Académique Documents
Professionnel Documents
Culture Documents
Iron Carbon Equilibrium Diagram
Transféré par
ganesh82Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Iron Carbon Equilibrium Diagram
Transféré par
ganesh82Droits d'auteur :
Formats disponibles
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
50
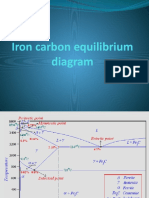
5. THE IRON-CARBON EQUILIBRIUM DIAGRAM
INTRODUCTION
The Iron-carbon diagram is the basis of two very important groups of alloys: steels and cast
irons (also termed ferrous alloys). Their importance derives from the following:
i) the relative abundance of iron which forms about 5% of the earth's crust.
ii) the ease with which the iron ore can be reduced to the metal. The reduction can be done
using carbon (which is cheap and is also the alloying element) as the reducing agent.
iii) their wide range of properties which makes them very versatile.
For this reason, ferrous alloys are relatively cheap and are the first choice as structural
material unless other considerations (weight, corrosion resistance, etc.,) dictate otherwise.
Their main disadvantage is their high susceptibility to atmospheric corrosion.
ALLOTROPIC FORMS OF IRON
Iron exhibits temperature based polymorphism (see chapter two): up to 912C, it has a bcc
crystal structure and this polymorph is termed iron. Between 912C and 1400C, the
structure changes to fcc - the polymorph being termed -- iron. The last polymorph, -- iron,
which also has a bcc structure, exists between 1400C and 1535C, (the melting point of
iron). Thus on heating, the volume change of iron with temperature will have the shape
shown below.
Fig 5.2 The variation of volume with temperature when iron is heated
There is normal thermal expansion up to 912C when a sudden drop occurs. This results due
to the closer packing of the fcc structure. A sudden rise in volume takes place at 1400C
when the structure reverts to bcc. This is followed by normal thermal expansion up to
melting at 1535C. Another important temperature is the Curie point, 769C at which iron
losses its magnetism (changes from ferromagnetic to paramagnetic).
Carbon dissolves interstitially in all the above polymorphs of iron. It has a maximum
solubility of 0.02% in --Fe at 723C. The resulting solid solution is given the special name
ferrite and designated by . The solubility in --Fe reaches a maximum of 2.06% at 1146C,
the resulting solid solution being given the name austenite and is designated by .
The higher solubility of carbon in --Fe (despite its higher atomic packing factor compared to
-Fe) results from the fact that the interstices in fcc are fewer but each one is bigger than
V
o
l
912
1535 1400
Temp (
o
C)
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
51
those in the bcc (which are more but smaller). A Carbon atom taking the (1/2, 0, 1/2)
position in a bcc lattice causes more distortion than one taking the (1/2, 1/2, 1/2) position in
an fcc). Finally, dissolves up to 0.1% carbon at 1492C. This solution, has no
technological importance and hence has no special name. It is designated by .
In addition to the above solid solutions, iron and carbon form an inter-metallic compound
Fe
3
C which is termed cementite or carbide. From equation
Ax wt at y wt at
y wt at
B Wt
. . . .
. .
% .
+
=
B
B
,
the weight percentage of carbon in Fe
3
C is 6.67%.
EUTECTIC, EUTECTOID AND PERITECTIC DECOMPOSITION IN THE
FE-C SYSTEM
Like most systems with an inter-metallic compound, only the part of the Fe-C diagram
between Fe and Fe
3
C has technological importance. In the Fe-Fe
3
C system, the peritectic
reaction takes place at 1492C (0.3%C) when reacts with excess liquid to form . The
eutectic reaction takes place at 1146C and 4.3%C when the liquid precipitates alternate
layers of and cementite. The cementite which results from the eutectic reaction is termed
primary cementite and the eutectic is termed ledeburite. The eutectoid reaction takes place at
723C, 0.8%C when iron changes polymorph from -Fe (fcc) to -Fe (bcc). Since the
solubility of carbon in -Fe is much higher than in -Fe, the excess carbon diffuses out of
solution to form Fe
3
C. Thus we have precipitating alternate layers of and cementite. The
product of the eutectoid reaction is termed pearlite.
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
52
With the information given above, the iron-carbon (or more correctly the Iron-Fe
3
C) diagram
looks as shown in fig. 5.2. Due to the importance of ferrous alloys all engineers must be well
conversant with the Fe-C diagram.
STABLE AND METASTABLE FE-C SYSTEMS
The Fe-Fe
3
C system described above is termed the metastable system. Under certain
conditions (slow cooling rate in presence of other elements especially silicon) Fe
3
C does not
form and graphite (carbon) precipitates instead. Fe
3
C already formed may also break down
to and free carbon in the form of graphite. The Fe- graphite system is referred to as the
stable system. In the stable system, only carbon in excess of 0.8%C can form graphite. The
rest will stay as Fe
3
C similar to the case in the metastable system.
