Académique Documents
Professionnel Documents
Culture Documents
Anodizing Techniques at Home PDF
Transféré par
palosotTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Anodizing Techniques at Home PDF
Transféré par
palosotDroits d'auteur :
Formats disponibles
Step by Step home anodizing of
aluminium
The exact steps any home anodizer is going to take are dictated by time, available
resources, attention to detail and various other factors. Here is my quick guide to home
aluminium anodizing - but don't blame me if it doesn't ork.
!ix up "# to $#% &ulphuric 'cid solution ith pure distilled ater. (nough to
fill hatever container you are going to use about $)* full. +eave to cool. This
mixture can be used many hundreds of times for anodizing runs. ,t ill eventually
pick up impurities any become less effective. -emember, never add ater to acid,
alays add acid to ater so it doesn't fizz and bite back. /o not let any extra
ater, caustic soda, sodium bicarbonate or similiar near the acid bath.
0repare your aluminium piece. 1inish is everything - anodizing does not hide a
poor finish. 2lean it up ith "$## paper and maybe polish.
2over your orking area in something disposable. 0utting the anodizing bath on a
big sheet of glass is a good idea - keeps any splashed acid off the orktop. !ake
sure the bucket of sodium barcarbonate solution is handy for dipping things in. ,
suggest getting a big 3ie several kilos4 carton of bicarbonate from a catering
suppler or cash and carry. ,f you do spill a serious amount of acid, its nice to have
some alkali handy to neutralise it.
1izz the aluminium in caustic soda solution until it looks a nice grey colour. ,f the
aluminium is already anodized, it is possible to remove the anodized layer by
leaving it in the caustic soda bath for longer. ,'ve not read of the correct strength
of the caustic soda bath for preparing the metal. 'n eggcup or to of caustic soda
granules in a pint of arm ater orks for me.
,f you have some desmut in nitric acid to clean of the other metals, then ash off
the part once more ith lots of ater. 5ithout nitric acid, 6ust try to clean up the
part as best you can ith hot soapy ater and then rinse.
&uspend the aluminium part in the acid so it is completely immersed using some
kind of aluminium ire or aluminium strut. The only metals alloed in the bath
are aluminium and lead. !ake sure you get a good electrical connection. 7ear in
mind that any parts here the suspending ire touches the part it ill not be
anodized, and ill not take up the dye. Tist a bit of ire into a tapped hole or
something. !ake sure that you don't touch the part. 8rease from finger prints can
leave a mark on the finished item. 8et some good gloves.
0lace a +ead cathode in the bath. This should have a surface area of at least tice
that of the aluminium part. /on't let it touch the aluminium part at the anode.
'ttach the positive connection of your poer supply to the aluminium anode and
the negative connection to the lead cathode.
-un the poer at "$ volts for about 9: minutes. The cathode ill fizz a lot, the
anode ill also sho some small bubbles. The acid ill heat up. ,f you are not
sure its orking, use an ammeter to see hats going on. ;ou should not allo the
acid to become arm - ideally it ants to stay at $#2. +et the acid cool beteen
anodizing runs, or rig up a cooler. -emember only lead or aluminium in the tank.
(ven a fan bloing on the tank helps. ,f you think about it, "$v at, say $ amps,
acts like a $9 att header, and thats before the heat created by the reaction.
There is a lot of ords ritten about hat current to anodize ith. 'pparently
you are supposed to anodize at beteen 9 and "$ amps per square foot of anode
surface area. 5ith most parts its almost impossible to estimate the surface area.
'fter etching in the caustic soda, you'll thro your calculations out even further.
1or my purposes , 6ust run the hole thing at "$ volts and let it dra as much
current.
-emove aluminium part from the acid and ash in distilled ater. Try not to drip
acid from the part over the kitchen hilst moving to the ater. ,f you must alk
around the house ith bits of aluminium covered in acid, hold a bol of
bicarbonate underneath.
/ip the part in the chosen dye for beteen " and ": minutes depending on ho
much colour you ant. Heating the dye ill increase the speed of colour uptake,
hoever no hotter than :#2 or you ill start to seal the layer. (xperiment is the
key. 5ith the /ylon dyes , normally mix them up ith about a litre of arm
ater and use that. The dye mix can be used over and over again. <eep the dye
mix out of sunlight.
