Académique Documents
Professionnel Documents
Culture Documents
NB PT I Blood Clotting-Gases
Transféré par
rajanOrajanTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
NB PT I Blood Clotting-Gases
Transféré par
rajanOrajanDroits d'auteur :
Formats disponibles
BASIC TISSUE DR.
LAVERS
NATIONAL BOARDS PART I
BLOOD CLOTTING
BLOOD CLOTTING
J78-20. A deficiency in vitamin K would affect blood clotting
chiefly by
*1. decreasing prothrombin production.
2. preventing the contraction of the clot.
3. preventing the reaction of thrombin with fibrinogen.
4. preventing the conversion of fibrinogen to fibrin.
5. preventing the conversation of prothrombin to thrombin.
J78-97. Which of the following is NOT required for the normal
blood clotting response?
1. Ca++
*2. Plasmin
3. Thrombin
4. Vitamin K
5. Phospholipid
6. Proteolysis
A/D79-2. The vitamin that promotes the synthesis of
prothrombin by the liver is
1. carotene
2. vitamin C *3. vitamin K
4. folic acid 5. vitamin B12
M81-73. Which of the following ion is involved in blood
clotting?
1. iron 2. sodium *3. calcium 4. potassium
82D-94. Which of the following agents is NOT likely to be
found in plasma?
*1. Thrombin
2. Fibrinogen
3. Prothrombin
4. Calcium ion 5. Ascorbic acid
D96-134. A derivative of vitamin K is the coenzyme for which
of the following?
A. Production of menadiol B. Esterification of retinol
C. Hydrolysis of peptide bonds
D. Cross-linking of fibrinogen
*E. Carboxylation of glutamate side chains
D98-113. Which of the following represents the normal
substrate of thrombin?
A. Fibrin B. Thrombospondin
C. Prothrombin
D. Thromboplastin
*E. Fibrinogen
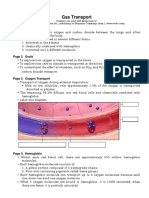
GAS TRANSPORT
J78-10. Some degree of alkalosis occurs temporarily as a result
of
1. ingestion of ammonium chloride.
*2. prolonged deep breathing. 3. severe muscular effort.
4. excessive smoking.
5. high fluid intake.
J78-30. The membrane of the human erythrocyte can be
correctly described as
1. directly catalyzing the reaction:
H2CO3 CO2 + H20
2. being composed of hemoglobin and other soluble
proteins necessary for gaseous respiratory exchange.
*3. containing lipoproteins and specific blood group
substances.
4. having a high histone content.
5. being a site of de novo hemoglobin synthesis.
Page 1 of 3
J78-38. The bicarbonate buffer system of the blood is very
efficient because
1. bicarbonate is rapidly excreted by the kidneys.
2. bicarbonate is able to be stored in the tissue.
3. carbon dioxide is able to combine with hemoglobin.
4. carbon dioxide forms a stable combination with base.
*5. carbon dioxide is rapidly eliminated through the lungs.
J78-42. The physiologic importance of hemoglobin lies in its
ability to combine
1. irreversibly with oxygen and CO2.
2. reversibly with oxygen at the ferric heme prosthetic
group.
3. irreversibly with oxygen at the ferrous heme prosthetic
group.
*4. reversibly with oxygen at the ferrous heme prosthetic
group.
5. None of the above
J78-71. Carbon dioxide is transported in four different chemical
forms. Which of the following is the principle mode of
transport?
1. Dissolved carbon dioxide 2. Carbamino compounds
*3. Bicarbonate ions
4. Carbonic acid
A/D79-17. Carbonic anhydrase in the kidney tubular cells is
known to be associated with the reabsorption of
1. urea 2. chloride *3. bicarbonate ion
4. carbohydrate
A/D79-18. Toxic effects of carbon monoxide are best explained
by the fact that carbon monoxide
1. depresses the rate of respiration
2. prevents dissociation of oxyhemoglobin
*3. competes with O2 for hemoglobin binding sites
4. poisons oxidative catalysts of the tissue cells
5. combines irreversibly with hemoglobin forming
methemoglobin
A/D79-45. During exercise, muscle tissue accumulates lactic
acid. As a results, erythrocytes passing through capillaries
in the muscle
1. release more CO2.
2. absorb more O2.
3. release more O2.