CLASSIFICATION OF FERROUS ALLOYS
PLAIN CARBON STEELS
The products of the eutectoid reaction are termed steels. They have less than 2.06%C and
have the distinct characteristic that they can be heated to a single solid phase . This makes
them suitable for hot working processes like forging, hot rolling, etc. The properties of steels
can be varied over a wide range by controlling the rate of the decomposition of austenite to
ferrite and cementite, and by adding other alloying elements in addition to carbon. This
accounts for the very widespread use of steel.
Fig 6.2 Fe-Fe
3
C equilibrium phase diagram
Peritectic
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
53
A steel which contains only carbon as alloying element (with up to 1% Mn) is termed a plain
carbon steel. If any other alloying elements are added, it is termed an alloy steel. The
following terminology is commonly used when dealing with plain carbon steels:
i) Ingot iron -- carbon content less than 0.05%. Hence high ductility.
ii) dead soft mild steels -- carbon content between 0.05% and 0.15%.
iii) mild steels - carbon content between 0.15% and 0.3%. These have adequate
plasticity, good machineability and weldability. Mild steel is therefore the most
commonly used structural material and is used for making structural shapes: I--
beams, channels and angles.
iv) medium carbon steels -- carbon content between 0.3-0.5%.
v) high carbon steel -- carbon content higher than 0.5%.
5.5.2 Hypo and Hyper-eutectoid Steels
As stated above, steels are the product of the eutectoid reaction in the Fe-C system. The
eutectoid reaction gives simultaneous precipitation of and cementite from . The resulting
product, which consists of alternate layers of and cementite is termed pearlite (from its
appearance of alternate black and white streaks when viewed under the microscope). A steel
with 0.8%C will consist entirely of pearlite when cooled under equilibrium conditions and is
termed an eutectoid steel. A steel with carbon content below 0.8% is termed a hypo-
eutectoid steel. If cooled under equilibrium conditions, the microstructure of hypo-eutectoid
steels will consist of a mixture of ferrite (which precipitates before the eutectoid reaction and
is termed pro-eutectoid ferrite) and pearlite. The ferrite and cementite in pearlite are termed
eutectoid ferrite and eutectoid cementite respectively. A steel with carbon content in excess
of 0.8%C is termed hyper-eutectoid steel and will consist of pro-eutectoid or secondary
cementite (usually precipitated at the grain boundaries of austenite -- the prior-austenite grain
boundaries) and pearlite. Fig. 5.3, which is an enlargement of the steel part of the Fe-C
diagram and fig. 6.4 showing schematically the grain structure of the various steels help to
illustrate these points. In fig. 5.3, points along PS are termed lower critical point, A
1
, those
along GS, the uper critical point, A
3
, while A
2
represents the Curie point. A
1
, A
2
and A
3
are also termed arrest temperatures.
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
54
Fig 5.3- Iron-carbon diagram
If the cooling rate is rather fast, pro-eutectoid (secondary) cementite will precipitate inside
the grains along certain preferred crystal plains -- forming a needle like network termed a
Widmamnstatten structure.
(A) (B) (C)
723
o
C
A
3
A
2
A
1
A
cm
+L
+
+ Pearlite
+L
+
+ C
P
e
a
r
l
i
t
e
1535
912
1134
0.86%C
2.01%C
P S
G
Ferrite
Pearlite
Cementite
Fig 5.4 The structures of (a) Hypoeutectoid (b) Eutectoid (c) Hyper-eutectoid steels
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
55
5.5.3 Quantities of Phases in the Fe-C System
The ratios of the phases in the Fe-C system can be easily calculated using the lever rule. For
a hypo-eutectoid steel with x% carbon just below the eutectoid temperature:
ratio of pro-eutectoid ferrite:
2 0 . 0 - 8 . 0
x - 8 . 0
ratio of pearlite = ratio of just above eutectoid temperature:
2 0 . 0 - 8 . 0
2 0 . 0 - x
ratio of eutectoid ferrite:
e t i l r a e p . t a u q
8 8 . 0 =
2 0 . 0 - 7 6 . 6
8 . 0 - 7 6 . 6
eutectoid cementite:
= 0.12 of quantity of Pearlite
The total quantity (ratio) of ferrite:
=(6.67 - x)/( 6.67 - 0.02)
and that of cementite:
= (x - 0.02)/ (6.67 - 0.02 )
Similarly for a hyper-eutectoid steel containing x%C:
ratio of pro-eutectoid cementite:
= (x - 0.8)/ ( 6.67 - 0.8)
ratio of pearlite:
= (6.67 - x)/ (6.67 - 0.8)
The ratios of eutectoid ferrite and cementite can be calculated from the same equations as for
hypo-eutectoid steel. As the temperature changes, the ratios also change and hence for any
steel, a phase transformation diagram can be plotted. Such a diagram is shown in figure 6.5
for a 0.6% C steel. Phase transformation diagrams are easily constructed from the phase
diagram using the above equations as appropriate.
Decrease in the solubility of carbon in ferrite as the temperature is decreased leads to
precipitation of ternary cementite which segregate to the grain boundaries or form plate-like
needles inside the ferrite matrix. Quantities of phases in the metastable system and for
products of the eutectic reaction can be determined easily in a manner similar to what is done
above for steels.