7oil the part in distilled ater for *# minutes to seal the surface. &ome of the dye
ill leak out into the ater before the surface is sealed, but its not too much of a
problem. ;ou might ant to hold the part in hot steam for a hile before you put
it in the ater. &tart the ater at about =:2 and bring it to a simmering boil over
the course of a fe minutes. ;ou can buy anodizing sealers to add to the ater,
but ,'ve not needed this. , have an unconfirmed suspicion that commerical
anodizing dyes need a special sealer.
8ive it a good rub ith a very soft hite cloth. &ometimes a get a bit of colour
coming off the sealed part, but this stops after a fe moments rubbing. , find a
good long boil reduces this problem.
Nitric Acid: The Chemical for Preparing
and Desmutting Aluminium
The Chemical formula for Nitric Acid
>itric 'cid is H>?
*
.
About Nitric Acid
>itric 'cid is really horrible stuff. ,n my opinion it is much nastier than sulfuric acid. The
bottles , get are a sort of light yello ee colour. ;ou unscre the cap, and a visible
fume of hite vapour comes out the top. >asty. The yello colour is due to the oxides of
nitrogen forming over time. -eally strong nitric acid 3over @A%4 is knon as fuming
nitric acid. This can either be hite fuming or red fuming depending on ho much
nitrogen dioxide is present.
>itric acid is used as a lab reagent, and more famously in the making of exposives like
T>T. ,t is also popular in making fertiliser, but that is far to dull to go into.
,'m alays careful ith this acid, and keep a lot of alkaline nearby for emergencies.
Where to buy Nitric Acid
>itric acid is not easy to buy. , use an online shop in the uk to buy bottles of B#%
solution. They send this stuff through the postC !adness, but , love it.
Nitric acid and aluminium anodising: Desmutting
The purpose of nitric acid in the aluminium anodising process is not immediately aparent.
>itric acid is used to prepare the surface of the aluminium prior to the anodising itself.
The first stage in preparing the aluminium for anodising is to clean off the existing oxide
layer in a bath of caustic soda. This is very exciting and smellyD lots of frothing and
steaming etc. 0roper chemistry. >ot of this mucking about ith pathetic bits of litmus
paper.
'fter the caustic soda bath, the aluminium is ashed. 't this point, depending on the
alloy of aluminium used, the surface appears to be covered in smut. 'luminium is not
normally used in the pure form. ,t is an alloy of many different metals. 'lthough the
caustic soda removes the aluminium and aluminium oxide, it does not remove the other
metals, and these remain on the surface.
(nter the >itric acid. 'll these other metals ill be dissolved by nitric acid.. but not the
aluminium. Therefore, after the caustic soda stage, the part is soaked in a bath of made of
half ater and half B#% nitric acid. This cleans up the part lovely.
'gain, the part is ashed in ater, and it is ready for anodising.
Some hints and tips on home aluminium
anodising Dye
This pages contains some small comments to help you on your path to home anodising -
6ust some small remarks that ,'ve noticed during my anodizing aluminium experiments.
The caustic soda phase is very critical. ' minute or so in the bath is required.
+eaving it a bit longer gives a very matt finish to the final aluminium partD 5hich
is a 8ood Thing 3T!4 if you are anodising an aluminium optical part.
The thing to remember ith the caustic soda bath is that it is dissolving the
aluminium. +eave it too long and it the part gets smaller. This is generally noticed
hen to closely fitting parts no longer closely fit. 'lso, after "# minutes in the
bath, tapped holes ill no longer hold their correct sized scres.
;ou can remove anodising ith a fe extra minutes in the caustic soda bath. ,
find that a mug of caustic soda granules in $ litres of hot ater ill strip off the
anodising in about : minutes. The caustic soda solution ends up looking very
yucky. 'lso see the point above.
