDFDocument390 pagesAbstracts Actas Iagod 2019 PDFPedro Carlos Picapiedra100% (2)
- Todo Sobre ApatitosDocument76 pagesTodo Sobre ApatitosCatalina Castro CárdenasPas encore d'évaluation
- Jurnal The Tin Ores of Banca, Billiton, and Singkep-Westerveld 1937 OkkDocument23 pagesJurnal The Tin Ores of Banca, Billiton, and Singkep-Westerveld 1937 OkkNadila Juang PutriPas encore d'évaluation
- Geology of Bangka and BilitonDocument8 pagesGeology of Bangka and BilitondeadromeoPas encore d'évaluation
- Hot Spring Au AgDocument15 pagesHot Spring Au AgJose Aranibar Aguilar100% (2)
- SGA Abstractvolym-2 DigitalDocument468 pagesSGA Abstractvolym-2 Digitalmalibrod100% (2)
- Ash 1989 Paper LiswaniteDocument6 pagesAsh 1989 Paper LiswaniteThomas Sant100% (1)
- Porphyry Copper DepositsDocument13 pagesPorphyry Copper DepositsIrwan EPPas encore d'évaluation
- 02-02 - Ruelo and AngelesDocument87 pages02-02 - Ruelo and AngelesLouie Bedes100% (2)
- Epithermal SystemDocument44 pagesEpithermal SystemEdo GondressPas encore d'évaluation
- Outcrops in Natuna Island New Insights of Reservoir Potential and Sediment Provenance of The East Natuna BasinDocument9 pagesOutcrops in Natuna Island New Insights of Reservoir Potential and Sediment Provenance of The East Natuna BasinHerry SuhartomoPas encore d'évaluation
- An Evaluation of Plate Tectonics Models PDFDocument28 pagesAn Evaluation of Plate Tectonics Models PDFBayu Aji WicaksonoPas encore d'évaluation
- T.M. Porter (Editor) - Super Porphyry Copper and Gold Deposits - A Global Perspective. 2-PGC Publishing (2005) PDFDocument276 pagesT.M. Porter (Editor) - Super Porphyry Copper and Gold Deposits - A Global Perspective. 2-PGC Publishing (2005) PDFtonicors_806375834100% (1)
- EleMents (Diamond) HeaneyDocument76 pagesEleMents (Diamond) HeaneyRudiny FarabyPas encore d'évaluation
- Furnace Upgrade With Hatch Technology at PT Antam Feni-Ii in Pomalaa, IndonesiaDocument16 pagesFurnace Upgrade With Hatch Technology at PT Antam Feni-Ii in Pomalaa, IndonesiaAbdul Aziz AmmarPas encore d'évaluation
- 2005 StratigraphyDocument31 pages2005 Stratigraphyivan dhermawanPas encore d'évaluation
- Economic Aspects of Carbonatites of India: P. Krishnamurthy, S.Q. Hoda, R.P. Sinha, D.C. Banerjee, K.K. DwivedyDocument7 pagesEconomic Aspects of Carbonatites of India: P. Krishnamurthy, S.Q. Hoda, R.P. Sinha, D.C. Banerjee, K.K. DwivedyPritam RajPas encore d'évaluation
- Epithermal Gold SynthesisDocument27 pagesEpithermal Gold SynthesisMohammad Wildan ArifinPas encore d'évaluation
- (Cluzel, 2008) Syntectonic Mobility of Supergene Nickel Ores of New Caledonia (Southwest Paci C) - Evidence From GarnieriteDocument10 pages(Cluzel, 2008) Syntectonic Mobility of Supergene Nickel Ores of New Caledonia (Southwest Paci C) - Evidence From GarnieriteTryana RahmatPas encore d'évaluation
- Malkanietal 2016MineralResourcesofPakistan-anupdateDocument26 pagesMalkanietal 2016MineralResourcesofPakistan-anupdateAnonymous h5OeGoX3MTPas encore d'évaluation
- Eem 10 - Placer DepositDocument23 pagesEem 10 - Placer DepositKevinPas encore d'évaluation
- SGA Abstractvolym-1 DigitalDocument455 pagesSGA Abstractvolym-1 Digitalmalibrod100% (1)
- 5 - Epithermal Gold Deposits - 2Document43 pages5 - Epithermal Gold Deposits - 2chengsrunPas encore d'évaluation