5.5.4 Properties of Annealed Plain-Carbon Steels
The mechanical properties of plain carbon steels are affected by:
i) the size and number of phases present;
ii) the ratio of the phases (which is a function of the carbon content);
iii) shape and distribution of the minor phase.
Ferrite, being a solid solution is soft and ductile. Pearlite has moderate strength which
depends on the size of the lamellae. The size of the lamellae in turn depends on the cooling
rate. The slower the cooling rate, the coarser the lamellae and the lower the hardness
(approximately. 170 HB). Finer lamellae can have hardness up to 340 HB since slip is more
difficult in the finer structure. With other factors kept constant therefore, the strength
(hardness) of a steel will vary with carbon content as shown in fig. 5.5. As the carbon
content increases, the ratio of pearlite increases. Maximum hardness is achieved at 100%
pearlite (0.86%C).
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
56
The shape and distribution of the minor phase also affects the properties. If the minor phase
(cementite in this case) is distributed as fine particles inside the ductile ferrite, the toughness
is increased. If the particles are larger or if they segregate to the grain boundaries, both the
hardness and toughness are reduced.
Steels are classified according to chemical composition of alloying ingredients:-
Carbon steels
Alloy steels
Stainless steels
Carbon Steels
Carbon steels as explained earlier contain mainly iron and carbon. Other elements such as
silicon, manganese, sulphur, phosphorous are considered as impurities. A deoxidizer can ce
added in order to remove the impurities.
Carbon is the most important element that determines the properties of steel
0.035% Tensile strength increases upto 0.83% Carbon
Properites:-
Good machinability
Deep hardening properties
Dont easily fail due to fatigue
Employed in forgings, crankshaft pins, gears, railway axels, connecting rods
High carbon steels
These contain over 0.6% C
They posses good machinability and high strength
The presence of silicon in carbon steels :-
Acts as a major deoxidizer
Promotes grain size
Imparts deep hardening properties
Facilitates carburization
0
0.5
1.0
%C
H
a
r
d
n
e
s
s
(b)
(a)
0.8
Fig 5.5 The
variation of
hardness with
carbon content in
plain carbon steels
(a) normalized
(b) quenched.
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
57
ALLOY STEELS
These are steels with special alloying elements like Nickel, Chromium, Molybdenum,
tungsten, vanadium, titanium, aluminium, cobalt e.t.c.
Silicon and aluminium in excess of 0.5% and 1.5% respectively may be considered as
alloying elements.
Low Alloy Steels
Those that may require heat treatments
They have similar microstructure and may have the same contents as plain carbon steels.
High alloy steels
These dont require similar heat treatments as plain carbon steels
STAINLESS STEELS
These are iron based alloys having great resistance to corrosion due to addition of Nickel and
chromium. Oxidation of the chromium on the surface creates a protective oxide film (that
prevents further attack).
Stainless steels are selected for their excellent resistance to corrosion. All true stainless steels
contain a minimum of about 12 % Cr, which permits a thin, protective surface layer of
chromium oxide to form when the steel is exposed to oxygen. Chromium is also a ferrite
stabilising element. Chromium causes the austenite region to shrink, while the ferrite region
increases in size. For high-chromium, low-carbon compositions, ferrite is present as a single
phase up to the solidus temperature.
There are several categories of stainless steels based on crystal structure and strengthening
mechanism.
Classes:-
Martensitic stainless steels
Cr 16%, 0.7 1.5% Carbon
These are hardenable and magnetic
They are referred to as chromium stainless steels
They are resistant to corrosion
Tempering at 500
o
C does not affect tensile strength.
They are used in the making of utensils
A 17% Cr-0.5%-C alloy heated to 1 200C forms 100% austenite, which transforms to
martensite on quenching in oil. The martensite is then tempered to produce high strengths and
hardnesses. The chromium content is usually less than 17% Cr; otherwise, the austenite field
becomes so small that very stringent control over both austenitising temperature and carbon
content is required. Lower chromium contents also permit the carbon content to vary from
about 0.1 % to 1.0%, allowing martensites of different hardnesses to be produced. The
combination of hardness, strength, and corrosion resistance makes the alloys attractive for
applications such as high quality knives, ball bearings, and valves.
Ferritic stainless steels
12 - 25% Cr
These are non-hardenable because - transformation temperature is raised by the presence
of chromium
Properties
Considerable ductility
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
58
Excellent corrosion resistance
Relatively cheap
In order to resist corrosion at high temperatures, Cr can be increased to 30%
Used in kitchen ware, chemical equipment (can resist nitric acid)
Ferritic Stainless Steels
Ferritic stainless steels contain up to 30% Cr and less than 0.12% C. Because of the BCC
structure, the ferritic stainless steels have good strengths and moderate ductilities derived
from solid solution strengthening and strain hardening. Ferritic stainless steels have excellent
corrosion resistance and moderate formability and are relatively inexpensive.
