Académique Documents
Professionnel Documents
Culture Documents
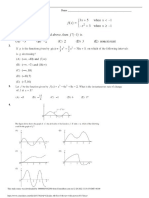
Answers 1996 Free Response
Transféré par
mohammedosamarashidDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Answers 1996 Free Response
Transféré par
mohammedosamarashidDroits d'auteur :
Formats disponibles
Advanced Placement Chemistry: 1996 Free Response Answers
Question 1 is question 4 in previous years, question 2 is question 1 in previous years and questions 3&4 are
questions 2&3 in previous years.
students are now allowed 10 minutes to answer question 1, after which they must seal that portion of the
test.
[delta is used to indicate the capital !ree" letter.
[square root applies to the num#ers enclosed in parenthesis immediately followin$
%ll simplifyin$ assumptions are &ustified within '(.
)ne point deduction for a si$nificant fi$ure or math error, applied only once per pro#lem.
*o credit earned for numerical answer without &ustification.
1+ ,eaction question
-a+ .a.)3 ///0 .a) 1 .)2
-#+ *i 1 .u
21
///0 *i
21
1 .u
hydrated ions accepta#le with correct char$e
1 point for *i-)2+2 as product
-c+ 23)4
2
4 1 2
1
///0 223044
incorrect char$e on 223044 when only one product occurs, 1 point only
1 product point for transfer if 2
1
from an ionic reactant to product when a phosphate
species is incorrectly #ut consistently written.
-d+ .l2 1 5r/ ///0 .l4 1 5r2
no credit for monatomic .l as reactant or 5r as product
-e+ *23 1 2.223)2 ///0 .223)24 1 *24
1
1 product point for *24.223)2
1 point for *23 1 2
1
///0 *24
-f+ -*24+2.)3 1 5a
21
1 )24 ///0 *23 1 5a.)3 1 22)
1 product point for either *23 or 5a.)3
2 product points for all three species correct
-$+ *2)3 1 22) ///0 2*)2
1 product point for 2
1
1 *)24
-h+ 6n)44 1 .2)4
2
4 ///0 6n)2 1 .)2
no penalty for )24 or 22) in equation
no point earned for 6n
21
as product
2+
a+ two points total 7 one point for correct su#stitutions7 one point for computation
[2
1
8 [).l4 8 square root -0.14 9 3.2 9 104
:
+ 8 ;.< 9 104
'
6
since =a
8 - [2
1
[).l4 + > [2).l
8 [2
1
2
> c2).l
-#+ two points total? one point each
).l4 1 22) @8880 2).l 1 )24 -or *a).l 1 22) ///0 *a
1
1 2).l 1 )24+
=# 8 =w > =a 8 1 9 104
14
A 3.2 9 104
:
8 3.1 9 104
<
-c+ two points total7 one for concentrations and one for p2 calc.
.oncentrations #efore reaction?
[2).l 8 [-0.0400+ -0.14+ > 0.0'0 8 0.11 6
[)24 8 [-0.0100+ -0.';+ > 0.0'0 8 0.11 6
Bhus reaction is essentially complete and e9actly equals a solution of *a).l and [).l4 8 0.11 6 -or reaction is at
equivalence point+.
Bhen
[)24 8 [2).l
=# 8 [)24
2
> 0.11 8 3.1 9 104
<
[)24 8 square root [-0.11+ -3.1 9 104
<
+ 8 1.: 9 104
4
p)2 8 3.<3
p2 8 14 / 3.<3 8 10.2<
-d+ two points7 one for half/neutraliCed7 one for mmol calcs.
p2 8 <.4D therefore [2
1
8 3.2 9 104
:
p2 8 p=a , or [2
1
8 =a.