'fter the caustic soda bath phase, it is very important to clean the part. 7ecause
aluminium is normally in an alloy ith lots of other metals, these metals ill smut
the surface. ;ou can buy de-smutting solution if you like. Hoever, , find a good
ash orks onders. 'fter the caustic soda bath, fill the sink ith hot ater ith
lots of ashing up liquid. 5ith rubber gloves on, scrub the part ith a toothbrush
until it is an nice even finish. /on't use a Egreen scrubby thingE or a 7rilo pad -
this ill scratch the surface 3hich you might ant, so hey.4. This scrubbing is
F(-; important to the final finish.
8etting a good electrical connection is vital. ,t is orth spending some time
making something out of aluminium that has a threaded section to scre into
tapped holes in your aluminium part. ,f you are anodising several things at once,
you'll find the one ith the poorest connection doesn't anodise ell
/on't use steel scres to hold the part in the acid - steel in the acid ill dissolve...
/on't use those pretty coloured aluminium bolts sold for bicycles either. These are
anodised... , supposed you could caustic soda them in the bath first and then use
them... , haven't tried.
'nodised aluminium does not conduct electricity ell. ,f you have used a bit of
scrap aluminium to hold a part in the acid bath, clean off the anodising ith a file
before you use it again.
, find about " hour in the acid bath gives a good anodized layer.
7e very very careful not to touch a freshly anodised part ith your fingers. The
grease ill ruin the final part. ;ou end up ith a nice anodised fingerprint on the
part.
,f you use steel bulldog)alligator clips to attach the poer to the acid bath, be sure
to dip them in the bicarb solution before you put them aay, other ise they ill
sloly dissolve.
7oil mercilessly. 7oiling for 9: minutes is not going to harm the part. This
ensures a good seal on the anodising.
,t doesn't matter if the part being anodised touchs the plastic sides of the acid
bath.
,t does matter if it touchs the lead cathode.
!ake the cathode as big as you reasonably can. 1old the lead around on itself
several times to get a good surface area.
'fter a fe sessions the acid bath ill have a lot of muck at the bottom. This
doesn't effect the anodising qualities.
,f you snap a hardened steel tap off in your 6ob, anodise it anyay. 5hen the
anodising is finished, you ill find the broken end of the tap that as embedded
in the aluminium part has dissolved. /on't do this too often otherise you'll end
up ith very mucky acid.
5ith larger parts, the acid bath ill fizz lots. ,t is best to do the anodising outside.
;ou are going to use a lot of distilled ater. 7attery topup ater can be purchased
in Halfords in gallon containers, but it is about * quid. This gets expensive. ,'ve
yet to figure out a ay of getting hold of lots cheaply. , suppose , could make my
on ater distiller.
/on't keep the caustic soda in the same box)draer)cupboard as the acid bath.
/o put a arning label on the box to say it contains nasty stuff.
/on't leave lead cathodes here animals are going to lick them.
&hutting your pet cat in the living room during anodising sessions is a4' really
good idea b4-eally pisses the cat off. This also applies to small children.
Anodizing aluminium manual 2
Some three years ago I took to wondering if it would not be practical for the home
workshop enthusiast to renew those faded or damaged anodised parts which
contribute so much to the good looks of a completed project and or new parts.
Since practical advice seems to be rather difficult to come by, I read a few books,
followed some eperimentation, achieved much discovery of an artful process, and
Success !!!
"he process itself, though chemically comple, is rather simple.
#irst it involves the transformation of the surface aluminium oide to
aluminium hydroide $anodise%, then to a hydroide monohydrate.
An interesting property of hydroide is its ability to absorb dyes into the
microscopic porosity&s of its surface. After impregnating, or dyed with a colour
medium, the surface is then 'sealed' into a monohydrate and the surface becomes
very hard and resistant to wear.
Method And Materials
"he process re(uires the use of either chromic or sulphuric acid in the
anodising electrolysis bath. I have limited my use to the sulphuric process
because of the ready availability of battery acid and the ability of the
process to absorb a wide variety of dye ."he acid used is ')2*+ S,lead
acid$, battery electrolyte obtainable wherever you buy new car batteries.
-ut this .+/.+ with distilled water to obtain the anodising solution.
01", Always wear eye protection and rubber gloves when working with acid.
-A2"I10 Always add the acid to the water never the reverse.