Austenitic stainless steels
Contain Chromium and Nickel totaling up to 24% (Combined total)
They are non-hardenable and non-magnetic
Properties:-
Resistant to acids
Addition of tungsten and molybdenum increase strength at elevated temperatures
Selenium and sulphur improve machinability
Used in parts subjected to severe stresses at elevated temperatures.
Nickel, which is an austenite stabilising element, increases the size of the austenite field,
while nearly eliminating ferrite from the iron-chromiumcarbon alloys. If the carbon content is
below about 0.03%, the carbides do not form and the steel is virtually all austenite at room
temperature.
The FCC austenitic stainless steels have excellent ductility, formability, and corrosion
resistance. Strength is obtained by extensive solid solution strengthening, and the austenitic
stainless steels may be cold-worked to higher strengths than the ferritic stainless steels. The
steels have excellent low-temperature impact properties, since they have no transition
temperature. Furthermore, the austenitic stainless steels are not ferromagnetic. Unfortunately,
the high nickel and chromium contents make the alloys expensive.
Precipitation-Hardening (PH) Stainless Steels
The precipitation-hardening (or PH) stainless steels contain AI, Nb, or Ta and derive their
properties from solid-solution strengthening, strain hardening, age hardening, and the
martensitic reaction. The steel is first heated and quenched to permit the austenite to
transform to martensite. Reheating permits precipitates such as Ni3Al to form from the
martensite. High mechanical properties are obtained even with low carbon contents.
Duplex Stainless Steels
In some cases, mixtures of phases are deliberately introduced into the stainless steel structure.
By appropriate control of the composition and heat treatment, a duplex stainless steel
containing approximately 50% ferrite and 50% austenite can be produced. This combination
provides a set of mechanical properties, corrosion resistance, formability, and weldability not
obtained in any one of the usual stainless steels.
Effect of alloying elements
Generally tend to improve properties
Molybdenum, chromium, vanadium provide hardness
Nickel, chromium improve corrosion resistance.
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
59
Vanadium, Molybdenum, chromium provide additional har abrasive particles to improve
wear resistance. They also act as deoxidisers and reduce blow holes in processed steel.
Sulphur, lead, phosphorous improve machinability
Manganese minimizes brittleness resulting form excess sulphur
Managenese molybdenum, chromium, nickel and silicon slow down the rate of
transformation of to pearlite upon cooling thus allowing thick sections to be hardened.
5.6 CAST IRON
The products of the eutectic reaction (1146C, 4.3%C) are termed cast irons. They contain at
least 2.1% carbon. The eutectic reaction gives a simultaneous precipitation of and
cementite (in the metastable system) which is termed ledeburite. These alloys are suitable for
casting due to the low melting point (eutectic temperature); their castability (ability to fill
moulds), their high elastic modulus (rigidity) and their high damping capacity (ability to
absorb vibration energy). Some grades of cast iron also have good machineability.
5.6.1 White Cast Iron
If the precipitation takes place within the metastable system (this is favoured by a relatively
fast cooling rate, absence of silicon and nickel), then the structure will consist of ferrite,
cementite and pearlite at room temperature (the austenite originally precipitated at 1146C
undergoes the eutectoid reaction at 723C). Cast iron which contains all its carbon in the
form of cementite is termed white cast iron. Due to the hard cementite; white cast iron is
hard, brittle ( practically 0%) and virtually unmachineable. It may therefore be used only
in those applications where wear resistance is the prime requirement. As was the case with
steels, we can have hypo-- and hyper--eutectic white cast irons. In hyper--eutectic white cast
irons, the primary cementite (cementite precipitated before the eutectoid reaction) can only
be retained if the material is quenched. The cementite which precipitates as part of ledeburite
is termed ledeburitic or "free" cementite.
If the cooling rate is fairly slow and if appreciable amounts of silicon and/or nickel are
present in the melt, the carbon will precipitate within the stable system as graphite in the
form of graphite flakes. Cast iron which contains all its carbon as graphite is termed grey
cast iron ( the graphite gives a grey appearance to a broken piece of this form of cast iron).
The tendency to form graphite is also increased by increase in carbon content.
Grey cast iron is weak and has no shock-resistance. This results due to the tips of the
graphite flakes acting as areas of stress concentration. For the same reason, grey cast iron has
no notch sensitivity (i.e., the presence of notches does not severely reduce its strength). Grey
cast iron usually contains its iron as pearlite (plate 6.1). If however it is quenched after
solidification, the austenite forms martensite (see chapter nine) and we end up with
martensitic grey cast iron which is hard.
