Académique Documents
Professionnel Documents
Culture Documents
Thermal Decomposition of MgO Nanoparticles
Transféré par
Ivy JoyceTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Thermal Decomposition of MgO Nanoparticles
Transféré par
Ivy JoyceDroits d'auteur :
Formats disponibles
Magnesium oxide nanocrystals via thermal decomposition
of magnesium oxalate
Fatemeh Mohandes
a
, Fatemeh Davar
b
, Masoud Salavati-Niasari
a,b,n
a
Department of Inorganic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, P.O. Box. 87317-51167, Islamic Repubic of Iran
b
Institute of Nano Science and NanoTechnology, University of Kashan, Kashan, P.O. Box. 87317-51167, Islamic Republic of Iran
a r t i c l e i n f o
Article history:
Received 21 February 2009
Received in revised form
22 August 2010
Accepted 29 August 2010
Keywords:
A. Nanostructures
A. Magnetic materials
B. Chemical synthesis
a b s t r a c t
The present work reports the synthesis of magnesium oxide (MgO) nanocrystals via a thermal
decomposition route and the study of physicochemical properties of products. The MgO nanocrystals
were prepared from magnesium oxalate powders as precursor. Transmission electron microscopy
(TEM) analysis demonstrated MgO nanocrystals with an average diameter of about 2025 nm. The
products were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM),
selected area electronic diffraction (SAED), and Fourier transform infrared (FT-IR) spectroscopy. Optical
absorption and photoluminescence emission properties of MgO nanocrystals were investigated.
& 2010 Elsevier Ltd. All rights reserved.
1. Introduction
Over the past few decades many advances have been made in
the area of preparation of nanomaterials. Nanotechnology has
made nanocrystalline materials become an area of intense
research activity [14]. Nanocrystalline materials are polycrystal-
line materials with grain size below 100 nm [5,6]. The change in
the crystalline size and shape will alter the properties, which were
formerly thought to be constant for a given material. Nanocrystals
of common metal oxides have been shown to be highly efcient
and active adsorbents for many toxic chemicals, including air
pollutants, and chemical warfare agents [7]. Magnesium oxide
(MgO, periclase), as an exceptionally important material for using
in catalysis [8,9], toxic waste remediation, or as additives in
refractory, paint, and superconductor products [10] has been
attracting both fundamental and application studies [11].
Many different synthetic routes provide nanoscale MgO
including solgel [12], hydrothermal/solvothermal [13,14], laser
vaporization [15], chemical gas phase deposition [16], aqueous
wet chemical [17], surfactant methods [18], polyol-mediated
thermolysis process [19], and microwave-assisted method [20].
One method to prepare nanosized MgO is the thermal
decomposition. Aramendia et al. [21] prepared MgO via decom-
position of magnesium hydroxide. Stark and Klabunde synthe-
sized MgO from magnesium carbonate by thermal decomposition
[22]. Novaro and coworkers have prepared nanocrystalline MgO
by thermal decomposition of brucite (Mg(OH)
2
) at different
temperature. When the calcination temperature is increased from
540 to 773 K, brucite decomposes to form periclase, from needle
shape morphology (hexagonal structure) to small crystallites with
the cubic structure [23].
In our group, for a few years we have been interested in the
synthesis of metal, metal oxide, metal suldes, and magnetic
nanoparticles using new inorganic precursors [2428]. Using the
novel compounds can be useful and opens a new way for
preparing nanomaterials to control shape, crystallinity, and size
distribution [29]. Herein, we report the facile synthesis of MgO
nanocrystals via thermolysis of magnesium oxalate
[Mg(O
4
C
2
) 2H
2
O] in presence oleylamine and triphenylpho-
sphine (TPP). To the best of our knowledge, this is the rst report
on the synthesis of magnesium oxide nanocrystals with this
method. In this process, oleylamine was used as both the medium
stabilizing reagent.
2. Experimental
Materials. All the chemicals in our experiments were used as
received without further purication with analytical grade.
Magnesium oxalate [Mg(O
4
C
2
) 2H
2
O], precursor was synthesized
according to this procedure: the magnesium acetate tetrahydrate
(CH
3
COO)
2
Mg 4H
2
O (2 mmol) was dissolved into 10 mL of
distilled water to form a homogeneous solution. A stoichiometric
amount of potassium oxalate was dissolved in an equal volume of
distilled water was dropwise added into the above solution under
Contents lists available at ScienceDirect
journal homepage: www.elsevier.com/locate/jpcs
Journal of Physics and Chemistry of Solids
0022-3697/$ - see front matter & 2010 Elsevier Ltd. All rights reserved.
doi:10.1016/j.jpcs.2010.08.014
n
Corresponding author at: Department of Inorganic Chemistry, Faculty of
Chemistry, University of Kashan, Kashan, P.O. Box. 87317-51167, Islamic Republic
of Iran. Tel.: +98 361 5555333; fax: +98 361 5552935.
E-mail address: salavati@kashanu.ac.ir (M. Salavati-Niasari).
Journal of Physics and Chemistry of Solids 71 (2010) 16231628
magnetic stirring. The solution was stirred for about 15 min and
white precipitate was isolated, washed with ethanol several times
and dried at 50 1C. The magnesium oxalate [Mg(O
4
C
2
) 2H
2
O],
was characterized by elemental analysis, FT-IR, powder X-ray
diffraction (XRD), and thermogravimetric analysis (TGA). Anal.
Calcd. for [Mg(O
4
C
2
) 2H
2
O]: C, 16.19; Mg, 16.38. Found: C, 16.10;
Mg, 15.68%.
Characterization. XRD patterns were recorded by a Rigaku
D-max C III, X-ray diffractometer using Ni-ltered Cu Ka
radiation. Elemental analyses were obtained from Carlo ERBA
Model EA 1108 analyzer. Transmission electron microscopy (TEM)
images and selected area electronic diffraction (SAED) pattern
were obtained on a Philips EM208 transmission electron micro-
scope with an accelerating voltage of 200 kV. Fourier transform
infrared (FT-IR) spectra were recorded on Shimadzu Varian 4300
spectrophotometer in KBr pellets. The UV diffuse reectance
spectra were acquired using a PerkinElmer Lambda 15 spectro-
photometer equipped with an integrating sphere and converted
to absorption spectra via the KubelkaMunk transform procedure.