4. both 1 and 3 above
*5. both 2 and 3 above
A/D79-65. The buffer system most important in maintaining the
physiological pH of plasma is
1. protein/proteinate. 2. acetic acid/acetate.
*3. carbonic acid/bicarbonate.
4. phosphoric acid/phosphate.
5. hydroxybutyric acid/hydroxybutyrate.
BASIC TISSUE DR. LAVERS
NATIONAL BOARDS PART I
BLOOD CLOTTING
M81-8. The consequence of appreciable conversion of
hemoglobin to methemoglobin is
1. a significant increase in carbon dioxide combining power
2. a significant decrease in carbon dioxide combining
power
3. no effect on the ability of blood to pick up oxygen
4. a noticeable increase in the ability of blood to pick up
oxygen
*5. a noticeable decrease in the ability of blood to transport
oxygen
M81-34. The normal blood bicarbonate-carbonic acid ratio is
20:1. A patient with a 10:1 ratio is in
1. compensated alkalosis.
2. compensated acidosis.
3. uncompensated alkalosis.
*4. uncompensated acidosis.
5. none of the above. This patients ratio is within normal
limits.
M81-36. Iron-porphyrin protein structures are components of
1. myoglobin.
2. B vitamins.
3. hemoglobin.
*4. cytochromes. 5. pyridine dinucleotides
M81-40. The physiological importance of hemoglobin lies in its
ability to combine
1. irreversibly with oxygen and CO2.
2. reversibly with oxygen at the ferric heme prosthetic
group.
3. irreversibly with oxygen at the ferrous heme prosthetic
group.
*4. reversibly with oxygen at the ferrous heme prosthetic
group.
5. None of the above.
M81-42. Blood leaving the lungs is saturated with O2 to the
extent of approximately
1. 5 per cent.
2. 25 per cent. 3. 50 per cent.
4. 75 per cent.
*5. 98 per cent.
M81-43. CO2 is transported in the blood predominantly in the
form of
1. carbonic acid.
2. carbaminohemoglobin.
*3. bicarbonate in plasma.
4. bicarbonate in erythrocytes.
5. CO2 physically dissolved in plasma.
M81-66. In severe degenerative disease of the liver, such as
advanced alcoholic cirrhosis, a marked deficiency of which
of the following factors essential to blood coagulating
mechanisms is likely?
(a) prothromboplastin (b) prothrombin
(c ) thrombin (d) vitamin K (e) fibrinogen
1. a and e 2. b and c *3. b and e 4. c and d
Page 2 of 3
82D-18. During respiration, carbon dioxide and oxygen are
exchanged at the junction of blood and alveolar spaces and
the junction of blood and tissue. The actual diffusion of the
gases is primarily controlled by the
1. atmospheric pressue.
2. elasticity of blood capillaries.
3. bicarbonate content of the blood.
4. hemoglobin content of erythrocytes.
*5. differentials in partial pressures of the gases.
82D-22. The function of vitamin K is involved directly with
*1. synthesis of prothrombin.
2. activation of the Stuart factor.
3. regulation of calcium in the blood.
4. conversion of fibrinogen to fibrin.
5. transcriptional control for fibrinogen synthesis.
82D-67. The greatest concentration of carbonic anhydrase is
found
1. in plasma.
2. in platelets.
3. in leukocytes.
*4. in erythrocytes.
5. equally distributed between plasma and erythrocytes.
D85-40. Carbon monoxide decreases the amount of
1. bicarbonate in the blood plasma.
2. carbonic anhydrase available in the lungs.
*3. oxygen that can be transported by hemoglobin.
4.alveolar surface available for gaseous exchange.
5. carbon dioxide that can be transported by the blood.
D87-45. Carbonic anhydrase in erythrocytes increases
1. rate of dissociation of H2CO3.
2. rate of formation of oxyhemoglobin.
3. permeability of erythrocytes to HCO3.
4. formation of carbamino compounds with hemoglobin.
D87-61. Normal hemoglobin concentration is about 15 gm./dl.
Blood and normal arterial oxygen content is about 20 ml.
O2/dl. Blood. AN anemic individual breathing room air with
a hemoglobin concentration of 10 gm./dl. Blood is expected
to have
1. normal arterial oxygen tension and normal arterial
oxygen content.
2. reduced arterial oxygen tension and normal arterial
oxygen content.
*3. normal arterial oxygen tension and reduced arterial
oxygen content.
4. reduced arterial oxygen tension and reduced arterial
oxygen content.