5.6.3 Malleable Cast Iron
The extreme properties of grey and white cast iron make their fields of application fairly
limited. Malleable cast iron is produced by heat treatment of white cast iron to reduce its
brittleness and hardness. There are two industrial processes used for the heat treatment: In
the "Blackheart" process, white cast iron is heated at about 900C for 2-3 days and cooled
very slowly in a neutral atmosphere. The cementite breaks down to ferrite plus spheroidal
graphite. Thus the final microstructure is ferrite + pearlite (from austenite) + spheroidal
graphite. In the "Whiteheart" process, the heating is done as above but in the presence of iron
CHP 316 MATERIALS SCIENCE - MOI UNIVERSITY
60
ore. Again the cementite breaks down as above but near the surface, the ore oxides the
carbon such that the structure at the surface becomes ferrite while the centre is ferrite +
pearlite + graphite nodules. Malleable cast iron has adequate ductility and toughness while
retaining adequate strength. It also has good machineability. Since the density of graphite is
less than that of cementite, formation of malleable cast iron is accompanied by expansion.
This is termed "growth" of cast iron.
The production of malleable iron requires several steps. Graphite nodules nucleate as the
white iron is slowly heated. During the first stage graphitization (FSG) cementite
decomposes to the stable austenite and graphite phases as carbon in Fe3C diffuses to the
graphite nuclei. Following FSG, the austenite transforms during cooling.
5.6.4 Other Types of Cast Iron
Other commercially available forms of cast iron include spheroidal cast iron and alloy cast
iron. In spheroidal cast iron (also called nodular or ductile cast iron) magnesium and nickel
are added to the melt. Cementite then solidifies in the form of spherical nodules inside a
matrix of ferrite and pearlite. The resulting structure is tougher and harder. In alloy cast iron,
other alloying elements are added to achieve specific properties. Chromium, nickel or
molybdenum are added to increase the strength and wear resistance, 15-25% Ni is added to
produce wear resistant cast iron, etc.
Fig 5.6 The variation of microstructure of cast iron with carbon content and silicon content.
2 4 6
%
C
%Si
White
Pearlite +
Ferrite
Ferrite
Mot Pearlite
4
3
2
1
Vous aimerez peut-être aussi
- Chapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMDocument13 pagesChapter 5, THE IRON-CARBON EQUILIBRIUM DIAGRAMPAUL NDIRITUPas encore d'évaluation
- University of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedDocument13 pagesUniversity of Babylon, College of Engineering, Engineering Metallurgy, Maithem H-RasheedAris BulaongPas encore d'évaluation
- Ch-27.3 Iron Carbon Equilibrium DiagramDocument58 pagesCh-27.3 Iron Carbon Equilibrium DiagramasjfgauojfgfPas encore d'évaluation
- Iron Carbon DiagramDocument10 pagesIron Carbon DiagramsivakumarPas encore d'évaluation
- Iron Carbon Phase DiagramDocument4 pagesIron Carbon Phase DiagramMizanur RahmanPas encore d'évaluation
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument52 pagesCh-27.5 Iron Carbon Equilibrium DiagramManojPas encore d'évaluation
- Steels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceDocument39 pagesSteels: Ii Beng (Hons) Mech Eng (Well Eng) Metallurgy & Manufacturing ScienceKareem YasserPas encore d'évaluation
- Fe-Fe3C Phase Diagram and MicrostructuresDocument42 pagesFe-Fe3C Phase Diagram and MicrostructuresTalaat Ahmed Mohamed El-Benawy100% (2)
- Weldability of Metals - NPTELDocument18 pagesWeldability of Metals - NPTELKaushal Gandhi0% (1)
- 9 Engineering AlloysDocument17 pages9 Engineering AlloysdavidtomyPas encore d'évaluation
- Introduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihDocument42 pagesIntroduction To Fe-C Equilibrium Phase Diagram: Chia-Chang ShihTuấnPhạmPas encore d'évaluation
- Ch-27.5 Iron Carbon Equilibrium DiagramDocument53 pagesCh-27.5 Iron Carbon Equilibrium DiagramSmruti Ranjan PattanayakPas encore d'évaluation
- Iron Carbon Equilibrium DiagramDocument52 pagesIron Carbon Equilibrium DiagramSohan Lal100% (2)
- Handout Chapter 5 Iron Carbon SystemDocument7 pagesHandout Chapter 5 Iron Carbon SystemBikram MuduliPas encore d'évaluation
- EMM 2312 - Fe-CDocument53 pagesEMM 2312 - Fe-CCalebPas encore d'évaluation