A pulsed Xe discharge lamp served as excitation light source
in a PerkinElmer LS 50B system for photoluminescence mea-
surements. Thermogravimetric analysis (TGA) was carried out
using a thermal gravimetric analysis instrument (Shimadzu TGA-
50 H) with a ow rate of 20.0 mLmin
1
and a heating rate of
10 1C min
1
.
Synthesis of MgO nanocrystals. The current synthetic procedure
is a modied version of the method developed by Hyeon and
others for the synthesis of nanocrystals for metals that employs
the thermal decomposition of transition metal complexes
[3032]. The synthetic pathway is shown in Scheme 1. In this
synthetic procedure, MgO nanocrystals were prepared by the
thermal decomposition of magnesium(oxalate)oleylamine,
[Mg(O
4
C
2
)]oleylamine complex as precursor. First, the
[Mg(O
4
C
2
)]oleylamine complex was prepared by reaction of
0.5 g of [Mg(O
4
C
2
) 2H
2
O] and 5 mL oleylamine. The mixed
solution was placed in a 50 mL three-neck distillation ask and
heated up to 145 1C for 120 min. The resultant metal-complex
solution was injected into 5 g of triphenylphosphine (TPP) at
240 1C. The color of solution was changed from white to dark,
which indicates colloidal products were generated. The black
solution was aged at 240 1C for 90 min and then cooled to room-
temperature. The white nanonparticles products were precipi-
tated by adding excess ethanol to the solution. The nal products
were washed with ethanol for at least three times to remove
impurities and dried at 100 1C. These products could easily be re-
dispersed in nonpolar organic solvents, such as hexane or toluene
(Scheme 1). The synthesized nanocrystals were characterized by
XRD, TEM, FT-IR, UV, and PL techniques.
3. Results and discussion
The TGA curve of the as-prepared precursor is shown in Fig. 1.
It can be seen that there are two distinct weight loss steps. The
rst weight loss occurs at 110170 1C, which corresponds to the
evaporation of crystallization water. According this result, it is
found that magnesium oxalate contains two crystallization water
molecules. The second weight loss step occurs at the temperature
range 270380 1C. The weight loss at 270380 1C may be ascribed
to the decomposition of the oxalate. The weight loss of two steps
is about 17.60% and 40.45% which is close to the theoretical value.
Fig. 2(a) and (b) shows the XRD patterns of magnesium oxalate
and as-synthesized MgO. In Fig. 2(a), all the reections in the
pattern could be clearly indexed on the basis of a monoclinic cell
reported for magnesium oxalate (JCPDS no. 25-1029) which
indicates the pure magnesium oxalate precursor was obtained.
All of the reections of the XRD pattern in Fig. 2(b) can be indexed
to the standard pattern of the pure cubic phase of MgO (space
group: Fm3m (2 2 5)) with crystalline cell constant a4.19
A
which is in good agreement with the reported data (JCPDS no.
75-1525). With this result, we came to know that the MgO
samples have a single-phase cubic structure. The strong intensity
related to the background signal indicates the high purity of MgO
cubic phase of the resultant product. From XRD data, the
crystallite size (D
c
) of as-prepared MgO particles was calculated
Scheme 1. Schematic diagram illustrating the formation of MgO nanocrystals. .
Fig. 1. TGA of the magnesium oxalate precursor.
F. Mohandes et al. / Journal of Physics and Chemistry of Solids 71 (2010) 16231628 1624
using the DebeyScherrer equation [33]
D
c
Kl
bcosy
1
where b is the breadth of the observed diffraction line at its half-
intensity maximum, K the so-called shape factor, which usually
takes a value of about 0.9 [34], and l the wavelength of X-ray
source used in XRD. The estimated particle size of MgO using the
DebeyScherrer equation is 25 nm.
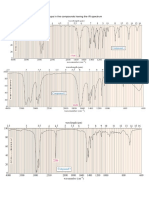
FT-IR spectroscopy is a useful tool to understand the functional
group of any organic molecule. The infrared spectra of free TPP,
oleylamine , magnesium oxalate, and MgO nanocrystals coated
with oleylamine and TPP are shown in Fig. 3. By comparing the
infrared spectrum of the nanoparticles to free surfactant one in
Fig. 3, we can see that the organic molecules have indeed become
a part of the nanoparticles. As shown in Fig. 3(a) and (b) peaks
corresponding to Pph stretching at 1710 cm
1
, rocking and
bending mode of the methylene group o(CH
2
) at 1270 cm
1
,
bending mode of the phenyl group g(CH) at 742 cm
1
and u
(benzene ring) at 697 cm
1
can clearly be seen. The only
difference among these characteristic peaks is either the peak
intensity or a slight shift in the peak position. For example, the
peak position of the longitudinal modes of oleylamine and TPP
shifts to lower wavenumbers after TPP and oleylamine are
adsorbed on the surface of the MgO nanoparticles. The FT-IR of
the MgO nanocrystals shows the intense adsorption peaks at 2920
and 2850 cm
1
attributed to the alkyl chains of oleylamine, which
indicates oleylamine is bound on the surface through unpaired
electron couple of the amine group [35,36]. The peaks at 3426 cm
1
has been assigned to the EtOH used in the separation step. From
these results, a chemical bond can be formed between N and Mg
atoms and a coordinate bond between P and Mg atoms on the
surface of the nanocrystals. Thus, the phenyl stretching mode
u(Pph) at 1430 cm
1
is still visible in Fig. 3(c) so that, the
oleylamine and TPP act as capping ligands that control the growth
of particles. The phenyl groups in TPP can provide greater steric
hindrance than straight-chained alkyl groups such as tributyl and
trioctyl to control the size of nonmagnetic metal and metal oxide
nanoparticles and to stabilize efciently the magnetic nanopar-
ticles. In Fig. 3(d) double absorption peaks appeared at 1667 and
1623 cm
1
are attributed to symmetric and asymmetric vibrations
of the carbonyl group which indicate the existence of the
[Mg(O
4
C
2
) 2H
2
O] compound. In pure potassium oxalate, only
one band at 1700 cm
1
can be observed but when oxalate
coordinates to metal ions, this absorption band will shift toward
Fig. 2. XRD patterns of the obtained (a) magnesium oxalate precursor and (b) MgO
nanocrystals.