D87-62. The hemoglobin dissociation curve is shifted to the
right by
1. a decreased in temperature.
2. an increase in arterial PCO2.
3. an increase in arterial hydrogen ion concentration.
4. both 1 and 3 above.
*5. both 2 and 3 above.
BASIC TISSUE DR. LAVERS
NATIONAL BOARDS PART I
BLOOD CLOTTING
J89-30. In sickle cell aneamia, a variation in an amino acid of
hemoglobin is detected. This substitution of valine for
glutamic acid
1. results in no change in solubility.
2. results in no change in isoelectric pH.
*3. is a result of a change in DNA coding.
4. has no noticeable effect on the O2 transport behavior of
the erythrocyte.
J89-70. the affinity of hemoglobin for oxygen diminishes as
which of the following is decreased?
*1. pH
2. PCO2
3. Temperature
4. Hydrogen ion concentration.
5. 2,3-diphosphoglyceric acid (DPG)
D96-198. MOST of the CO2 in blood is combined as
A . H2CO3.
*B. HCO3 . C. CH3COOH.
D. carbonic acid. E. carbaminohemoglobin.
D98-148. In respiratory acidosis, arterial CO2 content and pH
become abnormal. Which of the following BEST
describes their respective changes?
CO2
pH
A. Increases
Increases
*B. Increases
Decreases
C. Decreases
Increases
D. Decreases
Decreases
D98-174. Hyperventilation alters the acid-base balance of
arterial blood by
A. increasing CO2 and increasing pH.
B. increasing CO2 and decreasing pH.
C. decreasing CO2 and decreasing pH.
*D. decreasing CO2 and increasing pH.
D98-188. The rate of diffusion across the alveolar wall is
inversely proportional to which of the following?
A. The surface area for gaseous exchange
B. The thickness of the alveolar wall
*C. The difference in the partial pressures of the gas
D. The solubility of the gas.
D98-197. Absense of which of the following blood enzymes
drastically reduces blood CO2 carrying capacity?
*A. Carbonic anhydrase
B. Alkaline phosphatase
C. Pyruvate carboxykinase
D. Histidine decarboxylase
E. Serum glutamic-oxaloacetate transaminase
Page 3 of 3
Vous aimerez peut-être aussi
- Redox Chemistry and Biology of ThiolsD'EverandRedox Chemistry and Biology of ThiolsBeatriz AlvarezPas encore d'évaluation
- Anatomy Science Dentist 2Document1 pageAnatomy Science Dentist 2Assignment AbroadPas encore d'évaluation
- Protein Carbonylation: Principles, Analysis, and Biological ImplicationsD'EverandProtein Carbonylation: Principles, Analysis, and Biological ImplicationsJoaquim RosPas encore d'évaluation
- Physiology, Oxygen Transport: April 2019Document5 pagesPhysiology, Oxygen Transport: April 2019Soha SonaPas encore d'évaluation
- Subject ChemistryDocument12 pagesSubject ChemistrykottooranjohnbPas encore d'évaluation
- Seleksi Impho MCQDocument16 pagesSeleksi Impho MCQahmadnatsirrPas encore d'évaluation
- Glutamine MetabolismDocument14 pagesGlutamine MetabolismCristóbal Miño MoralesPas encore d'évaluation
- Physiology I Blood & ImmunityDocument13 pagesPhysiology I Blood & Immunitynadine azmyPas encore d'évaluation
- Hema Local Post TestDocument6 pagesHema Local Post TestJaymih Santos AbasoloPas encore d'évaluation
- Topic - 6 - QR of D-ElementsDocument14 pagesTopic - 6 - QR of D-Elementststtwa LyoaPas encore d'évaluation
- 001assessment Exam ImmunohemaDocument25 pages001assessment Exam ImmunohemaFrankenstein MelancholyPas encore d'évaluation
- Cell InjuryDocument9 pagesCell InjuryAccount for EverythingPas encore d'évaluation
- Topic 13. Metabolism of Erythrocytes. Antioxidant Systems.Document3 pagesTopic 13. Metabolism of Erythrocytes. Antioxidant Systems.Manar BehiPas encore d'évaluation
- 003assessment Exam ImmunohemaDocument41 pages003assessment Exam ImmunohemaFrankenstein MelancholyPas encore d'évaluation