- M. Tech. (FFT) Technology of Ferrous Casting Phase DiagramDocument7 pagesM. Tech. (FFT) Technology of Ferrous Casting Phase DiagramRajulapati Sunil KumarPas encore d'évaluation
- Capili Jefferson 10Document5 pagesCapili Jefferson 10Christian Al EncarnacionPas encore d'évaluation
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument79 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualdextrermachete4amgqgPas encore d'évaluation
- Foundations of Materials Science and Engineering 5th Edition Smith Solutions ManualDocument39 pagesFoundations of Materials Science and Engineering 5th Edition Smith Solutions Manualcacoonnymphaea6wgyct100% (13)
- Callister7E - pp290 301 (The Iron Carbon System)Document12 pagesCallister7E - pp290 301 (The Iron Carbon System)iglumacPas encore d'évaluation
- Iron Carbon Diagram (ChE Handbook)Document21 pagesIron Carbon Diagram (ChE Handbook)Mohamed Ismail100% (1)
- Metallury of SteelsDocument10 pagesMetallury of SteelsDalitso MwanzaPas encore d'évaluation
- Phase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramDocument46 pagesPhase Diagrams:: The Iron-Iron Carbide (Fe-Fe3C) Diagram or Iron-Carbon (Fe-C) Equilibrium DiagramUsman FarooqPas encore d'évaluation
- Iron Carbon Equillibrium Diagram GandhidhamDocument22 pagesIron Carbon Equillibrium Diagram Gandhidhamcal2_uniPas encore d'évaluation
- Fe-C Phase Diagram ExplainedDocument5 pagesFe-C Phase Diagram Explainedحسين كاظم ياسينPas encore d'évaluation
- Engineering Metallurgy: Misan University-College of EngineeringDocument27 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manPas encore d'évaluation
- MSM GTU Study Material E-Notes Unit-5 23112020052908AMDocument14 pagesMSM GTU Study Material E-Notes Unit-5 23112020052908AMVijayPas encore d'évaluation
- Cast Steel: The Iron-Carbon Equilibrium Diagram: AbstractDocument5 pagesCast Steel: The Iron-Carbon Equilibrium Diagram: Abstractchacha4500Pas encore d'évaluation
- Fec DiagramDocument15 pagesFec DiagramShankarPas encore d'évaluation
- Composition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofDocument14 pagesComposition of Plain Carbon Steel Carbon Steel or Plain-Carbon Steel, Is A Metal Alloy. It Is A Combination ofkayodePas encore d'évaluation
- Iron-Carbon DiagramDocument3 pagesIron-Carbon DiagramnaniPas encore d'évaluation
- Iron Carbon Note 1 2023Document23 pagesIron Carbon Note 1 2023gerrard samuelPas encore d'évaluation
- Iron-IronCarbide Phase DiagramDocument3 pagesIron-IronCarbide Phase Diagramumangmodi32Pas encore d'évaluation
- Unit Cell Cubic StructuresDocument8 pagesUnit Cell Cubic StructuresGuilherme Dos Santos MoreiraPas encore d'évaluation
- The Iron-Iron Carbide Equilibrium DiagramDocument15 pagesThe Iron-Iron Carbide Equilibrium DiagramjhangeerPas encore d'évaluation
- Structure and Properties of Plain Carbon SteelDocument4 pagesStructure and Properties of Plain Carbon Steelsatish_trivediPas encore d'évaluation
- Iron-Carbon Phase Diagram Explained BrieflyDocument4 pagesIron-Carbon Phase Diagram Explained BrieflyZicoPas encore d'évaluation
- Constr Materials B PDFDocument72 pagesConstr Materials B PDFAgniva DuttaPas encore d'évaluation
- Iron Carbon Phase DiagramDocument7 pagesIron Carbon Phase Diagrampratap biswasPas encore d'évaluation
- Lesson 5 - Fe-C Diagram - Rev. 0Document11 pagesLesson 5 - Fe-C Diagram - Rev. 0Arga SetyaPas encore d'évaluation
- MEC 414 - Iron Phase Diagram Experiment 2Document7 pagesMEC 414 - Iron Phase Diagram Experiment 2boatcomPas encore d'évaluation
- Theoretical Part: Chapter-1Document10 pagesTheoretical Part: Chapter-1hayder1920Pas encore d'évaluation
- Dokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dDocument16 pagesDokumen - Tips - Iron Iron Carbide Phase Diagram 58ac3a092bd8dAfrizal Adithya PPas encore d'évaluation
- Iron-Carbon Phase Diagram (A Review) See Callister Chapter 9Document34 pagesIron-Carbon Phase Diagram (A Review) See Callister Chapter 9Zefa Erliana YullahPas encore d'évaluation
- Iron Carbon Equilibrium DiagramDocument4 pagesIron Carbon Equilibrium DiagramParameshwari PrabakarPas encore d'évaluation
- Engineering Material-II: Iron Carbide Phase DiagramDocument16 pagesEngineering Material-II: Iron Carbide Phase DiagramAla ZiPas encore d'évaluation
- The Iron-Carbon Equilibrium Diagram: AbstractDocument4 pagesThe Iron-Carbon Equilibrium Diagram: Abstractleodavid87Pas encore d'évaluation