3istilled water is recommended because the use of ordinary tap water invariably
contains some minerals which will cause smutty deposits on the work and generally
not contribute to consistent results. "he acid anodising solution needs to be stored
and used in a suitably sized plastic, or glass, open ended container.
A lead strip cathode plate$s% is re(uired. "he lead plate$s% should be about twice
the surface area of the largest workpiece $anode% to be anodised.
I use a variable 3- power supply $2 to 4+ 5 surplus unit% which I find ideal but any
charged car battery will do the job. I find that most small parts re(uire around )+
5 to maintain the re(uired current density through the bath6 so )2 5 should
suffice for most work.
An ammeter reading from + to 4 A78S $for 2+ s(uare inch, maimum% Is a must,
as well as a heavy duty rheostat in series with the supply and the anodising tank.
"he resistance of the rheostat will, of course, vary with the size of the work
contemplated, but it can be calculated from the re(uired
current density of )9. to )*. milliamperes $ma% per s(uare inch of the anode
workpiece. "he workpiece surface area must be calculated In order to set the
anodising current. And, a surplus wirewound rheostat $variable resistor% of a few
hundred ohms will do.
:et&s assume that we want to anodise a propeller spinner, or flywheel, the surface
of which, is calculated to be 2.. s(. inches . "he anodising current density re(uired
will be;
7inimum; +.)9. < 2.. = +.4>2 Amps
7aimum; +.)*. < 2.. = +.94* Amps
So, the current must be between 4>2 and 94* ma. In anodising this part I would
maintain my adjustment at 9oo ma.
"he part to be anodised must be chemically clean. 0o effort must be spared
buffing and cleaning prior to complete degreasing in hot water using a strong
detergent. At this stage handle the part with rubber gloves or not at all.
2se only the aluminium contact strip fied to the part. ?inse well and you&re ready
to anodise.
"he anodising tank is set up as follows;
Process Notes
A good anodised coating thickness will be built up after 9+ minutes at the
calculated current density. @eep a running check on the current reading as this will
tend to vary during the process.
"oo low a current setting will result in a surface that will have difficulty
absorbing the colour dye.
"oo high a current setting will result in overheating the tank solution and a porous
finish which will leach out the dye during fiing.
A good anodised surface will have a slightly milky appearance when ready for
fiing6 or colour dyed and fied.
-opper , brass. or iron will contaminate the tank and degrade the process.
2se only lead or aluminium contact strips. I use wooden clothes pegs to set my
workpiece height in the tank.
#ew, if any, parts you make will be made from pure aluminium. 7ost will be made
from aluminium alloys which contain varying proportions of copper, manganese,
silicon, and sometimes, other elements in the mi. "hese alloys have an effect on
the ultimate colour shade obtainable with a given dye and process. If colour shade
repeatability is re(uired, the same alloy, process times, and temperatures must be
carefully duplicated. 8reviously anodised parts must first be stripAcleaned in a
strong Akali to remove all traces of prior anodising oides.
COLOUR DYES
1rganic dyes are usually used because of their great variety and depth of colours.
Industrial dyes can be obtained, but only in too large a (uantity for our purposes.
-oloured artist Inks are generally suitable, as are food colour
3yes with varying results. A yellow dye gives the 'gold' anodised look because of
the translucence of the anodic coating and the metallic reflection.
Some writing Inks are also suitable such as Skip or -arter, which gives a great
'black'.
"he trick is to find a colour with a pigment size small enough to enter the
microscopic anodic oide coating and be sealed there. ,perimentation and
patience are both recommended.
COLOUR PROCESS
3yes may be used hot, usually ).+ #, or at room temperature. "he dye and the
effect re(uired will determine the choice. I usually use mine at room temperature
and an immersion time of between one minute to ). minutes, depending on the
depth of shade re(uired. Agitation is re(uired. A 'coarse' dye will just accumulate
on the surface and will wash off during fiing.
FIXING PROCESS
#iing is done in plain old B21 $near boiling% for about 2+ minutes.
8referably, use distilled water to avoid those nasty mineral deposits
on our nice parts A temperature; 2++ #.
A certain amount of dye will leach out into the water before the surface seals. It
is best to avoid actively boiling water since this agitation will accelerate the colour
loss.
-hemical additives for the fiing bath are available, but I haven&t found any to
recommend.