Eo [).l4 > [2).l 8 1 , or solution must #e half neutraliCed.
initial mmol 2).l 8 '0.0 9 0.20 8 10.0 mmol
mmol *a)2 required 8 10.0 A 2 8 '.0 mmol
-e+ one point
From equation, 1 mol 2
1
produced for each 1 mole of 2).l produced, thus [2
1
8 [2).l 8 0.0;' therefore p2 8
1.1D
3+
-a+ two points7 one for line of answer
/ 232.< G>= 8 EH -.22;+ / -2;1.4 1 200.D+ G.>=
EH -.22;+ 8 22D.; G>=
units i$nored7 1 point earned for D:.D G>=7 1 point lost if stoichiometry is not implied in
process
-#+ three points total7 one point each portion7 any value for B -e.$., 2<3 = or 2D: =+ is allowa#le?
[delta2H 8 -/ :4.< "G+ / -22;.< "G+ 8 /311.4"G
8 / 311.4"G / -2D: =+ -/ 0.232<"G>=+
8 / 311.4 "G 1 ;D.3 "G
8 / 242.1 "G
*e$ative [delta!H therefore reaction is spontaneous, or =eq 0 1 therefore reaction is spontaneous, or products are
favored at equili#rium.
-c+ two points
ln = 8 242.1 A [-:.31 9 104
3
+ -2D:+ 8 D<.<
= 8 3 9 10
42
-1,2,or 3 si$nificant fi$ures accepta#le+
-d+ two points7 first point earned for correct su#stitution and correct num#er of #onds, second point earned for
settin$ equal to [delta2r9n and correct calculation of answer7 no points earned for Ie9trapolationI techniques to find
car#on/car#on triple #ond ener$y7 JK is the ener$y of the car#on/car#on triple #ond.
/ 311.4 "G 8 [-2+ -43;+ 1 JK 1 -2+ -414+ / [-34<+ 1 -;+ -414+
JK 8 :20 "G
4+
-a+ one point
L161 8 L262
-1.00M+ -'.20 mol>M+ 8 -9+ -1:.4 mol>M+
9 8 '.2 mol > -1:.4 mol>M+ 8 0.2:3 M -or 2:3 mM+
-#+ two points
mass 1 liter of concentrated 22E)4 8 1 M 9 -1.:4 $>mM+ 9 -1,000 ml>M+ 8 1,:40 $ 22E)4
1:.4 mol 22E)4 9 D:.1 $>mol 8 1,:0' $ 22E)4
mass percent 22E)4 8 -1,:0' $ > 1,:40 $+ 9 100 8 D:.1(
-c+ three points
Etoichiometric ratio of *a2.)3 to 22E)4 8 2?1
10.' $ *a2.)3 9 -1 mol *a2.)3 > :4.0 $ *a2.)3+ 8 0.12' mol *a2.)3
Eince 1 mol 22E)4 reacts with 2 mol *a2.)3, 0.12' mol *a2.)3 reacts with 0.0;2' mol 22E)4
0.0;2' mol 22E)4 8 L 9 6 8 -L+ -'.20 6+
L 8 0.0;2' mol > -'.20 mol>M+ 8 0.0120 M -or 12.0 mM+
-d+ three points
molality 8 moles solute > 1,000 $ solvent 8 moles solute > 1 "$ solvent
mass of 1 M of '.20 6 22E)4 8 1 M 9 -1,000 mM > 1 M+ 9 -1.3: $ 1 mM+ 8 1,3:0 $
mass of 22E)4 in 1 M 8 -'.20 mol>M+ -D:.1 $.mol+ 8 '10 $
mass of 22) in 1 M 8 1,3:0 / '10 8 :<0 $
molality 8 -'.20 mol 22E)4 > :<0 $+ 9 -1,000 $ > 1 "$+ 8 '.D: m
*ote? no credit earned for '.20 mol > 1.3: "$ 8 '.<< m
'+ -a+ two points
.)2
#ecause all contain same num#er of molecules -moles+, and .)2 molecules are the heaviest
*ote? total of 1 point earned if .)2 not chosen #ut same num#er of molecules -moles+ is specified
-#+ two points
%ll are equal
#ecause same temperature, therefore same avera$e "inetic ener$y
*ote? &ust restatement of Isame conditions, etc.I does not earn second point
-c+ two points
.)2
either one?
it has the most electrons, hence is the most polariCa#le
it has the stron$est intermolecular -Mondon+ forces
*ote? also allowa#le are Ipolar #ondsI, Iinelastic collisionsI7 claimin$ lar$er siCe or lar$er molecular volume does
not earn second point
-d+ two points
2e
%ny one?