- Blood and Related PhysiologyDocument22 pagesBlood and Related Physiologysebastianpaez8Pas encore d'évaluation
- Gas Transport PDFDocument19 pagesGas Transport PDFMarilia BonorinoPas encore d'évaluation
- RBC, Structure and FunctionDocument15 pagesRBC, Structure and FunctionstellatrsPas encore d'évaluation
- 1 Blood: Composition of Blood (Viva)Document21 pages1 Blood: Composition of Blood (Viva)Priyanka MhPas encore d'évaluation
- Gas TransportDocument19 pagesGas TransportJesus GutierrezPas encore d'évaluation
- Carbon Monoxide PoisoningDocument16 pagesCarbon Monoxide PoisoningMARIA TELLEZPas encore d'évaluation
- Biochem Final Exam MCQs PDFDocument173 pagesBiochem Final Exam MCQs PDFzeeshan jaskaniPas encore d'évaluation
- Hematopoiesis (Occurs in The Liver) Is Considered As The Counterpart in Fetal DevelopmentDocument17 pagesHematopoiesis (Occurs in The Liver) Is Considered As The Counterpart in Fetal DevelopmentEG Ongteco Gonzaga OngtecoPas encore d'évaluation
- Review: Running Head: Nitric Oxide and Energy Balance in HypoxiaDocument70 pagesReview: Running Head: Nitric Oxide and Energy Balance in HypoxiaAprilihardini LaksmiPas encore d'évaluation
- Which of The Following Molecules Can Obligate Lithotrophs Not Oxidize? A. H B. Fe C. Nadh D. NH E. None of The AboveDocument6 pagesWhich of The Following Molecules Can Obligate Lithotrophs Not Oxidize? A. H B. Fe C. Nadh D. NH E. None of The AboveKatie MariePas encore d'évaluation
- Calculated Osmolality (Mosm/kg) 2C (Mmol/liter) + C (MG/DL) + C (MG/DL) 18 2.8Document3 pagesCalculated Osmolality (Mosm/kg) 2C (Mmol/liter) + C (MG/DL) + C (MG/DL) 18 2.8Jana LacuestaPas encore d'évaluation
- Physiology - Guyton BLOODDocument6 pagesPhysiology - Guyton BLOODIsra K. SoomroPas encore d'évaluation
- Mockboards Reviewer FinalDocument161 pagesMockboards Reviewer FinalRoselle Joyce Arce CalubanPas encore d'évaluation
- Dr. Ligia Eliya Matti: College of Health Sciences Clinical Biochemistry Department Theoretical Toxicology 24-4-2019Document7 pagesDr. Ligia Eliya Matti: College of Health Sciences Clinical Biochemistry Department Theoretical Toxicology 24-4-2019aveen rasulPas encore d'évaluation
- British Biology Olympiad Part A QuestionsDocument22 pagesBritish Biology Olympiad Part A Questionsmartynapet25% (4)
- Mario Comporti Department of Pathophysiology, Experimental Medicine and Public Health University of SienaDocument14 pagesMario Comporti Department of Pathophysiology, Experimental Medicine and Public Health University of SienaKomang F Gita TriesnandaPas encore d'évaluation
- Biochem 2014 Exam 1Document16 pagesBiochem 2014 Exam 1Thomas B.Pas encore d'évaluation
- Unit Test 1 QUIZZES 1-11 Sir M (Lec Vid) Notes Laboratory Manual Lecture Notes & QuizzesDocument490 pagesUnit Test 1 QUIZZES 1-11 Sir M (Lec Vid) Notes Laboratory Manual Lecture Notes & Quizzesrealeliazar0531Pas encore d'évaluation
- Australian Biology Olympiad 2008Document31 pagesAustralian Biology Olympiad 2008Science Olympiad BlogPas encore d'évaluation
- 103-The Physiology of Red Blood Cells and Haemaglobin Variants PDFDocument9 pages103-The Physiology of Red Blood Cells and Haemaglobin Variants PDFChoiri Khumaidah FikriyahPas encore d'évaluation
- Imcq 11 - KeyDocument36 pagesImcq 11 - KeyReggiePas encore d'évaluation
- Bio Chem EssaysDocument20 pagesBio Chem EssaysEINSTEIN2DPas encore d'évaluation
- Human Physiology From Cells To Systems Canadian 4th Edition Sherwood Test BankDocument27 pagesHuman Physiology From Cells To Systems Canadian 4th Edition Sherwood Test Bankdulciethanhonjja4100% (34)