- Lithium - Source - India - World - Price & Consumption Trends 5septDocument8 pagesLithium - Source - India - World - Price & Consumption Trends 5septnitesh.costmastersPas encore d'évaluation
- MPC Book of Abstracts - 0 PDFDocument120 pagesMPC Book of Abstracts - 0 PDFFranciscoZegarraPas encore d'évaluation
- Low Iron Silicasand For GlassmakingDocument9 pagesLow Iron Silicasand For GlassmakingenjaegerPas encore d'évaluation
- Preliminary Model of Porphiry Copper DepositsDocument62 pagesPreliminary Model of Porphiry Copper DepositsPeritajes Sociales GuanajuatoPas encore d'évaluation
- VMS Usgs2Document363 pagesVMS Usgs2HEBERT JHON RAFAEL AGUILAR100% (1)
- VMS DepositDocument39 pagesVMS DepositrifkyPas encore d'évaluation
- M. Cuney and K. Kayser - 2008, Recent and Not-So-Recent Developments in Uranium Deposits and Implications For ExplorationDocument259 pagesM. Cuney and K. Kayser - 2008, Recent and Not-So-Recent Developments in Uranium Deposits and Implications For ExplorationDomenik Elizabeth Calderon VPas encore d'évaluation
- Uranium Geology and Resources of The Gas Hills District, Wind River Basin, Central WyomingDocument40 pagesUranium Geology and Resources of The Gas Hills District, Wind River Basin, Central WyomingDaniel FrancoPas encore d'évaluation
- Controversies On The Origin of World-Class Gold Deposits Part I Carlin-Type Gold Deposits in Nevada PDFDocument9 pagesControversies On The Origin of World-Class Gold Deposits Part I Carlin-Type Gold Deposits in Nevada PDFjunior.geologiaPas encore d'évaluation
- The Porphyry Copper Deposit at El Salvador Chile L.gustAFSON, J.huntDocument57 pagesThe Porphyry Copper Deposit at El Salvador Chile L.gustAFSON, J.huntDánisa Urrutia ContrerasPas encore d'évaluation
- The Phalaborwa Complex Is Palaeoproterozoic in AgeDocument12 pagesThe Phalaborwa Complex Is Palaeoproterozoic in Agethanyani gudani0% (1)
- Big IV BorneoDocument194 pagesBig IV BorneoMuliandhy_Baranta_G100% (1)
- Epithermal Gold DepositsDocument43 pagesEpithermal Gold DepositsMatías Rodríguez100% (1)
- Paper Pongkor, Ephitermal SystemDocument16 pagesPaper Pongkor, Ephitermal SystemisniantoPas encore d'évaluation
- PirometalurgiDocument2 pagesPirometalurgieltonPas encore d'évaluation
- Greenstone-Hosted Quartz-Carbonate Vein DepositsDocument25 pagesGreenstone-Hosted Quartz-Carbonate Vein DepositsCarlos Gallego100% (1)
- Principle of XRFDocument25 pagesPrinciple of XRFLizbeth AvenaPas encore d'évaluation
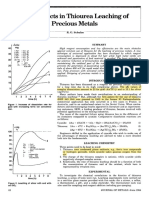
- New Aspects Thiourea Leaching of Precious Metals: R. G. SchulzeDocument4 pagesNew Aspects Thiourea Leaching of Precious Metals: R. G. SchulzeLizbeth AvenaPas encore d'évaluation
- Systematic Variation in Galena Solid-Solution Compositions at Santa Eulalia, Chihuahua, MexicoDocument16 pagesSystematic Variation in Galena Solid-Solution Compositions at Santa Eulalia, Chihuahua, MexicoLizbeth AvenaPas encore d'évaluation
- Ruiz 1985Document10 pagesRuiz 1985Lizbeth AvenaPas encore d'évaluation
- Garzanti 2018Document55 pagesGarzanti 2018Lizbeth Avena100% (1)
- English 2 Q3 Week 7 DLLDocument7 pagesEnglish 2 Q3 Week 7 DLLEste R A BulaonPas encore d'évaluation
- List of Institutions With Ladderized Program Under Eo 358 JULY 2006 - DECEMBER 31, 2007Document216 pagesList of Institutions With Ladderized Program Under Eo 358 JULY 2006 - DECEMBER 31, 2007Jen CalaquiPas encore d'évaluation