- Metallurgical Principles in The Heat Treatment of SteelsDocument21 pagesMetallurgical Principles in The Heat Treatment of SteelsRajeev MaheshwariPas encore d'évaluation
- Chapter 2 - TTA - TTT - DiagramsDocument13 pagesChapter 2 - TTA - TTT - DiagramsPrasad Mhatre100% (1)
- Iron Carbon Part1 PDFDocument33 pagesIron Carbon Part1 PDFErick HoganPas encore d'évaluation
- Smart materials adapt like biologyDocument8 pagesSmart materials adapt like biologyOmar Adel MohammedPas encore d'évaluation
- The Iron-Carbon Equilibrium Diagram: AbstractDocument4 pagesThe Iron-Carbon Equilibrium Diagram: AbstractRama Krishna Reddy DonthireddyPas encore d'évaluation
- Fe CDocument34 pagesFe CZaza ArifinPas encore d'évaluation
- The Iron-Iron Carbide (Fe-Fe C) Phase DiagramDocument32 pagesThe Iron-Iron Carbide (Fe-Fe C) Phase DiagramNisaPas encore d'évaluation
- Engineering Metallurgy: Misan University-College of EngineeringDocument26 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manPas encore d'évaluation
- Lecture 9 - Ferrous AlloysDocument31 pagesLecture 9 - Ferrous Alloysmahmoud foudaPas encore d'évaluation
- Iron-Carbide Phase Diagram AnalysisDocument26 pagesIron-Carbide Phase Diagram AnalysisHiral HiraniPas encore d'évaluation
- 3 Iron Carbon DiaDocument21 pages3 Iron Carbon DiaChhavi SharmaPas encore d'évaluation
- Hardening From The Liquid StateDocument5 pagesHardening From The Liquid StateSinhrooPas encore d'évaluation
- Ganglion Cyst en INDocument2 pagesGanglion Cyst en INganesh82Pas encore d'évaluation
- Channel List EngDocument8 pagesChannel List Engstudyurself0% (1)
- Previews 2082971 Pre PDFDocument6 pagesPreviews 2082971 Pre PDFganesh82Pas encore d'évaluation
- Valve Material & General PurposeDocument36 pagesValve Material & General PurposeOky Andytya PratamaPas encore d'évaluation
- Inconel Alloy 617Document12 pagesInconel Alloy 617ganesh820% (1)
- Banking OmbudsmanDocument96 pagesBanking OmbudsmanVijay RajaPas encore d'évaluation
- Et 2019 Detailed NotificationDocument6 pagesEt 2019 Detailed NotificationAyush ChoudharyPas encore d'évaluation
- Valve Material & General PurposeDocument36 pagesValve Material & General PurposeOky Andytya PratamaPas encore d'évaluation
- +44 (0) 1786 475 662 Sales@amsmetals - Co.ukDocument2 pages+44 (0) 1786 475 662 Sales@amsmetals - Co.ukganesh82Pas encore d'évaluation
- Boiler SafetyDocument16 pagesBoiler SafetyTGUlabreaPas encore d'évaluation
- VdTUV Data Sheet 485Document1 pageVdTUV Data Sheet 485ganesh82Pas encore d'évaluation
- Pressure MeasurementDocument37 pagesPressure MeasurementSaumya GoelPas encore d'évaluation
- BPVC P-3aDocument2 pagesBPVC P-3aganesh82Pas encore d'évaluation
- Form P-4A Manufacturer'S Data Report For Fabricated Piping As Required by The Provisions of The ASME Code Rules, Section IDocument2 pagesForm P-4A Manufacturer'S Data Report For Fabricated Piping As Required by The Provisions of The ASME Code Rules, Section Iganesh82Pas encore d'évaluation
- 19750011340Document54 pages19750011340ganesh82Pas encore d'évaluation
- IbrDocument119 pagesIbrganesh82Pas encore d'évaluation
- BPVC Ix QW-483Document2 pagesBPVC Ix QW-483ganesh82Pas encore d'évaluation
- BPVC Ix QW-482Document2 pagesBPVC Ix QW-482ganesh82Pas encore d'évaluation
- Safety Valve SelectionDocument56 pagesSafety Valve Selectionganesh82Pas encore d'évaluation
- Form P-4 Manufacturer'S Partial Data Report As Required by The Provisions of The ASME Code Rules, Section IDocument2 pagesForm P-4 Manufacturer'S Partial Data Report As Required by The Provisions of The ASME Code Rules, Section Iganesh82Pas encore d'évaluation
- Form P-4A Manufacturer'S Data Report For Fabricated Piping As Required by The Provisions of The ASME Code Rules, Section IDocument2 pagesForm P-4A Manufacturer'S Data Report For Fabricated Piping As Required by The Provisions of The ASME Code Rules, Section Iganesh82Pas encore d'évaluation
- Form P-4A Manufacturer'S Data Report For Fabricated Piping As Required by The Provisions of The ASME Code Rules, Section IDocument2 pagesForm P-4A Manufacturer'S Data Report For Fabricated Piping As Required by The Provisions of The ASME Code Rules, Section Iganesh82Pas encore d'évaluation
- 1 214 BoilerSafetyValveRegulationsDocument18 pages1 214 BoilerSafetyValveRegulationsganesh82Pas encore d'évaluation