Fig. 3. FT-IR spectra of (a) TPP, (b) oleylamine, (c) MgO nanocrystals, and (d)
magnesium oxalate precursor.
F. Mohandes et al. / Journal of Physics and Chemistry of Solids 71 (2010) 16231628 1625
low frequency region and splits into two bands, giving rise to
some structure vacancies [22]. As shown in Fig. 3(c), there is no
evidence for the existence of free precursor ([Mg(O
4
C
2
)2H
2
O]) in
the MgO nanocrystals because the stretching vibrations of
CO(u
CO
) and CO(u
CO
) oxalate have been disappeared. Hence
the oleylamine serves as the capping ligand that controls growth
[37,38]. Two new peaks are shown at 443 and 528 cm
1
in
spectrum (Fig. 3c). These peaks were undoubtedly assigned to
MgO stretching as was reported in the literature [39].
The mechanism of growth could well be understood on the
basis of the following reactions and the crystal habits of MgO. In
the presence of oleylamine as solvent, [Mg(O
4
C
2
)]oleylamine are
synthesized. After addition of TPP to these solutions at high
temperature, [Mg(O
4
C
2
)]oleylamine will be decomposed. TPP is
a high-boiling point surfactant with a patulous long-chain
structure providing greater steric hindrance. The addition of TPP
into the mixture of oleylamine and complex as an additional
surfactant reduces the particle size further more and provides
nanocrystals with very thin arrays. Oleylamine is known as a
ligand that binds tightly to the metal nanoparticles surface. The
combined effects of TPP and oleylamine were much more
profound than those of individual contributions. On the other
hand, these complexes transform to MgO. When the triphenyl-
phopsphine (TPP) was used as the complexing agent, the
circumference of metallic nuclei was capped by this surfactant,
leading to the decrease in surface energy of some crystallographic
faces. The growth of the MgO nanocrystals can be explained on
the basis of the schematic view presented in Scheme 1. The MgO
nanocrystals agglomerated together to form a hexagonal nucleus.
In order to observe the detailed morphology and particle size
of the MgO, the TEM and SAED images of as-synthesized MgO
nanocrystals were recorded and are seen in Fig. 4. The images
indicate that MgO powders consist of nanometric particles with
diameter from 20 to 25 nm which is agreeable to determined
particle size by the DebeyScherrer equation. The expected shape
factor K of the average crystallite in the present case is considered
to be 0. 9 due to the presence of spherical grains of the nal
product. It is seen that the crystallite size calculated from TEM
micrographs is smaller than the calculated one using Scherrers
equation, due to the obvious fact that all the errors were not
eliminated while calculating the crystallite size. Since powder
samples were used to obtain the XRD pattern, the broadening due
to mechanical strain and instrument errors were ignored too.
For UV diffuse reectance and photoluminescence measure-
ments the sample material was transferred into quartz glass cells
which provide a vacuum better than po10
6
mbar. In the UV
diffuse reectance spectrum of MgO nanocrystals (Fig. 5a),
absorption bands at 230 nm (5.4 eV) and 260 nm (4.7 eV) were
identied with the excitation of 4-fold and 3-fold coordinated
O
2
anions in edges and corners, respectively [40]. On pure MgO,
the optical excitation of 5-fold coordinated O
2
anions in (1 0 0)
planes lies at 185 nm (6.6 eV), i.e., below the wavelength
accessible in nonevacuated spectrometers (l4200 nm). Fig. 4. (a and b) TEM images and (c) SAED pattern of MgO nanocrystals.
Fig. 5. Room-temperature (a) DR-UVvis spectra and (b) photoluminescence spectrum of MgO nanocrystals.
F. Mohandes et al. / Journal of Physics and Chemistry of Solids 71 (2010) 16231628 1626
Although MgO is a typical wide bandgap insulator, the PL
properties of its nanocrystals have been studied because of the
presence of defects [14,41]. The PL spectrum of the MgO
nanocrystals is shown in Fig. 5(b). The excitation wavelength
was 290 nm. A little emission peak at around 370 nm can be
observed in the PL spectrum. Clearly, the PL peak is not the
band gap emission, and can be ascribed to crystal defects or
defect levels associated with oxygen vacancies that have been
formed during the production process, which is similar to the
literatures [42].
For further investigation, the effects of oleylamine and TPP on
particle size and morphology of MgO were studied. Therfore, the
magnesium oxide nanocrystals have been prepared under
different condition (Table 1). By controlling the quality of
nanocrystals, MgO with various size and morphology can be
formed. [Mg(O
4
C
2
)2H
2
O] (sample 1) was calcined at 800 1C for 4 h
under nitrogen atmosphere. In this case, spherical MgO nanopar-
ticles with irregular particle size were obtained under rather
difcult conditions with particle size about 60 nm (see Fig. 6a).
We decided to use a suitable solvent for the preparation of
magnesium oxides with the smallest particle size under mild
conditions. Therefore, 0.5 g of [Mg(O
4
C
2
) 2H
2
O] and 5 mL of
oleylamine, (sample 2), were mixed and heated at 240 1C for
90 min. After thermal treatment reaction, sheet-like MgO nano-
crystals were prepared with particle size 52 nm (see Fig. 6b). The
MgO nanocrystals of sample 2 are very regular in size and shape.
According to these images, it is found that oleylamine has an
effective role on particle size and morphology because the
nitrogen atoms of oleylamine provide an active surface which
protects magnesium ions against agglomeration. After all, we
decided to test the effect of oleylamine in the presence TPP on
MgO nanocrystals. Final, MgO nanocrystals (sample 3), were
synthesized according to the procedure mentioned in experi-
mental section. The SEM image of MgO synthesized according to
the procedure mentioned in experimental section is shown in
Fig. 6(c). The plate-like materials contain nanoparticle MgO with
the smallest particle size (25 nm by XRD and 2025 nm by TEM)
were obtained. Therefore, using both oleylamine and TPP as
capping agent, we can reach desired nanostructures with reason-
able particle size and shape. Therefore, it is better that we prot
the particle size and shap of MgO naocrystals using amine and
phosphine agents each other. MgO synthesized according to the
procedure mentioned in experimental section (sample 3) was
introduced as optimum nanosized magnesium oxides.