- Acute Kidney Injury Due To Rhabdomyolysis and Renal Replacement Therapy A Critical ReviewDocument1 pageAcute Kidney Injury Due To Rhabdomyolysis and Renal Replacement Therapy A Critical ReviewAmmar QuasmiPas encore d'évaluation
- LS 1 Test 2 16-17Document8 pagesLS 1 Test 2 16-17Kelly LeePas encore d'évaluation
- Chapter 13: Alterations in Oxygen Transport Banasik: Pathophysiology, 6th EditionDocument10 pagesChapter 13: Alterations in Oxygen Transport Banasik: Pathophysiology, 6th Editionqwivy.com100% (1)
- RBC 1Document32 pagesRBC 1Oriade TaiwoPas encore d'évaluation
- Carbon Monoxide Poisoning: Ivan BlumenthalDocument3 pagesCarbon Monoxide Poisoning: Ivan BlumenthalVivi Evita DewiPas encore d'évaluation
- Critical Thinking QuestionsDocument7 pagesCritical Thinking QuestionsYashal KhanPas encore d'évaluation
- Acid-Base Balance: AnswerDocument2 pagesAcid-Base Balance: AnswerJoan MecijaPas encore d'évaluation
- Gas Transport PH in The Lung - pptx11Document92 pagesGas Transport PH in The Lung - pptx11زينب هانيPas encore d'évaluation
- Non Respi QuizDocument1 pageNon Respi QuizRicky JalecoPas encore d'évaluation
- Widdop 2002 Analysis of Carbon MonoxideDocument14 pagesWiddop 2002 Analysis of Carbon Monoxidepsibedi02Pas encore d'évaluation
- Biochem 1Document55 pagesBiochem 1Pragadeesh ChakravarthyPas encore d'évaluation
- Exercise 1: (6pts) Transport of Respiratory GasesDocument3 pagesExercise 1: (6pts) Transport of Respiratory GasesMajed MashmoushyPas encore d'évaluation
- Asidosis Metabolik PDFDocument6 pagesAsidosis Metabolik PDFafifurrahman_rizalPas encore d'évaluation
- Respiration Physiology SEQs With KeyDocument12 pagesRespiration Physiology SEQs With KeyMudassar Roomi100% (1)
- 2008 Article 50Document4 pages2008 Article 50danielmartinezPas encore d'évaluation
- Formative Assessment (For Term Test) : Topics 3 & 4: Section A MCQ (2 Marks Each)Document5 pagesFormative Assessment (For Term Test) : Topics 3 & 4: Section A MCQ (2 Marks Each)RaysonChooPas encore d'évaluation
- 17uch08 Inorganic ChemistryDocument72 pages17uch08 Inorganic ChemistryVadivelanPas encore d'évaluation
- RBC FunctionDocument25 pagesRBC FunctionGabriela SolanoPas encore d'évaluation
- البحث.doc AliDocument33 pagesالبحث.doc AliMohamed MisbahPas encore d'évaluation
- Respiratory Physiology Part 2Document5 pagesRespiratory Physiology Part 2kabir musa ladanPas encore d'évaluation
- Physiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaDocument65 pagesPhysiology of Blood: Ratna Kusumawati Department of Physiology, Faculty of Medicine Universitas Sebelas Maret SurakartaRizal AbdurrahmanPas encore d'évaluation
- Review Phys 2Document5 pagesReview Phys 2John RoylePas encore d'évaluation
- ErytHroCyte ProduCtionDocument8 pagesErytHroCyte ProduCtionMichelle CaamicPas encore d'évaluation
- Direct Measuremet of Forces BTW Surfaces in Liquids at Molecular LevelDocument3 pagesDirect Measuremet of Forces BTW Surfaces in Liquids at Molecular LevelrajanOrajanPas encore d'évaluation
- The Explosion of Acetylene and NitrogenDocument12 pagesThe Explosion of Acetylene and NitrogenrajanOrajanPas encore d'évaluation
- Electric Double Layer-Electrostatic Control of Ions and Molescules in Nanofluidic-Transistor PDFDocument6 pagesElectric Double Layer-Electrostatic Control of Ions and Molescules in Nanofluidic-Transistor PDFrajanOrajanPas encore d'évaluation
- Boards7 7 08Document6 pagesBoards7 7 08ritabagadiaPas encore d'évaluation
- Questions (Multiple Correct Answers, Extended Matching and Ordering)Document111 pagesQuestions (Multiple Correct Answers, Extended Matching and Ordering)gary_singh_198195% (19)