"o keep colour loss at a minimum I have found that rotating the part in
steam for ten minutes before total immersion does a considerable job in reducing
leaching probably by closing the pores and sealing the dye before washing it out.
"he finished part is buffed with a clean cloth to remove any smutty deposits.
A little wa brings out the colour.
?eproduced from an article by ,d -o in Strictly I- Aug/Sept )CDD....2SA
8ublication
$0o -opyright infringement intended%
Vous aimerez peut-être aussi
- Anodizing InstructionsDocument15 pagesAnodizing InstructionsCarl J. Wilkey67% (3)
- LCD Anodizing 2020Document30 pagesLCD Anodizing 2020donny reborn100% (1)
- How To AnodizeDocument4 pagesHow To Anodizedannyjan5080Pas encore d'évaluation
- Nickel Electroplating: Power SupplyDocument6 pagesNickel Electroplating: Power Supplyyonathan fausaPas encore d'évaluation
- AnodizingDocument11 pagesAnodizingwcw111Pas encore d'évaluation
- How To Make Aluminum Powder Weekend Science Project PDFDocument5 pagesHow To Make Aluminum Powder Weekend Science Project PDFJohnny ChingasPas encore d'évaluation
- Aluminium AnodizingDocument14 pagesAluminium AnodizingMonique SamehtiniPas encore d'évaluation
- Metal Coloring and FinishingDocument48 pagesMetal Coloring and FinishingMikePas encore d'évaluation
- Rust Bluing TutorialDocument9 pagesRust Bluing TutorialStefan BadicaPas encore d'évaluation
- Material Finish GuideDocument7 pagesMaterial Finish GuideRomie CubalPas encore d'évaluation
- Low Current Density Anodizing Rev 0ADocument18 pagesLow Current Density Anodizing Rev 0AtootalldeanPas encore d'évaluation
- Diy Hard Anodizing Chemicals KitDocument7 pagesDiy Hard Anodizing Chemicals KitOtter1z100% (1)
- Anodize TitaniumDocument9 pagesAnodize TitaniumJuan Andrés Hdez SuárezPas encore d'évaluation
- Anodized Aluminum ColorsDocument10 pagesAnodized Aluminum ColorsIvy Li100% (1)
- Anodizing Basics: Mechanical & Chemical Surface TreatmentsDocument4 pagesAnodizing Basics: Mechanical & Chemical Surface TreatmentsSporkx100% (1)
- Electroplating:: How Electroplating Is DoneDocument4 pagesElectroplating:: How Electroplating Is DoneRakshaMahaPas encore d'évaluation
- ElectroplatingDocument18 pagesElectroplatingRahul Pandey100% (2)
- PVD Coatings PDFDocument18 pagesPVD Coatings PDFetamil87Pas encore d'évaluation
- Electroplating 101Document118 pagesElectroplating 101Mike NichlosPas encore d'évaluation
- NX 9 for Beginners - Part 5 (Sheet Metal Design)D'EverandNX 9 for Beginners - Part 5 (Sheet Metal Design)Évaluation : 5 sur 5 étoiles5/5 (1)
- Electro-Plating For The AmateurDocument114 pagesElectro-Plating For The AmateurmangyanPas encore d'évaluation
- Anodizing AluminumDocument7 pagesAnodizing AluminumyunitaparerPas encore d'évaluation
- Blackening Antiquing BrochureDocument8 pagesBlackening Antiquing BrochureMala PrasadPas encore d'évaluation
- Heat Treatment of MetalsDocument8 pagesHeat Treatment of MetalsDr. L. Bhanuprakash ReddyPas encore d'évaluation
- Aluminium Mig WeldingDocument5 pagesAluminium Mig WeldingManish SharmaPas encore d'évaluation
- Indestructible Corner Clamp (Jig) For Welding ProjectsDocument1 pageIndestructible Corner Clamp (Jig) For Welding ProjectsTomas Tom MlPas encore d'évaluation
- MIL-C-26074E Electroless Nickel CoatingsDocument11 pagesMIL-C-26074E Electroless Nickel CoatingsAbdelhamied ElkadyPas encore d'évaluation