4. Conclusion
In summary, we have demonstrated the synthesis of MgO
nanocrystals from the magnesium oxalate powder via a thermal
decomposition process. The as-synthesized product is highly
crystallized with cubic structure. The crystallite size of the
nanocrystals can be estimated between 2025 nm. The experi-
mental results show that the use of oleylamine and TPP as solvent
and capping agent in the chemical process play an important role
Table 1
Preparation of MgO under different conditions.
Sample Capping agent Temperature (1C) Time Particles size (according to XRD) (nm)
1 800 4 h 60
2 Oleylamine 240 90 min 52
3 Oleylamine+TPP 240 90 min 25
Fig. 6. SEM images of (a) Sample 1, (b) Sample 2, and (c) Sample 3.
F. Mohandes et al. / Journal of Physics and Chemistry of Solids 71 (2010) 16231628 1627
in the formation of nal products. This method is known as an
easy reproducible process for the large-scale synthesis of MgO
nanocrystals. We expect that it will be a suitable route for
preparation of other metal oxides with various nanostructures.
Acknowledgement
The authors are grateful to Council of University of Kashan for
providing nancial support to undertake this work.
References
[1] J.H. Fendler, in: Nanoparticles and Nanostructured Films Preparation,
Characterization and Application, WileyVCH, Weinheim, 1994.
[2] T. Trindade, P. OBrien, N.L. Pickett, Chem. Mater. 13 (2001) 38433858.
[3] C.N.R. Rao, G.U. Kulkarni, P.J. Thomas, P.P. Edwards, Chem. Eur. J. 8 (2002)
2935.
[4] M. Green, Curr. Opin. Solid State Mater. Sci. 6 (2002) 353361.
[5] M. Salavati-Niasari, Z. Fereshteh, F. Davar, Polyhedron 28 (2009) 126130.
[6] A. Ahniyaa, G.A. Seisenbaeva, L. H aggstr om, S. Kamali, V.G. Kessler, P
Nordblad, C. Johansson, L. Bergstr om, J. Magn. Magn. Mater. 320 (2008)
781787.
[7] G.W. Wagner, P.W. Bartram, O. Koper, K.J. Klabunde, J. Phys. Chem. B 103
(1999) 32253228.
[8] S.H.C. Liang, I.D. Gay, J. Catal. 101 (1986) 293300.
[9] H. Tsuji, F. Yagi, H. Hattori, H. Kita, J. Catal. 148 (1994) 759770.
[10] Y.S. Yuan, M.S. Wong, S.S. Wong, J. Mater. Res. 11 (1996) 817.
[11] M. Sterrer, O. Diwald, E. Knozinger, J. Phys. Chem. B 104 (2000) 36013607.
[12] H. Fang, B. Hu, L. Wang, R. Lu, C. Yang, Front. Chem. China 3 (2008) 193197.
[13] Y. Ding, G. Zhang, H. Wu, B. Hai, L. Wang, Y. Qian, Chem. Mater. 13 (2001)
435440.
[14] H. Niu, Q. Yang, K. Tang, Y. Xie, J. Nanoparticle Res. 8 (2006) 881888.
[15] M. El-Shall, W. Slack, W. Vann, D. Kane, D. Hanley, J. Phys. Chem. 98 (1998)
30673070.
[16] J.S. Matthews, O. Just, B. Obi-Johnson, W.S. Rees Jr., Chem. Vapour Deposition
6 (2000) 129132.
[17] A. Bhargava, J. Alarco, I. Mackinnon, D. Page, A. Ilyushechkin, Mater. Lett. 34
(1998) 133142.
[18] F. Khairallah, A. Glisenti, J. Mol. Catal. Chem. 274 (2007) 137147.
[19] A. Subramania, G.V. Kumar, A.R.S. Priya, T. Vasudevan, Nanotechnology 18
(2007) 225601225605.
[20] S. Makhluf, R. Dror, Y. Nitzan, Y. Abramovich, R. Jelinek, A. Gedanken, Adv.
Funct. Mater. 15 (2005) 17081715.
[21] M.A. Aramendia, V. Borau, C. Jimenez, J.M. Marinas, A. Porras, F.J. Urbano,
J. Mater. Chem. 6 (1996) 19431949.
[22] J.V. Stark, K. Klaunde, J. Chem. Mater. 8 (1996) 19131918.
[23] J.A. Wang, O. Novaro, X. Bokhimi, T. Lo pez, R. Go mez, J. Navarrete, M.E. Llanos,
E. Lo pez-Salinas, Mater. Lett. 35 (1998) 317323.
[24] M. Salavati-Niasari, F. Davar, Mater. Lett. 63 (2009) 441443.
[25] M. Salavati-Niasari, F. Davar, M.R. Loghman-Estarki, J. Alloys Compd. 494
(2010) 199204.
[26] F. Davar, M. Salavati-Niasari, Z. Fereshteh, J. Alloys Compd. 496 (2010)
638643.
[27] F. Davar, Z. Fereshteh, M. Salavati-Niasari, J. Alloys Compd. 476 (2009)
797801.
[28] M. Salavati-Niasari, N. Mir, F. Davar, J. Alloys Compd. 476 (2009)
908912.
[29] S. Baskoutas, P. Giabouranis, S.N. Yannopoulos, V. Dracopoulos, L. Toth, A.
Chrissanthopoulos, N. Bouropoulos, Thin Solid Films 515 (2007)
84618464.
[30] T. Hyeon, S.S. Lee, J. Park, Y. Chung, H.B. Na, J. Am. Chem. Soc. 123 (2001)
1279812801.
[31] F. Davar, F. Mohandes, M. Salavati-Niasari, Inorg. Chim. Acta 362 (2009)
36633668.