$reatest movement throu$h the #alloom wall
smallest siCe
$reatest molecular speed
most rapid effusion -!rahamNs law+
;+ for e9planation point in D -a+, -c+, and -d+, credit is earned at step indicted in boldface type.
-a+ two points
.alculated 6m-2%+ too low
6-*a)2+ 80 L-*a)2+ 80 n(Na!" 80 n-2%+ 80 6m-2%+
-6 8 n A L+ and -6m 8 mA n+
-#+ two points
.alculated 6m-2%+ not affected
%ny one of the followin$ reasons. Oater?
does not chan$e n-2%+
chan$es only 6-2%+ // sense of dilution
is not a reactant
-c+ two points
.alculated 6m-2%+ too hi$h
e#$ivalence point 80 n-*a)2+ 80 n-2%+ 80 6m-2%+
(e%pected p! hi&her"
*ote? Ino effect if *a)2 standardiCed with same indicatorI earns 2 points7 no credit earned if p28< or neutral
-d+ two points
.alculated 6m-2%+ too low
L-*a)2+ 80 n(Na!" 80 n-2%+ 80 6m-2%+
*ote? point earned for L-*a)2+ only if?
-i+ no e9planation point is earned in -a+
-ii+ e9planation in -a+ also includes L-*a)2+
<+
-a+ two points
Bhe si$n of the cell potential will #e positive
#ecause -any one is sufficient+?
= is $reater than 1
the reaction is spontaneous -occurs+
JH for Er
21
is more positive
Etandard reduction potential for Er more ne$ative
JH 8 1 0.'2 L
*ote? only 1 point earned for &ust JH positive #ecause =eq positive.
-#+ one point
Bhe o9idiCin$ a$ent is 6$
21
-c+ two point
Bhe cell potential would increase
Eince all ions are at 1 6, Q for the system is 1 and JH 8 -,B>nF+ ln =
so as B increases, so should JH
*ote? no credit lost if student reco$niCes =eq dependence on B. For temperature chan$e in this pro#lem, decrease in
ln = term is small relative to the term ,B>nF
),
*o chan$e, #ecause in the *ernst equation Jcell 8 JH / -,B>nF+ ln Q
ln Q 8 0, and Jcell 8 JH
*ote? this second approach earns 1 point only
-d+ two points
Jcell will increase
Pn the equation Jcell 8 JH / -0.0'D2 > n+ lo$ Q
Q 8 0.1 therefore lo$ Q is ne$ative therefore term after JH is positive therefore Jcell increases
),
with the concentration of 6$
21
lar$er than that of Er
21
, Me .hatelierNs principle predicts the reaction will have a
lar$er drivin$ force to the ri$ht and a more positive Jcell
-e+ one point
%t equili#rium, Jcell 8 0
*ote? I#alancedI, IneutralI, or Ino net reactionI not accepted
:+
-a+ one point
2 *) 1 2 22 ///0 *2 1 2 22)
-#+ two points
*2)2 and *2) are intermediates
#ecause they appear in the mechanism #ut not in the overall products -or reactants+
-c+ three points7 one for each half of conclusion -1+ answer, third for conclusion -2+ answer
Etudent indicates conclusion -1+ is correct,
#ecause the sum of the e9ponents in rate law is 2 1 1 8 3
Etudent indicates conclusion -2+ is incorrect,
#ecause no step involves two *) molecules and a 22 molecule
-d+ two points7 B $oes up therefore " $oes up?