- Chrome Plating EngDocument24 pagesChrome Plating EngNikita OkochaPas encore d'évaluation
- Zinc Nickel Electroplating Guide ZyliteDocument9 pagesZinc Nickel Electroplating Guide ZyliteBryan DixPas encore d'évaluation
- Foundry ToolsDocument5 pagesFoundry ToolsChristian VicentePas encore d'évaluation
- Chrome PlatingDocument14 pagesChrome Platingsonnu151Pas encore d'évaluation
- Drop Forging, Die Sinking and Machine Forming of Steel - Modern Shop Practice, Processes, Methods, Machines, Tools and DetailsD'EverandDrop Forging, Die Sinking and Machine Forming of Steel - Modern Shop Practice, Processes, Methods, Machines, Tools and DetailsÉvaluation : 5 sur 5 étoiles5/5 (1)
- AnodizingDocument57 pagesAnodizingชนพัทธ์ คงพ่วงPas encore d'évaluation
- Dog Tag/ Key ChainDocument13 pagesDog Tag/ Key ChainAidan O'HaraPas encore d'évaluation
- Chrome PlatingDocument11 pagesChrome PlatingMahesh Babu100% (1)
- Feedstock Technology for Reactive Metal Injection Molding: Process, Design, and ApplicationD'EverandFeedstock Technology for Reactive Metal Injection Molding: Process, Design, and ApplicationPas encore d'évaluation
- Aluminum Anodizing ProcessDocument31 pagesAluminum Anodizing ProcessSenthil Kumar100% (2)
- Electroplating Copper and NickelDocument9 pagesElectroplating Copper and NickelJuan Fernando DíezPas encore d'évaluation
- ANKURDocument20 pagesANKURDevashish JoshiPas encore d'évaluation
- Blackening Processes For ZincDocument13 pagesBlackening Processes For Zincvasudev_nPas encore d'évaluation
- The Art of Casting in Iron: How to Make Appliances, Chains, and Statues and Repair Broken Castings the Old-Fashioned WayD'EverandThe Art of Casting in Iron: How to Make Appliances, Chains, and Statues and Repair Broken Castings the Old-Fashioned WayÉvaluation : 5 sur 5 étoiles5/5 (1)
- ElectroplatingDocument23 pagesElectroplatingJayne Kazandra P. Ortega67% (3)
- Electroplating in The Home WorkshopDocument21 pagesElectroplating in The Home WorkshopBhalchandra Rupe0% (1)
- Titanium Anodizing: An in House Evaluation by METALAST International, IncDocument6 pagesTitanium Anodizing: An in House Evaluation by METALAST International, IncGian GianPas encore d'évaluation
- Recovery of Silver From Industrial Wastes Cassava Solution EffectsDocument4 pagesRecovery of Silver From Industrial Wastes Cassava Solution EffectsTanawat JansengPas encore d'évaluation
- Metal Casting Basics Book 1Document57 pagesMetal Casting Basics Book 1browar444100% (1)
- Electroplating in The Home WorkshopDocument21 pagesElectroplating in The Home WorkshopcdslicPas encore d'évaluation
- 06 - Sheet-Metal Forming PDFDocument52 pages06 - Sheet-Metal Forming PDFmandakini baskeyPas encore d'évaluation
- Faculdade Estadual de Engenharia Química de Lorena - FAENQUILDocument56 pagesFaculdade Estadual de Engenharia Química de Lorena - FAENQUILLuis Gustavo PachecoPas encore d'évaluation
- Metalcastingprocess 110925103638 Phpapp02 PDFDocument51 pagesMetalcastingprocess 110925103638 Phpapp02 PDFramesh tPas encore d'évaluation
- Retail: Idealpos ForDocument20 pagesRetail: Idealpos ForpalosotPas encore d'évaluation
- Read MeDocument1 pageRead MepalosotPas encore d'évaluation
- Manual Charge 2 en USDocument43 pagesManual Charge 2 en UStomPas encore d'évaluation
- Installation Guide V3.20Document2 pagesInstallation Guide V3.20palosotPas encore d'évaluation