[32] F. Mohandes, F. Davar, M. Salavati-Niasari, J. Magn. Magn. Mater. 322 (2010)
872877.
[33] R. Jenkins, R.L. Snyder, in: In: Chemical Analysis: Introduction to X-ray
Powder Diffractometry, John Wiley & Sons, Inc., New York, 1996, p. 90.
[34] H.P. Klug, L.E. Alexander, in: X-ray Diffraction Procedures, second edn., Wiley
(Interscience), New York, 1974, p. 656.
[35] M. Salavati-Niasari, F. Davar, Z. Fereshteh, J. Alloys Compd. 494 (2010)
410414.
[36] B.A. Korgel, S. Fullam, S. Connolly, D. Fitzmaurics, J. Phys. Chem. B 102 (1998)
83798388.
[37] M. Salavati-Niasari, F. Mohandes, F. Davar, K. Saberyan, Appl. Surface Sci. 256
(2009) 14761480.
[38] M. Salavati-Niasari, N. Mir, F. Davar, Appl. Surface Sci. 256 (2010) 40034008.
[39] H. Thoms, M. Epple, A. Reller, Solid State Ionics 101103 (1997) 7984.
[40] S. Stankic, M. Sterrer, P. Hofmann, J. Bernardi, O. Diwald, E. Kn ozinger, Nano
Lett. 5 (2005) 18891893.
[41] S. Stankic, J. Bernardi, O. Diwald., E. Kn ozinger, J. Phys. Chem. B 110 (2006)
1386613871.
[42] H. Niu, Q. Yang, K. Tang, Y. Xie, Scripta Mater. 54 (2006) 17911796.
F. Mohandes et al. / Journal of Physics and Chemistry of Solids 71 (2010) 16231628 1628
Vous aimerez peut-être aussi
- 8 - Chapter 30 PDFDocument32 pages8 - Chapter 30 PDFarian hedayati farPas encore d'évaluation
- 1 FiguresofMerit ExamplesDocument12 pages1 FiguresofMerit ExamplesIvy JoycePas encore d'évaluation
- Principles of Plant Nutrition: 5th EditionDocument10 pagesPrinciples of Plant Nutrition: 5th EditionQian QianPas encore d'évaluation
- Accepted Manuscript: Applied Surface ScienceDocument21 pagesAccepted Manuscript: Applied Surface ScienceAlina BurduceaPas encore d'évaluation
- 1783 Pui PDFDocument9 pages1783 Pui PDFKhuyen VoPas encore d'évaluation
- Polyhedron: Masoud Salavati-Niasari, Fatemeh Davar, Noshin MirDocument5 pagesPolyhedron: Masoud Salavati-Niasari, Fatemeh Davar, Noshin MirNILTHON FRANCO POMA HUARINGAPas encore d'évaluation
- Bright Blue Pigment Coal O Nanocrystals Prepared by Modified Sol-Gel MethodDocument7 pagesBright Blue Pigment Coal O Nanocrystals Prepared by Modified Sol-Gel Methodjeremy parkerPas encore d'évaluation
- Preparation of Nano Particle MG Fe Oby Solution Combustion Method and Their CharacterizationDocument3 pagesPreparation of Nano Particle MG Fe Oby Solution Combustion Method and Their CharacterizationDinh PhucPas encore d'évaluation
- Assessment of Novel MgCe4P6O24 Ceramic Electrolyte in Fabricating Mg-Sensors: Focus On Structure and Electrochemical Impedance SpectrosDocument10 pagesAssessment of Novel MgCe4P6O24 Ceramic Electrolyte in Fabricating Mg-Sensors: Focus On Structure and Electrochemical Impedance SpectrosMohammed AdamuPas encore d'évaluation
- Manganese Oxides Synthesized Via Microwave-Assisted Hydrothermal Method: Phase Evolution and Structure RefinementDocument16 pagesManganese Oxides Synthesized Via Microwave-Assisted Hydrothermal Method: Phase Evolution and Structure Refinementneto DibPas encore d'évaluation
- Syntheses and Characterization of MG (OH) 2 and MgO Nanostructures by Ultrasonic MethodDocument6 pagesSyntheses and Characterization of MG (OH) 2 and MgO Nanostructures by Ultrasonic MethodTahseen AlHattabPas encore d'évaluation
- 300058.factors That May Influence The Microemulsion SynthesisDocument8 pages300058.factors That May Influence The Microemulsion SynthesisPilar Collado MartinezPas encore d'évaluation
- Kumpulan Jurnal Nano MagnetDocument120 pagesKumpulan Jurnal Nano MagnetRachmad Almi PutraPas encore d'évaluation
- Formation Pathways of Magnetite Nanoparticles by CoprecipitationDocument8 pagesFormation Pathways of Magnetite Nanoparticles by CoprecipitationShweta KaurPas encore d'évaluation
- Room Temperature Stable CO - Free H Production From Methanol With Magnesium Oxide NanophotocatalystsDocument9 pagesRoom Temperature Stable CO - Free H Production From Methanol With Magnesium Oxide NanophotocatalystsNasirPas encore d'évaluation
- A Comparative Study of Hydrothermal and Sol-Gel Methods MnO2Document6 pagesA Comparative Study of Hydrothermal and Sol-Gel Methods MnO2hilzone_Pas encore d'évaluation
- Materials Chemistry and PhysicsDocument7 pagesMaterials Chemistry and PhysicsHarun AydınPas encore d'évaluation
- Dye DegradationDocument5 pagesDye DegradationPrasad DPas encore d'évaluation
- 48 - 461 JMES 2264 ElAoufirDocument18 pages48 - 461 JMES 2264 ElAoufirKHLIFI Abdelilah Safi PrimairePas encore d'évaluation
- Exploring The Assembly Of Supramolecular Polyoxometalate Triangular Morphologies With Johnson Solid Cores: ( (Mn (H O) ) (K ⊂ (Α-Gew Mn O) ) )Document6 pagesExploring The Assembly Of Supramolecular Polyoxometalate Triangular Morphologies With Johnson Solid Cores: ( (Mn (H O) ) (K ⊂ (Α-Gew Mn O) ) )Johnny SandovalPas encore d'évaluation
- Dehydrogenation CatalystDocument4 pagesDehydrogenation CatalystWoon Xuet WeiPas encore d'évaluation