- MasterDental Anatomy FactsDocument10 pagesMasterDental Anatomy FactsrajanOrajanPas encore d'évaluation
- 30 TH October Micrscope FinalDocument229 pages30 TH October Micrscope FinalrajanOrajanPas encore d'évaluation
- Nbde-1 Test-1Document45 pagesNbde-1 Test-1rajanOrajan100% (4)
- Biochemistry: Proteins and Amino AcidsDocument8 pagesBiochemistry: Proteins and Amino AcidsrajanOrajanPas encore d'évaluation
- Remembered Questions Part 1Document4 pagesRemembered Questions Part 1Neeraj Singh100% (2)
- 7 OsmfDocument20 pages7 OsmfrajanOrajanPas encore d'évaluation
- Tru StoryDocument1 pageTru StoryrajanOrajanPas encore d'évaluation
- Remembered Question Part - 1 2007Document3 pagesRemembered Question Part - 1 2007rajanOrajanPas encore d'évaluation
- Mnemonic SDocument9 pagesMnemonic SrajanOrajanPas encore d'évaluation
- Youtube Videos NBDE1Document12 pagesYoutube Videos NBDE1rajanOrajanPas encore d'évaluation
- Dental Boards QuestionsDocument2 pagesDental Boards QuestionsBia BezerraPas encore d'évaluation
- Inserto Fibrinogeno SIEMENSDocument5 pagesInserto Fibrinogeno SIEMENSedson floresPas encore d'évaluation
- Data Interpretation For Medical StudentDocument18 pagesData Interpretation For Medical StudentWee K WeiPas encore d'évaluation
- J. DURANTEAU - PpsDocument58 pagesJ. DURANTEAU - PpsElena ChitoiuPas encore d'évaluation
- Mclarty 2012Document7 pagesMclarty 2012Gisele CanelaPas encore d'évaluation
- Anp2001 Test 1 Questions 2014Document7 pagesAnp2001 Test 1 Questions 2014Jerilee SoCute WattsPas encore d'évaluation
- Introduction To Immunity and The Immune System: HistoryDocument149 pagesIntroduction To Immunity and The Immune System: HistoryMarissa CordovaPas encore d'évaluation
- Thrombin Time TestDocument3 pagesThrombin Time Testhorray2333Pas encore d'évaluation
- Opm M2 V117UV EN Rev11Document58 pagesOpm M2 V117UV EN Rev11Jose Rolando Orellana Rodriguez0% (1)
- Hemostasis Reagent Portfolio PDFDocument6 pagesHemostasis Reagent Portfolio PDFAlma FloresPas encore d'évaluation
- Disseminated Intravascular CoagulationDocument8 pagesDisseminated Intravascular CoagulationMade NoprianthaPas encore d'évaluation
- Humaclot Duo PlusDocument54 pagesHumaclot Duo PlusPatricia Tauran0% (1)
- Helena C-4 - 5060169855786 - EN - Operation ManualDocument58 pagesHelena C-4 - 5060169855786 - EN - Operation ManualДенис СахноPas encore d'évaluation
- The Thrombin Time Test and Reptilase Test - What Is Their Role in Coagulation TestingDocument7 pagesThe Thrombin Time Test and Reptilase Test - What Is Their Role in Coagulation TestingTuan NguyenPas encore d'évaluation
- Disseminated Intravascular Coagulation: Dr. Rudy Afriant, Sppd-KhomDocument22 pagesDisseminated Intravascular Coagulation: Dr. Rudy Afriant, Sppd-Khomsonnya morisaPas encore d'évaluation
- June 2015 (IAL) QP - Unit 1 Edexcel BiologyDocument20 pagesJune 2015 (IAL) QP - Unit 1 Edexcel BiologyYogesh GaneshPas encore d'évaluation
- Mark Scheme (Results) Summer 2016: Pearson Edexcel GCE in Biology Spec B (8BI0) Paper 02 Core Physiology and EcologyDocument24 pagesMark Scheme (Results) Summer 2016: Pearson Edexcel GCE in Biology Spec B (8BI0) Paper 02 Core Physiology and EcologyLorenzo Von MatterhornPas encore d'évaluation
- Apheresis ProceduresDocument34 pagesApheresis ProceduresyamtotlPas encore d'évaluation
- Pre-Analytical Variables in Coagulation TestingDocument20 pagesPre-Analytical Variables in Coagulation TestingTaylorPas encore d'évaluation
- Coagulation Tests: Clotting Time, Bleeding Time, Prothrombin Time, Activated Partial Thromboplastin TimeDocument15 pagesCoagulation Tests: Clotting Time, Bleeding Time, Prothrombin Time, Activated Partial Thromboplastin TimeAnastasiaPas encore d'évaluation