- Description - GB - 98926286 - Hydro EN-Y 40-250-250 JS-ADL-U3-ADocument6 pagesDescription - GB - 98926286 - Hydro EN-Y 40-250-250 JS-ADL-U3-AgeorgePas encore d'évaluation
- Pitch AnythingDocument8 pagesPitch AnythingDoland drumb100% (1)
- Prof Ed 3 Module 1Document9 pagesProf Ed 3 Module 1alexa dawatPas encore d'évaluation
- CompTIA A+ Lesson 3 Understanding, PATA, SATA, SCSIDocument8 pagesCompTIA A+ Lesson 3 Understanding, PATA, SATA, SCSIAli Ghalehban - علی قلعه بانPas encore d'évaluation
- Bus105 Pcoq 2 100%Document9 pagesBus105 Pcoq 2 100%Gish KK.GPas encore d'évaluation
- TMA GuideDocument3 pagesTMA GuideHamshavathini YohoratnamPas encore d'évaluation
- Analog Electronics-2 PDFDocument20 pagesAnalog Electronics-2 PDFAbhinav JangraPas encore d'évaluation
- Development of PBAT Based Bio Filler Masterbatch: A Scientific Research Proposal OnDocument15 pagesDevelopment of PBAT Based Bio Filler Masterbatch: A Scientific Research Proposal OnManmathPas encore d'évaluation
- Boq Cme: 1 Pole Foundation Soil WorkDocument1 pageBoq Cme: 1 Pole Foundation Soil WorkyuwonoPas encore d'évaluation
- Busbusilak - ResearchPlan 3Document4 pagesBusbusilak - ResearchPlan 3zkcsswddh6Pas encore d'évaluation
- Perbandingan Implementasi Smart City Di Indonesia: Studi Kasus: Perbandingan Smart People Di Kota Surabaya Dan Kota MalangDocument11 pagesPerbandingan Implementasi Smart City Di Indonesia: Studi Kasus: Perbandingan Smart People Di Kota Surabaya Dan Kota Malanglely ersilyaPas encore d'évaluation
- Approximate AnalysisDocument35 pagesApproximate AnalysisSyahir HamidonPas encore d'évaluation
- EceDocument75 pagesEcevignesh16vlsiPas encore d'évaluation
- Ems Speed Sensor Com MotorDocument24 pagesEms Speed Sensor Com MotorKarina RickenPas encore d'évaluation
- Master Thesis On Smart GridDocument6 pagesMaster Thesis On Smart Gridsandraandersondesmoines100% (2)
- IOM - Rampa Hidráulica - Blue GiantDocument32 pagesIOM - Rampa Hidráulica - Blue GiantPATRICIA HERNANDEZPas encore d'évaluation
- Solution Documentation For Custom DevelopmentDocument52 pagesSolution Documentation For Custom DevelopmentbayatalirezaPas encore d'évaluation
- SCHEMA - Amsung 214TDocument76 pagesSCHEMA - Amsung 214TmihaiPas encore d'évaluation
- 009 Attached-1 NAVFAC P-445 Construction Quality Management PDFDocument194 pages009 Attached-1 NAVFAC P-445 Construction Quality Management PDFSor sopanharithPas encore d'évaluation
- AURTTA104 - Assessment 2 Practical Demonstration Tasks - V3Document16 pagesAURTTA104 - Assessment 2 Practical Demonstration Tasks - V3muhammaduzairPas encore d'évaluation
- SAP Environment, Health, and Safety (EHS)Document13 pagesSAP Environment, Health, and Safety (EHS)SAFETY VOFPLPas encore d'évaluation
- Top249 1 PDFDocument52 pagesTop249 1 PDFCarlos Henrique Dos SantosPas encore d'évaluation
- File 1038732040Document70 pagesFile 1038732040Karen Joyce Costales MagtanongPas encore d'évaluation
- Module 1 Dynamics of Rigid BodiesDocument11 pagesModule 1 Dynamics of Rigid BodiesBilly Joel DasmariñasPas encore d'évaluation
- Rubric For Audio Speech DeliveryDocument2 pagesRubric For Audio Speech DeliveryMarie Sol PanganPas encore d'évaluation
- Application of A HAZOP Study Method To Hazard Evaluation of Chemical Unit of The Power StationDocument8 pagesApplication of A HAZOP Study Method To Hazard Evaluation of Chemical Unit of The Power Stationshinta sariPas encore d'évaluation