- Lumia Nokia User ManualDocument128 pagesLumia Nokia User ManualpalosotPas encore d'évaluation
- Configuring YAMon 2Document3 pagesConfiguring YAMon 2palosotPas encore d'évaluation
- Newsletter AUGUST 2016 2nd QDocument3 pagesNewsletter AUGUST 2016 2nd QpalosotPas encore d'évaluation
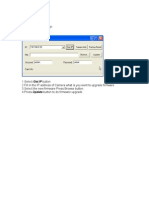
- Ucamupdate Firmware Upgrade Step: Run Ucamupdate - ExeDocument1 pageUcamupdate Firmware Upgrade Step: Run Ucamupdate - ExepalosotPas encore d'évaluation
- PLC Quick Installation GuideDocument6 pagesPLC Quick Installation GuidepalosotPas encore d'évaluation
- Configuring YAMon 2Document3 pagesConfiguring YAMon 2palosotPas encore d'évaluation
- School WebpageDocument16 pagesSchool WebpagepalosotPas encore d'évaluation
- Open School Installation GuideDocument16 pagesOpen School Installation GuidepalosotPas encore d'évaluation
- Forceware Graphics Driver: User'S GuideDocument222 pagesForceware Graphics Driver: User'S GuideOscar MirandaPas encore d'évaluation
- LinkageDocument44 pagesLinkagepalosotPas encore d'évaluation
- 01-09-15 Mac ListDocument4 pages01-09-15 Mac ListpalosotPas encore d'évaluation
- ReadmeDocument1 pageReadmeMilos MaTa MaticPas encore d'évaluation
- Nokia 3G Requirements For Installation and OperationDocument54 pagesNokia 3G Requirements For Installation and OperationpalosotPas encore d'évaluation
- Trace Mac AddressDocument8 pagesTrace Mac AddresspalosotPas encore d'évaluation
- Unlocking E1552 & Other Huawei 3g Modems ManualDocument8 pagesUnlocking E1552 & Other Huawei 3g Modems ManualpalosotPas encore d'évaluation
- PMP 320 Hardware Installation E2 0Document139 pagesPMP 320 Hardware Installation E2 0palosotPas encore d'évaluation
- PMP 320 Hardware Installation E2 0Document139 pagesPMP 320 Hardware Installation E2 0palosotPas encore d'évaluation
- Game InfoDocument2 pagesGame InfoBranislav SimsicPas encore d'évaluation
- Nokia 3G Requirements For Installation and OperationDocument54 pagesNokia 3G Requirements For Installation and OperationpalosotPas encore d'évaluation
- Change Log 1255Document1 pageChange Log 1255Harsha VardhanPas encore d'évaluation
- Yamaha Crypton Parts ManualDocument59 pagesYamaha Crypton Parts Manualsouheil_sou80% (5)
- Nokia 3G Requirements For Installation and OperationDocument54 pagesNokia 3G Requirements For Installation and OperationpalosotPas encore d'évaluation
- Schedule (20140904 1529)Document19 pagesSchedule (20140904 1529)palosotPas encore d'évaluation
- Network Update Ron Line HelpDocument118 pagesNetwork Update Ron Line HelppalosotPas encore d'évaluation
- Nokia 3G Requirements For Installation and OperationDocument54 pagesNokia 3G Requirements For Installation and OperationpalosotPas encore d'évaluation
- User Information 201408291332Document1 pageUser Information 201408291332palosotPas encore d'évaluation
- Half Life and Nuclear ProblemsDocument14 pagesHalf Life and Nuclear Problemsshabbir626Pas encore d'évaluation
- Physical Properties of Metals and Non-MetalsDocument2 pagesPhysical Properties of Metals and Non-MetalsShirley DenicePas encore d'évaluation
- Latihan Menjawab Kertas 3 1 A Student Carried Out An Experiment To Determine The Empirical Formula ofDocument5 pagesLatihan Menjawab Kertas 3 1 A Student Carried Out An Experiment To Determine The Empirical Formula ofMasitah Abu BakarPas encore d'évaluation