#ecause increasin$ num#er of collisions #etween reactants
are occurin$ with sufficient ener$y to form an activated comple9
),
B $oes up therefore rate $oes up
#ecause no chan$e in concentration of reactants, therefore " must increase
),
from %rrhenius equation -not required in %3 .hemistry curriculum, #ut noted in some student responses+?
as B $oes up, " $oes up
),
$raph as #elow with proper e9planation
D+
-a+ two points
2ydro$en #ondin$ -or dipole/dipole attraction+ in 2F is $reater than it is in 2.l
*ote? only one point earned if simply stated that 2F has $reater intermolecular forces than 2.l
-#+ two points
%sF3 has a tri$onal pyramid shape and #ond dipoles do *)B cancel -or asymmetric molecule+
%sF' has a tri$onal #ipyramid shape and #ond dipoles cancel -or symmetric shape+
*otes? e9planation must refer to shape in order to earn point7 one point earned if only correct Mewis structures are
$iven.
-c+ two points
*)24 has resonance structures
2*)2 has no resonance structures
),
one */) sin$le #ond, one *8) dou#le #ond
*ote? one point earned if only correct Mewis structures, includin$ resonance for *)24 $iven.
-d+ two points
Eulfur uses d or#itals -or e9panded octet+, o9y$en has no d or#itals in its valence shell
),
Eulfur is a lar$er atom, can accomodate more #onds.
Vous aimerez peut-être aussi
- Before the Application: How to Become the Ideal College Candidate (A Step-by-Step Guide to Making Each Year of High School Count)D'EverandBefore the Application: How to Become the Ideal College Candidate (A Step-by-Step Guide to Making Each Year of High School Count)Pas encore d'évaluation
- Tables of Laguerre Polynomials and Functions: Mathematical Tables Series, Vol. 39D'EverandTables of Laguerre Polynomials and Functions: Mathematical Tables Series, Vol. 39Pas encore d'évaluation
- University of Waterloo Physics 115 Final ExamDocument13 pagesUniversity of Waterloo Physics 115 Final ExamAashish GauravPas encore d'évaluation
- Che102 Chemistry For Engineers: Final Exam Review Package Waterloo SosDocument29 pagesChe102 Chemistry For Engineers: Final Exam Review Package Waterloo SosalyPas encore d'évaluation
- Math 2000Document4 pagesMath 2000rdialfinPas encore d'évaluation
- Ap10 FRQ Physics B FormbDocument11 pagesAp10 FRQ Physics B FormbrsbasuPas encore d'évaluation
- Math 103 Quiz EsDocument17 pagesMath 103 Quiz Esalfredomedardo100% (1)
- Syllabus Ap Calculus BC 2019Document6 pagesSyllabus Ap Calculus BC 2019api-318044188Pas encore d'évaluation
- Session 1 Calculus AB 2023 AP Daily Practice SessionsDocument1 pageSession 1 Calculus AB 2023 AP Daily Practice SessionsAdel AbdulrahmanPas encore d'évaluation
- AP Physics C Mechanics Sample Syllabus 1Document9 pagesAP Physics C Mechanics Sample Syllabus 1mahmoudPas encore d'évaluation
- Predicting Reaction NotesDocument4 pagesPredicting Reaction NotesCindy MoPas encore d'évaluation
- Math099 SyllabusDocument9 pagesMath099 SyllabustinomutendaPas encore d'évaluation
- AP Calculus AB Test 3 Review With Answers16 17 PDFDocument6 pagesAP Calculus AB Test 3 Review With Answers16 17 PDFEthan NguyenPas encore d'évaluation
- SAT Session 8Document58 pagesSAT Session 8Arjuna PaulPas encore d'évaluation