- 1 s2.0 S0304885315305205 MainDocument6 pages1 s2.0 S0304885315305205 MainHuckkey HuPas encore d'évaluation
- Facile Synthesis of N MgSb2O6 Trirutile Antimonate and Its Enhanced Photocatalytic PerformanceDocument16 pagesFacile Synthesis of N MgSb2O6 Trirutile Antimonate and Its Enhanced Photocatalytic PerformancejorgePas encore d'évaluation
- Tam Metin SYNTHESIS OF NANO-MANGANESE OXIDE (Mn2O3) PARTICLES BY USING HIGH FREQUENCY-INDUCTION SYSTEMDocument8 pagesTam Metin SYNTHESIS OF NANO-MANGANESE OXIDE (Mn2O3) PARTICLES BY USING HIGH FREQUENCY-INDUCTION SYSTEMLevent LeventPas encore d'évaluation
- 2015 - The Synthesis of Metallic β-Sn Nanostructures for Use as a Novel Pt Catalyst Support and Evaluation of Their Activity Toward Methanol ElectrooxidationDocument9 pages2015 - The Synthesis of Metallic β-Sn Nanostructures for Use as a Novel Pt Catalyst Support and Evaluation of Their Activity Toward Methanol ElectrooxidationLili LilithPas encore d'évaluation
- Farghali 2017Document22 pagesFarghali 2017Emerson SilvaPas encore d'évaluation
- Pelagia Research Library: Precipitation of Nano-Magnesium Hydroxide From Bittern Using Ultrasound IrradiationDocument13 pagesPelagia Research Library: Precipitation of Nano-Magnesium Hydroxide From Bittern Using Ultrasound IrradiationChemical Ferdi IzzecsonPas encore d'évaluation
- Synthesis Conditions of MG (OH) 2 Nanostructures by Hydrothermal RouteDocument4 pagesSynthesis Conditions of MG (OH) 2 Nanostructures by Hydrothermal RouteJuan Alejandro Menchaca RiveraPas encore d'évaluation
- Recovery of Nano-Sized Cobalt Powder From Cemented Carbide ScrapDocument8 pagesRecovery of Nano-Sized Cobalt Powder From Cemented Carbide ScrapEswara ReddyPas encore d'évaluation
- 1 s2.0 S1452398123062375 MainDocument13 pages1 s2.0 S1452398123062375 MainherayatiPas encore d'évaluation
- Art 3A10.1007 2Fs10853 009 3397 8Document7 pagesArt 3A10.1007 2Fs10853 009 3397 8Danesh AzPas encore d'évaluation
- Spectrochimica Acta Part A: Molecular and Biomolecular SpectrosDocument6 pagesSpectrochimica Acta Part A: Molecular and Biomolecular SpectrosHaneen WaleedPas encore d'évaluation
- The Synthesis of Doped Manganese Cobalt Ferrites by Autocombustion TehniqueDocument4 pagesThe Synthesis of Doped Manganese Cobalt Ferrites by Autocombustion TehniqueAlin DrucPas encore d'évaluation
- Facile Method To Synthesize Oleic Acid-Capped Magnetite NanoparticlesDocument4 pagesFacile Method To Synthesize Oleic Acid-Capped Magnetite Nanoparticles122866Pas encore d'évaluation
- Improved Ionic Conductivity of MgM4P6O24 (M HF, ZR) Ceramic Solid - State Electrolytes by Sol-Gel SynthesisDocument10 pagesImproved Ionic Conductivity of MgM4P6O24 (M HF, ZR) Ceramic Solid - State Electrolytes by Sol-Gel SynthesisMohammed AdamuPas encore d'évaluation
- Porous Znmno Plates Prepared From ZN/MN - Sucrose Composite As High-Performance Lithium-Ion Battery AnodesDocument4 pagesPorous Znmno Plates Prepared From ZN/MN - Sucrose Composite As High-Performance Lithium-Ion Battery AnodesjeffersonPas encore d'évaluation
- Materials Letters: Ming Sun, Bang Lan, Lin Yu, Fei Ye, Wei Song, Jun He, Guiqiang Diao, Yuying ZhengDocument3 pagesMaterials Letters: Ming Sun, Bang Lan, Lin Yu, Fei Ye, Wei Song, Jun He, Guiqiang Diao, Yuying ZhengDary LorenaPas encore d'évaluation
- A2BO4 Type PerovskiteDocument11 pagesA2BO4 Type PerovskiteMahmoud ȜliPas encore d'évaluation
- Spectrochimica Acta Part A: Molecular and Biomolecular SpectrosDocument8 pagesSpectrochimica Acta Part A: Molecular and Biomolecular SpectrosKristofer BonillaPas encore d'évaluation
- B-Doped Cryptomelane For BKYST-2023 - 2theta - Lamle's VersionDocument5 pagesB-Doped Cryptomelane For BKYST-2023 - 2theta - Lamle's VersionAndy LêPas encore d'évaluation
- Synthesis of Iron Nanoclusters by Pulsed Current Method: &) O. Rostami-OstadkalayehDocument14 pagesSynthesis of Iron Nanoclusters by Pulsed Current Method: &) O. Rostami-OstadkalayehsecatePas encore d'évaluation
- Minerals: Fabrication of Single-Crystalline Calcite Needle-Like Particles Using The Aragonite-Calcite Phase TransitionDocument9 pagesMinerals: Fabrication of Single-Crystalline Calcite Needle-Like Particles Using The Aragonite-Calcite Phase TransitionkrmktsPas encore d'évaluation
- Electrolytic Activity of Carbon-supportedPt-Au Nano Particles ForDocument7 pagesElectrolytic Activity of Carbon-supportedPt-Au Nano Particles ForAmitvikram DubeyPas encore d'évaluation
- Self-Assembled Palladium Nanoparticles On Carbon NanofibersDocument6 pagesSelf-Assembled Palladium Nanoparticles On Carbon NanofiberswwPas encore d'évaluation
- Nanosized Magnesium Doped Copper Chromites Spinel Particles Synthesis and CharacterizationDocument7 pagesNanosized Magnesium Doped Copper Chromites Spinel Particles Synthesis and CharacterizationSikander AzamPas encore d'évaluation
- Formation Pathways of Magnetite Nanoparticles by Coprecipitation MethodDocument8 pagesFormation Pathways of Magnetite Nanoparticles by Coprecipitation Method122866Pas encore d'évaluation