- Welding - Visual DefectsDocument19 pagesWelding - Visual Defectsbasita2Pas encore d'évaluation
- 4.7 PSV DesignDocument16 pages4.7 PSV Designganesh82Pas encore d'évaluation
- CreamyLayerOBC ClarificationsgDocument6 pagesCreamyLayerOBC Clarificationsgganesh82Pas encore d'évaluation
- B eDocument92 pagesB eganesh82Pas encore d'évaluation
- 5 04 FinancialFitness PDFDocument9 pages5 04 FinancialFitness PDFbook2mindPas encore d'évaluation
- UT Power PointDocument78 pagesUT Power PointAnonymous gFcnQ4goPas encore d'évaluation
- High-performance Cylinder Liners Materials for Diesel EnginesDocument2 pagesHigh-performance Cylinder Liners Materials for Diesel EnginesVaisakh PillaiPas encore d'évaluation
- Astm G 49 - 85 R00 - RZQ5Document6 pagesAstm G 49 - 85 R00 - RZQ5Samuel EduardoPas encore d'évaluation
- Ionic Puzzle ActivityDocument4 pagesIonic Puzzle ActivityEngr Mumtaz0% (1)
- Chemical Resistance Ohji Rubber Lining: 1. Inorganic AcidsDocument5 pagesChemical Resistance Ohji Rubber Lining: 1. Inorganic AcidsJavier Alejandro Rodriguez MelgozaPas encore d'évaluation
- Nium Heptamolybdate: Jump To Navigation Jump To SearchDocument6 pagesNium Heptamolybdate: Jump To Navigation Jump To SearchWilmer AlexanderPas encore d'évaluation
- AFM ER308 Afm Er308LDocument9 pagesAFM ER308 Afm Er308LKH NgPas encore d'évaluation
- Material Standard: IPS-M-PI-150Document30 pagesMaterial Standard: IPS-M-PI-150gurugovindanPas encore d'évaluation
- Aluminium Alloy 2014A T651 Sheet and Plate: Specifications Temper TypesDocument3 pagesAluminium Alloy 2014A T651 Sheet and Plate: Specifications Temper TypesMellierPas encore d'évaluation
- Chemistry - F3 To F4 Summer AssignmentsDocument24 pagesChemistry - F3 To F4 Summer AssignmentsCoolman PoonPas encore d'évaluation
- Quotation Portacabin-NormalDocument4 pagesQuotation Portacabin-NormalBhavanishankar ShettyPas encore d'évaluation
- 3 Classifications of Matter Lesson PlanDocument6 pages3 Classifications of Matter Lesson PlanShane CantelaPas encore d'évaluation
- Using of Calsium Magnesium Aluminate Flux With High MgO Content To Improve Secondary Steel Ladle LifetimeDocument6 pagesUsing of Calsium Magnesium Aluminate Flux With High MgO Content To Improve Secondary Steel Ladle LifetimeBagas Prasetyawan Adi NugrohoPas encore d'évaluation
- 400 Important Chemistry Questions and AnswerDocument28 pages400 Important Chemistry Questions and Answerhnin pwint100% (2)
- Hanger CatalogueDocument4 pagesHanger CatalogueDnyaneshwar100% (1)
- Ez 33 A PDFDocument18 pagesEz 33 A PDF孙俊磊Pas encore d'évaluation
- FILE NO 3 Exercise 2 Chemical Formula Writing and Naming of Compounds RevDocument2 pagesFILE NO 3 Exercise 2 Chemical Formula Writing and Naming of Compounds RevEJ TaylanPas encore d'évaluation
- Introduction 2 Study Mineralogy I ToDocument154 pagesIntroduction 2 Study Mineralogy I ToCaracude John100% (1)
- Effect of Welding Current On The Mechanical and StructuralDocument8 pagesEffect of Welding Current On The Mechanical and StructuralBhramandhikaNalendraGhuptaPas encore d'évaluation
- Welding-Standards 23-03-2014Document1 pageWelding-Standards 23-03-2014givali100% (7)
- Permalite Roofing Manual 5.3.2Document36 pagesPermalite Roofing Manual 5.3.2Peter FowlesPas encore d'évaluation
- IMDS ID / Version: 747476346 / 1 Radiator Assembly Material Composition and Substance DataDocument14 pagesIMDS ID / Version: 747476346 / 1 Radiator Assembly Material Composition and Substance Datajavier ortizPas encore d'évaluation
- Welding Procedure SpecificationDocument30 pagesWelding Procedure Specificationrahman196011100% (1)
- MMD Brochure CompressedDocument13 pagesMMD Brochure Compressedapi-254065454100% (1)
- Mixing Water For ConcreteDocument10 pagesMixing Water For ConcreteTausif AhmadPas encore d'évaluation
- Split Pattern of Green Sand Molding and Hollow Casting ProductionDocument11 pagesSplit Pattern of Green Sand Molding and Hollow Casting ProductionJorge Sánchez100% (2)
- ENER GEN Introduction To Orgonite Jon LoganDocument42 pagesENER GEN Introduction To Orgonite Jon LoganAmelia Zaccardi50% (2)
- MINERAL - RESOURCES (1) Class 10 CBSEDocument10 pagesMINERAL - RESOURCES (1) Class 10 CBSEfsdf ffdhrtPas encore d'évaluation
- Intec Gold ProcessDocument19 pagesIntec Gold ProcessSteven Tremol100% (1)
- Copper, Bronze, IronDocument15 pagesCopper, Bronze, IronDerick BrinPas encore d'évaluation