- Limiting Reactants RevisedDocument12 pagesLimiting Reactants Revisedmaryelle conejarPas encore d'évaluation
- The Complete Aqueous Hydrochloric Acid Solutions Density-Concentration CalculatorDocument2 pagesThe Complete Aqueous Hydrochloric Acid Solutions Density-Concentration CalculatorEngr. Md. Tipu SultanPas encore d'évaluation
- Unit 5 Organic ReactionsDocument2 pagesUnit 5 Organic ReactionsmcwwfcPas encore d'évaluation
- The Phosphorous Myth ExposedDocument24 pagesThe Phosphorous Myth ExposedAdvanced Nutrients100% (1)
- Din 17007Document8 pagesDin 17007Aditya Pratap100% (1)
- Minor09 QP DLP NEET2019 (Pmtcorner - In)Document33 pagesMinor09 QP DLP NEET2019 (Pmtcorner - In)jay danenjeyanPas encore d'évaluation
- ICSE Selina Solution For Class 9 Chemistry Chapter 5 Exercise QuestionsDocument15 pagesICSE Selina Solution For Class 9 Chemistry Chapter 5 Exercise QuestionsAnubrata SarkarPas encore d'évaluation
- Experiment 4. Water: Its Properties and PurificationDocument7 pagesExperiment 4. Water: Its Properties and PurificationOri SeinPas encore d'évaluation
- Electricity & Chemistry 1 MSDocument7 pagesElectricity & Chemistry 1 MSTanakaPas encore d'évaluation
- Terjemahan PatentDocument2 pagesTerjemahan Patentteknik kimiaPas encore d'évaluation
- June 2020 (9-1) (v1) QP - Paper 6 CIE Chemistry IGCSEDocument8 pagesJune 2020 (9-1) (v1) QP - Paper 6 CIE Chemistry IGCSENatalie RossettePas encore d'évaluation
- Chemistry Tricks and MagicDocument6 pagesChemistry Tricks and MagicDaniel Baylosis AsongPas encore d'évaluation
- ElectrochemistryDocument14 pagesElectrochemistryWillie WeiPas encore d'évaluation
- Chemical Reactions and Uses of AmmoniaDocument4 pagesChemical Reactions and Uses of AmmoniaReckless RiderPas encore d'évaluation
- April 16, 1968 L, 4, Derham Et Al 3,378,338: Production of High-Purity Aluminium ChlorideDocument4 pagesApril 16, 1968 L, 4, Derham Et Al 3,378,338: Production of High-Purity Aluminium ChlorideDimas MitraPas encore d'évaluation
- Ciclo de Born HaberDocument8 pagesCiclo de Born HaberJanaina LeitinhoPas encore d'évaluation
- Stoichiometry SL & HL (Core)Document6 pagesStoichiometry SL & HL (Core)Zuu3a Lauren ϟPas encore d'évaluation
- Color Code ChartDocument15 pagesColor Code ChartYel DG0% (1)
- Chapter - 11 - Practice - Test - CHEMICAL REACTIONDocument6 pagesChapter - 11 - Practice - Test - CHEMICAL REACTIONLourdesCorpusMendoza100% (1)
- Halogen and Noble PDFDocument33 pagesHalogen and Noble PDFPrabhakar BandaruPas encore d'évaluation
- Alchemists ConcordanceDocument108 pagesAlchemists Concordanceroger santosPas encore d'évaluation
- Tool Steels E28093 Molybdenum High Speed SteelsDocument5 pagesTool Steels E28093 Molybdenum High Speed Steelswulfgang66Pas encore d'évaluation
- C067Document49 pagesC067Gato Sesa0% (1)
- CBSE Class 10 Science MCQ Chapter 1 Chemical Reactions and EquationsDocument6 pagesCBSE Class 10 Science MCQ Chapter 1 Chemical Reactions and EquationsKarl MarxPas encore d'évaluation
- Chemical Res Chart 09 12bombalittlegiantDocument20 pagesChemical Res Chart 09 12bombalittlegiantjuanmanuel56Pas encore d'évaluation
- 304 DDQ SpecificationDocument1 page304 DDQ Specificationdac_angelovPas encore d'évaluation
- 11th Chemistry Practical Notes Analysis of 15 Simple Salts Xi STDDocument38 pages11th Chemistry Practical Notes Analysis of 15 Simple Salts Xi STDPratheeksha shalbinPas encore d'évaluation