- Chapter 35 Part 3Document11 pagesChapter 35 Part 3Gordon JamesonPas encore d'évaluation
- Platelet DisordersDocument9 pagesPlatelet DisordersSophia Gail ChingPas encore d'évaluation
- January 2016 (IAL) QP - Unit 1 Edexcel Biology A-LevelDocument24 pagesJanuary 2016 (IAL) QP - Unit 1 Edexcel Biology A-LevelkirthikaPas encore d'évaluation
- 06.1 High-Risk Postpartal ClientDocument22 pages06.1 High-Risk Postpartal ClientJAN CAMILLE LENONPas encore d'évaluation
- Blood Product Transfusions Key in Acute Promyelocytic LeukemiaDocument5 pagesBlood Product Transfusions Key in Acute Promyelocytic LeukemiaaymenPas encore d'évaluation
- Management of Bleeding PDFDocument25 pagesManagement of Bleeding PDFGlory Owens AgbonkpoloPas encore d'évaluation
- Libro - Biological Effects of Magnetic and Electromagnetic Fields (The Language of Science)Document243 pagesLibro - Biological Effects of Magnetic and Electromagnetic Fields (The Language of Science)Faiber Soto VargasPas encore d'évaluation
- Coagulation Profile in Diabetes MellitusDocument5 pagesCoagulation Profile in Diabetes MellitusAsfandyar RoghaniPas encore d'évaluation
- Coagulation DisordersDocument9 pagesCoagulation DisordersIS99057Pas encore d'évaluation
- Pediatric TransfusionDocument82 pagesPediatric TransfusionMia Lesaca-Medina100% (2)
- Anatomy and Physiology of Blood and Cardiovascular SystemDocument6 pagesAnatomy and Physiology of Blood and Cardiovascular SystemCharise LigoresPas encore d'évaluation
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincD'EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincÉvaluation : 3.5 sur 5 étoiles3.5/5 (137)
- Forever Strong: A New, Science-Based Strategy for Aging WellD'EverandForever Strong: A New, Science-Based Strategy for Aging WellPas encore d'évaluation
- The Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyD'EverandThe Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyÉvaluation : 4.5 sur 5 étoiles4.5/5 (2)
- Gut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)D'EverandGut: The Inside Story of Our Body's Most Underrated Organ (Revised Edition)Évaluation : 4 sur 5 étoiles4/5 (378)
- The Beck Diet Solution Weight Loss Workbook: The 6-Week Plan to Train Your Brain to Think Like a Thin PersonD'EverandThe Beck Diet Solution Weight Loss Workbook: The 6-Week Plan to Train Your Brain to Think Like a Thin PersonÉvaluation : 3.5 sur 5 étoiles3.5/5 (33)
- Summary: Fast Like a Girl: A Woman’s Guide to Using the Healing Power of Fasting to Burn Fat, Boost Energy, and Balance Hormones: Key Takeaways, Summary and AnalysisD'EverandSummary: Fast Like a Girl: A Woman’s Guide to Using the Healing Power of Fasting to Burn Fat, Boost Energy, and Balance Hormones: Key Takeaways, Summary and AnalysisÉvaluation : 3 sur 5 étoiles3/5 (2)
- Summary of Mary Claire Haver's The Galveston DietD'EverandSummary of Mary Claire Haver's The Galveston DietÉvaluation : 5 sur 5 étoiles5/5 (1)
- The Body Book: The Law of Hunger, the Science of Strength, and Other Ways to Love Your Amazing BodyD'EverandThe Body Book: The Law of Hunger, the Science of Strength, and Other Ways to Love Your Amazing BodyPas encore d'évaluation
- Metabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeD'EverandMetabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifePas encore d'évaluation
- Hungry for Change: Ditch the Diets, Conquer the Cravings, and Eat Your Way to Lifelong HealthD'EverandHungry for Change: Ditch the Diets, Conquer the Cravings, and Eat Your Way to Lifelong HealthÉvaluation : 4 sur 5 étoiles4/5 (7)