- Aplang 2007 Answer KeyDocument3 pagesAplang 2007 Answer KeyJorge LopezPas encore d'évaluation
- Ap23 Apc Chemistry q1Document15 pagesAp23 Apc Chemistry q1Dylan DanovPas encore d'évaluation
- Pedagogy Professional KnowledgeDocument6 pagesPedagogy Professional Knowledgemamumeed100% (3)
- Tissue EngineeringDocument8 pagesTissue EngineeringLnplsnPas encore d'évaluation
- CrackingSAT 2019 Study Guide 4-WeekDocument6 pagesCrackingSAT 2019 Study Guide 4-WeekTommyPas encore d'évaluation
- Ap Calculus Ab 2019 Practice Exam FRQDocument20 pagesAp Calculus Ab 2019 Practice Exam FRQJosh Albee0% (1)
- Chapter Test B: Teacher Notes and Answers Electric Forces and FieldsDocument7 pagesChapter Test B: Teacher Notes and Answers Electric Forces and Fieldsmahsan abbasPas encore d'évaluation
- ARCO SAT Subject Math Level 2 Practice TestDocument24 pagesARCO SAT Subject Math Level 2 Practice TestRekhaBhandariPas encore d'évaluation
- Calc 152 Qs Practice CollectionDocument80 pagesCalc 152 Qs Practice Collection策略王1Pas encore d'évaluation
- Chapter 4 Chemical Bonding and Molecular StructureDocument26 pagesChapter 4 Chemical Bonding and Molecular StructureYash PlayPas encore d'évaluation
- Collegeboard-Approved Ap Calculus Ab SyllabusDocument7 pagesCollegeboard-Approved Ap Calculus Ab Syllabusapi-292365857Pas encore d'évaluation
- ARCO SAT Subject Math Level 2 Practice TestDocument23 pagesARCO SAT Subject Math Level 2 Practice TestJam GeejeePas encore d'évaluation
- Ap Biology 2020 Practice Exam 1 Scoring WorksheetDocument1 pageAp Biology 2020 Practice Exam 1 Scoring WorksheetkaylaPas encore d'évaluation
- AP Chem Practice TestDocument14 pagesAP Chem Practice Testamrdeck1Pas encore d'évaluation
- Introduction To Differential EquationsDocument3 pagesIntroduction To Differential EquationsZarqa MiraniPas encore d'évaluation
- CA 2 MomentsDocument3 pagesCA 2 MomentsOctavius_19930% (5)
- Practice Momentum TestDocument5 pagesPractice Momentum TestKinalPatelPas encore d'évaluation
- Chemical Bonding - Study NotesDocument15 pagesChemical Bonding - Study NotesTamoghna DeyPas encore d'évaluation
- Angular MotionDocument21 pagesAngular Motionsuperman618Pas encore d'évaluation
- Physics Bowl ExamDocument7 pagesPhysics Bowl ExamShilpa GodbolePas encore d'évaluation
- AP Physics 1 Practice Test 1 KEYDocument1 pageAP Physics 1 Practice Test 1 KEYMohamed MohamedPas encore d'évaluation
- 2009 AP United States: Chapter III: Answers To The Government and Politics ExamDocument2 pages2009 AP United States: Chapter III: Answers To The Government and Politics ExamBob KeyPas encore d'évaluation
- SAT Product Knowledge - 20170220Document43 pagesSAT Product Knowledge - 20170220ZUPPPas encore d'évaluation
- SAT Math 1 NotesDocument47 pagesSAT Math 1 NotesSergioPas encore d'évaluation
- SAT II BiologyDocument4 pagesSAT II BiologysharabitarekPas encore d'évaluation
- AP PHYSICS B 1984 MC + SolutionsDocument19 pagesAP PHYSICS B 1984 MC + SolutionsbastardPas encore d'évaluation
- TB LoopsandConditionalsPracticeTestDocument34 pagesTB LoopsandConditionalsPracticeTestemi dPas encore d'évaluation
- Mccune Sandra Clark William Mcgrawhills 500 Precalculus QuesDocument174 pagesMccune Sandra Clark William Mcgrawhills 500 Precalculus QuesGwynbleidd WitcherPas encore d'évaluation