- synthesis of α-MnO2 using KIO4Document3 pagessynthesis of α-MnO2 using KIO4Đoàn Trí KhoaPas encore d'évaluation
- Abdur UmerSaeed 2Document9 pagesAbdur UmerSaeed 2Sari Ramadhani MeutuahPas encore d'évaluation
- Structural and Electrical Properties of La SR Co Fe O Powders Synthesized by Solid State ReactionDocument8 pagesStructural and Electrical Properties of La SR Co Fe O Powders Synthesized by Solid State ReactionShivaraj SubramaniamPas encore d'évaluation
- One Step Synthesis of Mn3O4 NanoparticlesDocument6 pagesOne Step Synthesis of Mn3O4 Nanoparticlessiti fatimahPas encore d'évaluation
- 2007 - 11 - Carbon-Supported, Nano-Structured, Manganese Oxide CompositeDocument5 pages2007 - 11 - Carbon-Supported, Nano-Structured, Manganese Oxide CompositeAndiQonitaPas encore d'évaluation
- Magnetite Coprecipitation MechanimDocument8 pagesMagnetite Coprecipitation MechanimonynhoPas encore d'évaluation
- Materials Science and Engineering C: Ting Zhang, Yaqin Chai, Ruo Yuan, Junxiang GuoDocument5 pagesMaterials Science and Engineering C: Ting Zhang, Yaqin Chai, Ruo Yuan, Junxiang GuoNadia MandasariPas encore d'évaluation
- A014 PDFDocument18 pagesA014 PDFRastraPatriaPas encore d'évaluation
- 10.1007@s10971 015 3696 2Document15 pages10.1007@s10971 015 3696 2Xiongyu LuoPas encore d'évaluation
- Electrodeposition of Maghemite ( - Fe O) NanoparticlesDocument5 pagesElectrodeposition of Maghemite ( - Fe O) NanoparticlesTia AdrianyputriPas encore d'évaluation
- Photocatalytic Degradation of Phenol On MWNT and Titania Composite Catalysts Prepared by A Modified Sol-Gel MethodDocument8 pagesPhotocatalytic Degradation of Phenol On MWNT and Titania Composite Catalysts Prepared by A Modified Sol-Gel MethodŞebnem Gül İlarslanPas encore d'évaluation
- Preparation of Meixnerite (Mg-Al-OH) Type Layered Double Hydroxide by A Mechanochemical RouteDocument6 pagesPreparation of Meixnerite (Mg-Al-OH) Type Layered Double Hydroxide by A Mechanochemical RouteDAVID LUCAS LIMA BARROSOPas encore d'évaluation
- Selective Self-Propagating Combustion Synthesis of Hexagonal and Orthorhombic Nanocrystalline Yttrium Iron OxideDocument9 pagesSelective Self-Propagating Combustion Synthesis of Hexagonal and Orthorhombic Nanocrystalline Yttrium Iron Oxideochimaru266Pas encore d'évaluation
- Revier-3 Spectrochimica Acta Part A Molecular and Biomolecular Spectroscopy 142, 405-409, 2015Document5 pagesRevier-3 Spectrochimica Acta Part A Molecular and Biomolecular Spectroscopy 142, 405-409, 2015Samita ThakurPas encore d'évaluation
- Heterogeneous Catalysis at Nanoscale for Energy ApplicationsD'EverandHeterogeneous Catalysis at Nanoscale for Energy ApplicationsPas encore d'évaluation
- Surface Plasmon Enhanced, Coupled and Controlled FluorescenceD'EverandSurface Plasmon Enhanced, Coupled and Controlled FluorescencePas encore d'évaluation
- Chem223 Quiz1Document1 pageChem223 Quiz1Ivy JoycePas encore d'évaluation
- Course Outline For Chem 114: Chemistry For Engineers: Intended Learning Outcomes TopicsDocument4 pagesCourse Outline For Chem 114: Chemistry For Engineers: Intended Learning Outcomes TopicsIvy JoycePas encore d'évaluation
- Effects of Electrolytes On Chemical EquilibriaDocument23 pagesEffects of Electrolytes On Chemical EquilibriaIvy Joyce100% (1)
- Organic Compounds - Handouts PDFDocument39 pagesOrganic Compounds - Handouts PDFIvy JoycePas encore d'évaluation
- Lesson: Stressed and Strained: Quick LookDocument7 pagesLesson: Stressed and Strained: Quick LookIvy JoycePas encore d'évaluation
- Crystals: Explain Why The Solution Had To Be Supersaturated For The Sugar Crystals To FormDocument1 pageCrystals: Explain Why The Solution Had To Be Supersaturated For The Sugar Crystals To FormIvy JoycePas encore d'évaluation
- 21-24. Indicate The Correct Material Class For Each Material ListedDocument1 page21-24. Indicate The Correct Material Class For Each Material ListedIvy JoycePas encore d'évaluation
- Experiment 10A Molar Mass of A Liquid From The Density of Its VaporDocument6 pagesExperiment 10A Molar Mass of A Liquid From The Density of Its VaporIvy JoycePas encore d'évaluation
- Components of MatterDocument60 pagesComponents of MatterIvy JoycePas encore d'évaluation
- An Introduction To Chromatographic Separations: Chromatography Permit The Scientist To Separate CloselyDocument45 pagesAn Introduction To Chromatographic Separations: Chromatography Permit The Scientist To Separate CloselyIvy JoycePas encore d'évaluation
- Problem Set Organic ChemistryDocument2 pagesProblem Set Organic ChemistryIvy JoycePas encore d'évaluation
- Problem Set #1 Answer The Following Questions. Write Your Solution On The Testbooklet. Deadline of Submission Is On or Before 9:00am April 8, 2019 1Document2 pagesProblem Set #1 Answer The Following Questions. Write Your Solution On The Testbooklet. Deadline of Submission Is On or Before 9:00am April 8, 2019 1Ivy JoycePas encore d'évaluation