- Eat & Run: My Unlikely Journey to Ultramarathon GreatnessD'EverandEat & Run: My Unlikely Journey to Ultramarathon GreatnessPas encore d'évaluation
- Instant Loss On a Budget: Super-Affordable Recipes for the Health-Conscious CookD'EverandInstant Loss On a Budget: Super-Affordable Recipes for the Health-Conscious CookÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- How to Be Well: The 6 Keys to a Happy and Healthy LifeD'EverandHow to Be Well: The 6 Keys to a Happy and Healthy LifeÉvaluation : 5 sur 5 étoiles5/5 (1)
- The Food Lover's Cleanse: 140 Delicious, Nourishing Recipes That Will Tempt You Back into Healthful EatingD'EverandThe Food Lover's Cleanse: 140 Delicious, Nourishing Recipes That Will Tempt You Back into Healthful EatingÉvaluation : 4 sur 5 étoiles4/5 (3)
- Sugar Crush: How to Reduce Inflammation, Reverse Nerve Damage, and Reclaim Good HealthD'EverandSugar Crush: How to Reduce Inflammation, Reverse Nerve Damage, and Reclaim Good HealthÉvaluation : 4 sur 5 étoiles4/5 (6)
- Body Love Every Day: Choose Your Life-Changing 21-Day Path to Food FreedomD'EverandBody Love Every Day: Choose Your Life-Changing 21-Day Path to Food FreedomÉvaluation : 4 sur 5 étoiles4/5 (1)
- How Not to Die by Michael Greger MD, Gene Stone - Book Summary: Discover the Foods Scientifically Proven to Prevent and Reverse DiseaseD'EverandHow Not to Die by Michael Greger MD, Gene Stone - Book Summary: Discover the Foods Scientifically Proven to Prevent and Reverse DiseaseÉvaluation : 4.5 sur 5 étoiles4.5/5 (83)
- Secrets From the Eating Lab: The Science of Weight Loss, the Myth of Willpower, and Why You Should Never Diet AgainD'EverandSecrets From the Eating Lab: The Science of Weight Loss, the Myth of Willpower, and Why You Should Never Diet AgainÉvaluation : 3.5 sur 5 étoiles3.5/5 (38)
- The Diet Trap Solution: Train Your Brain to Lose Weight and Keep It Off for GoodD'EverandThe Diet Trap Solution: Train Your Brain to Lose Weight and Keep It Off for GoodPas encore d'évaluation
- Keto Friendly Recipes: Easy Keto For Busy PeopleD'EverandKeto Friendly Recipes: Easy Keto For Busy PeopleÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- The Candida Cure: The 90-Day Program to Balance Your Gut, Beat Candida, and Restore Vibrant HealthD'EverandThe Candida Cure: The 90-Day Program to Balance Your Gut, Beat Candida, and Restore Vibrant HealthPas encore d'évaluation
- The Arm: Inside the Billion-Dollar Mystery of the Most Valuable Commodity in SportsD'EverandThe Arm: Inside the Billion-Dollar Mystery of the Most Valuable Commodity in SportsÉvaluation : 4 sur 5 étoiles4/5 (49)
- Find Your Path: Honor Your Body, Fuel Your Soul, and Get Strong with the Fit52 LifeD'EverandFind Your Path: Honor Your Body, Fuel Your Soul, and Get Strong with the Fit52 LifeÉvaluation : 4 sur 5 étoiles4/5 (3)
- Eat to Lose, Eat to Win: Your Grab-n-Go Action Plan for a Slimmer, Healthier YouD'EverandEat to Lose, Eat to Win: Your Grab-n-Go Action Plan for a Slimmer, Healthier YouPas encore d'évaluation
- Happy Gut: The Cleansing Program to Help You Lose Weight, Gain Energy, and Eliminate PainD'EverandHappy Gut: The Cleansing Program to Help You Lose Weight, Gain Energy, and Eliminate PainÉvaluation : 3.5 sur 5 étoiles3.5/5 (6)
- Molecules of Emotion: Why You Feel the Way You FeelD'EverandMolecules of Emotion: Why You Feel the Way You FeelÉvaluation : 4 sur 5 étoiles4/5 (128)
- Glucose Revolution: The Life-Changing Power of Balancing Your Blood SugarD'EverandGlucose Revolution: The Life-Changing Power of Balancing Your Blood SugarÉvaluation : 5 sur 5 étoiles5/5 (351)