- Ap Physics 1 - Summer AssignmentsDocument93 pagesAp Physics 1 - Summer AssignmentsNicole Saint-AubinPas encore d'évaluation
- APM263 GrossmanSolutionManualDocument196 pagesAPM263 GrossmanSolutionManualJared AllenPas encore d'évaluation
- Math Strategies QuizDocument18 pagesMath Strategies QuizS.L.L.CPas encore d'évaluation
- AP Practice ExamDocument18 pagesAP Practice ExamDSaulBPas encore d'évaluation
- SG Unit4ProgressCheckFRQ 63fef7b960f508 63fef7bac30bc9 07327279Document8 pagesSG Unit4ProgressCheckFRQ 63fef7b960f508 63fef7bac30bc9 07327279Qamariya AlbadiPas encore d'évaluation
- 002 - WorkbookDocument52 pages002 - WorkbookPeter LeePas encore d'évaluation
- Phys 121 f15 AllDocument5 pagesPhys 121 f15 AllAnonymous bZTdTpLPas encore d'évaluation
- Grading Policy-Ap Calculus BCDocument2 pagesGrading Policy-Ap Calculus BCapi-319065418Pas encore d'évaluation
- AP Statistics SyllabusDocument4 pagesAP Statistics SyllabusdarshPas encore d'évaluation
- AP Physics C Magnetism MCDocument21 pagesAP Physics C Magnetism MCShameem AkhterPas encore d'évaluation
- PDFDocument56 pagesPDFKEERTHANA SASIDHARANPas encore d'évaluation
- Sat SchedDocument2 pagesSat SchedTrishiaJustineBattungPas encore d'évaluation
- Determination of Composition of Complexes Using Jobs MethodDocument10 pagesDetermination of Composition of Complexes Using Jobs Methodnawal200750% (8)
- Exam2 Final Differential EquationsDocument7 pagesExam2 Final Differential EquationshellooceanPas encore d'évaluation
- PersonalDocument8 pagesPersonalAtanu Brainless RoyPas encore d'évaluation
- Quiz3 2015 SolutionsDocument8 pagesQuiz3 2015 SolutionsGerald RattichPas encore d'évaluation
- EC 400 Quizzes For Class 2Document14 pagesEC 400 Quizzes For Class 2Avijit PuriPas encore d'évaluation
- Mark Scheme (Results) June 2013: GCE Core Mathematics 4 (6666/01)Document28 pagesMark Scheme (Results) June 2013: GCE Core Mathematics 4 (6666/01)Abdulrahman JijawiPas encore d'évaluation
- Asme Code Qualification of Pipe Bends With Localized Wall Thinning PDFDocument8 pagesAsme Code Qualification of Pipe Bends With Localized Wall Thinning PDFZhiqiang GuPas encore d'évaluation
- IbnTaymiyyah Theological EthicsDocument361 pagesIbnTaymiyyah Theological EthicsDado Daki100% (1)
- Vanilla Farming: The Way Forward: July 2019Document6 pagesVanilla Farming: The Way Forward: July 2019mituPas encore d'évaluation
- The 50 Most Inspiring Travel Quotes of All TimeDocument4 pagesThe 50 Most Inspiring Travel Quotes of All Timeungku1Pas encore d'évaluation
- Full Download Test Bank For Environmental Economics and Management Theory Policy and Applications 6th Edition Callan PDF Full ChapterDocument27 pagesFull Download Test Bank For Environmental Economics and Management Theory Policy and Applications 6th Edition Callan PDF Full Chapterscissionrideau941m100% (20)
- Assembly Manual, Operation and Maintenance Round Vibrating Screen Model: Tav-Pvrd-120Document15 pagesAssembly Manual, Operation and Maintenance Round Vibrating Screen Model: Tav-Pvrd-120Sandro Garcia Olimpio100% (1)
- Cooking Off The Clock by Elizabeth Falkner - Recipes and ExcerptDocument16 pagesCooking Off The Clock by Elizabeth Falkner - Recipes and ExcerptThe Recipe ClubPas encore d'évaluation