- ChemistryDocument1 pageChemistryIvy JoycePas encore d'évaluation
- Problem Set #1 Answer The Following Questions. Write Your Solution On The Testbooklet. Deadline of Submission Is On or Before 3:00pm March 15, 2019Document2 pagesProblem Set #1 Answer The Following Questions. Write Your Solution On The Testbooklet. Deadline of Submission Is On or Before 3:00pm March 15, 2019Ivy JoycePas encore d'évaluation
- Answer The Following Questions Thoroughly. Submit Your Answers Written On The Chemistry Test Booklet Not Later Than 8AM On Wednesday, November 28, 2018Document1 pageAnswer The Following Questions Thoroughly. Submit Your Answers Written On The Chemistry Test Booklet Not Later Than 8AM On Wednesday, November 28, 2018Ivy JoycePas encore d'évaluation
- Determine The Functional Groups in The Compounds Having The IR SpectrumDocument2 pagesDetermine The Functional Groups in The Compounds Having The IR SpectrumIvy JoycePas encore d'évaluation
- Functional Group InterconversionDocument28 pagesFunctional Group InterconversionIvy JoycePas encore d'évaluation
- Problem Set Organic ChemistryDocument2 pagesProblem Set Organic ChemistryIvy JoycePas encore d'évaluation
- Adv Organic Chemistry Problem Set No. 2Document3 pagesAdv Organic Chemistry Problem Set No. 2Ivy JoycePas encore d'évaluation
- Islam Oxygen LTD RED HARRING PROSPECTUS P 322Document322 pagesIslam Oxygen LTD RED HARRING PROSPECTUS P 322Rumana SharifPas encore d'évaluation
- Phase DiagramsDocument19 pagesPhase Diagramsget2cs100% (1)
- Tuesday 22 October 2019: ChemistryDocument16 pagesTuesday 22 October 2019: ChemistryAsma AkterPas encore d'évaluation
- S02.Techno2.Hard Gelatin CapsuleDocument29 pagesS02.Techno2.Hard Gelatin CapsulearmstrongvinodrajPas encore d'évaluation
- Action of Metals With Water: O-LevelDocument3 pagesAction of Metals With Water: O-Levelleticia karungiPas encore d'évaluation
- Exploring The Potential of Metal-Doped Perovskite-Oxides As Oxygen Reduction Catalyst For Enhancing The Performance of Microbial Desalination CellsDocument13 pagesExploring The Potential of Metal-Doped Perovskite-Oxides As Oxygen Reduction Catalyst For Enhancing The Performance of Microbial Desalination CellsTahseena NaazPas encore d'évaluation
- Ferrous Metal - Iron and SteelDocument56 pagesFerrous Metal - Iron and Steelsubhash sureshPas encore d'évaluation
- Fundamental of Bioprospection AssignmentDocument19 pagesFundamental of Bioprospection AssignmentSalsabila LuqyanaPas encore d'évaluation
- Las ProteinsDocument25 pagesLas ProteinschennielafleurPas encore d'évaluation
- BromatometryDocument2 pagesBromatometryMohamed Dahmane0% (1)
- Lab 21Document3 pagesLab 21KeenanPas encore d'évaluation
- Biocompatibility of Dental MaterialsDocument100 pagesBiocompatibility of Dental Materialsjohnjpw 92Pas encore d'évaluation
- Chem 241 Lab Final Study Guide PDFDocument11 pagesChem 241 Lab Final Study Guide PDFFerriel SiapnoPas encore d'évaluation
- 1 s2.0 S0960894X2300241X MainDocument10 pages1 s2.0 S0960894X2300241X Mainc4ph5s5fjrPas encore d'évaluation
- TDS Crystar Saint-Gobain HiFlo - enDocument2 pagesTDS Crystar Saint-Gobain HiFlo - enSanja BjelicaPas encore d'évaluation
- Chapter One MCQ For EngversionDocument3 pagesChapter One MCQ For Engversionapi-271352412Pas encore d'évaluation
- TDS TW5001Document2 pagesTDS TW5001razamehdi3Pas encore d'évaluation
- Kondensasi Karbonil FarDocument71 pagesKondensasi Karbonil Faragus raharjaPas encore d'évaluation
- Determination of Gamma No and TSDocument3 pagesDetermination of Gamma No and TSAditya ShrivastavaPas encore d'évaluation
- 5070 w10 QP 21Document20 pages5070 w10 QP 21Awais JavedPas encore d'évaluation
- Classificatio N of Matter: Reference: Science For Active Learning-Grade 6Document17 pagesClassificatio N of Matter: Reference: Science For Active Learning-Grade 6Sharmaine TuazonPas encore d'évaluation
- HKDSE Chemistry MC Chapter 10Document7 pagesHKDSE Chemistry MC Chapter 10ScribdPas encore d'évaluation
- CartilageDocument3 pagesCartilagejose randyPas encore d'évaluation
- Ethylene-Propylene Diene Rubber As A Futuristic Elastomer For Insulation of Solid Rocket MotorsDocument12 pagesEthylene-Propylene Diene Rubber As A Futuristic Elastomer For Insulation of Solid Rocket MotorsbbnchemPas encore d'évaluation
- Asme P NumberDocument4 pagesAsme P NumberTanveer Rajput EngrPas encore d'évaluation
- Co JetDocument4 pagesCo JetJaime PaulPas encore d'évaluation
- HW5 EnzymesDocument2 pagesHW5 EnzymesFATIMA AIRA LEGASPIPas encore d'évaluation
- Media and Bioreactor Sterilization Techniques: Manam Walait Lecturer FLS, UCP LahoreDocument12 pagesMedia and Bioreactor Sterilization Techniques: Manam Walait Lecturer FLS, UCP LahoreJawadPas encore d'évaluation
- Expo Tanabe SuganeDocument8 pagesExpo Tanabe Suganesamir velezPas encore d'évaluation