- ADT Cat D400EDocument2 pagesADT Cat D400EEbran AndromedaPas encore d'évaluation
- Evaluation of Global Research Trends in The Area of Food Waste D - 2020 - Food CDocument10 pagesEvaluation of Global Research Trends in The Area of Food Waste D - 2020 - Food CAliPas encore d'évaluation
- Practice Exam 3 KEY (Solutions)Document13 pagesPractice Exam 3 KEY (Solutions)josePas encore d'évaluation
- Band Structure Engineering in Gallium Sulfde NanostructuresDocument9 pagesBand Structure Engineering in Gallium Sulfde NanostructuresucimolfettaPas encore d'évaluation
- Concrete ON MALDIVESDocument55 pagesConcrete ON MALDIVESKãrthìçk JkrPas encore d'évaluation
- BÀI TẬP TA 9 THEO CHUYÊN ĐỀ NGỮ PHÁPDocument213 pagesBÀI TẬP TA 9 THEO CHUYÊN ĐỀ NGỮ PHÁPhoangmaiPas encore d'évaluation
- 31. (NÂNG CAO) Đề soạn theo cấu trúc minh họa 2021 - Tiếng Anh - Đề 31 - DươngDocument15 pages31. (NÂNG CAO) Đề soạn theo cấu trúc minh họa 2021 - Tiếng Anh - Đề 31 - DươngNguyễn Quế Anh100% (1)
- Smart Dust Technology Seminar ReportDocument32 pagesSmart Dust Technology Seminar ReportSushan Upadhyay67% (3)
- Chapter 1 Cumulative Review: Multiple ChoiceDocument2 pagesChapter 1 Cumulative Review: Multiple ChoiceJ. LeePas encore d'évaluation
- Moving Money Box: Pig (Assembly Instructions) : The Movements Work Better With Heavier CoinsDocument6 pagesMoving Money Box: Pig (Assembly Instructions) : The Movements Work Better With Heavier CoinsjuanPas encore d'évaluation
- Graviola1 Triamazon, AntiCancer, InglesDocument2 pagesGraviola1 Triamazon, AntiCancer, InglesManuel SierraPas encore d'évaluation
- Audio Spot LightingDocument20 pagesAudio Spot LightingLavanya Vaishnavi D.A.Pas encore d'évaluation
- Miltel - Case Study, Steven Age, UKDocument2 pagesMiltel - Case Study, Steven Age, UKAnit SahuPas encore d'évaluation
- Massimo Cacciari, 1994. The Necessary AngelDocument133 pagesMassimo Cacciari, 1994. The Necessary AngelAbner J ColmenaresPas encore d'évaluation
- The Art of Logical ThinkingDocument210 pagesThe Art of Logical ThinkingAndyAyam100% (1)
- Agricultural Extension and CommunicationDocument173 pagesAgricultural Extension and CommunicationAlfredo Conde100% (1)
- FF0221 01 Free Corporate Slides For Powerpoint 16x9Document14 pagesFF0221 01 Free Corporate Slides For Powerpoint 16x9KevinChicoReyesPas encore d'évaluation
- PrionDocument22 pagesPrionAnushkaPas encore d'évaluation
- List of Irc Publications Referred To in The Specifications: AppendicesDocument17 pagesList of Irc Publications Referred To in The Specifications: AppendicesPrasad BoniPas encore d'évaluation
- RadarDocument65 pagesRadarAsifa LiaqatPas encore d'évaluation
- Flooding Deagon Flood Flag MapDocument1 pageFlooding Deagon Flood Flag MapNgaire TaylorPas encore d'évaluation
- Travelsinvarious03clar BWDocument522 pagesTravelsinvarious03clar BWSima Sorin MihailPas encore d'évaluation
- Tsang Mui Millennium School 2019-2020 English Worksheet: Fill in The Blanks With The Correct Form of The VerbsDocument46 pagesTsang Mui Millennium School 2019-2020 English Worksheet: Fill in The Blanks With The Correct Form of The VerbscocoyipPas encore d'évaluation