Académique Documents
Professionnel Documents
Culture Documents
Worksheet 21
Transféré par
Vijay Bhaskar0 évaluation0% ont trouvé ce document utile (0 vote)
95 vues3 pagesTitre original
worksheet_21.doc
Copyright
© © All Rights Reserved
Formats disponibles
DOC, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOC, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
95 vues3 pagesWorksheet 21
Transféré par
Vijay BhaskarDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOC, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 3
21 Worksheet (A2)
Specific heat capacity of water = 4200 J kg
1
K
1
Specific latent heat of fusion of water = 3.4 10
5
J kg
1
1 escri!e the arrange"ent of ato"s# the forces !etween the ato"s an$ the "otion of the
ato"s in%
a a soli$ &3'
b a li(ui$ &3'
c a gas. &3'
2 ) s"all a"ount of gas is trappe$ insi$e a container. escri!e the "otion of the gas ato"s
as the te"perature of the gas within the container is increase$. &3'
3 a efine the internal energy of a su!stance. &1'
b *he te"perature of an alu"iniu" !lock increases when it is place$ in the fla"e of a
+unsen !urner. ,-plain why this causes an increase in its internal energy. &3'
c ) lu"p of "etal is "elting in a hot o.en at a te"perature of /00 01.
,-plain whether its internal energy is increasing or $ecreasing as it "elts. &4'
4 2rite a wor$ e(uation for the change in the ther"al energy of a su!stance in ter"s of its
"ass# the specific heat capacity of the su!stance an$ its change of te"perature. &1'
5 *he specific heat capacity of a su!stance is "easure$ in the units J kg
1
K
1
# whereas
its specific latent heat of fusion is "easure$ in J kg
1
. ,-plain why the units are $ifferent. &2'
6 uring a hot su""er3s $ay# the te"perature of /.0 10
5
kg of water in a swi""ing pool
increases fro" 21 01 to 24 01. 1alculate the change in the internal energy of the water. &3'
7 ) 300 g !lock of iron cools fro" 300 01 to roo" te"perature at 20 01. *he specific heat
capacity of iron is 440 J kg
1
K
1
. 1alculate the heat release$ !y the !lock of iron. &3'
8 1alculate the energy that "ust !e re"o.e$ fro" 200 g of water at 0 01 to con.ert it all
into ice at 0 01. &2'
)S an$ ) 5e.el 6hysics 7riginal "aterial 8 1a"!ri$ge 9ni.ersity 6ress 2010 1
21 Worksheet (A2)
9 a 1hange the following te"peratures fro" $egrees 1elsius into kel.in.
i 0 01
ii :0 01
iii 120 01 &3'
b 1hange the following te"peratures fro" kel.in into $egrees 1elsius.
i 400 K
ii 2;2 K
iii 3 K &3'
10 )n electrical heater is use$ to heat 100 g of water in a well<insulate$ container at a stea$y
rate. *he te"perature of the water increases !y 15 01 when the heater is operate$ for a
perio$ of 5.0 "inutes. eter"ine the change of te"perature of the water when the sa"e
heater an$ container are in$i.i$ually use$ to heat%
a 300 g of water for the sa"e perio$ of ti"e &3'
b 100 g of water for a ti"e of 2.5 "inutes. &3'
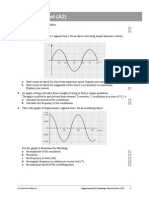
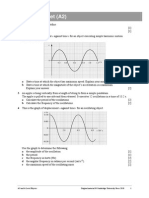
11 *he graph !elow shows the .ariation of the te"perature of 200 g of lea$ as it is heate$
at a stea$y rate.
a 9se the graph to state the "elting point of lea$. &1'
b ,-plain why the graph is a straight line at the start. &1'
c ,-plain what happens to the energy supplie$ to the lea$ as it "elts at a constant
te"perature. &1'
d *he initial te"perature of the lea$ is 0 01. 9se the graph to $eter"ine the total
energy supplie$ to the lea$ !efore it starts to "elt. &3'
=*he specific heat capacity of lea$ is 130 J kg
1
K
1
.>
e 9se your answer to d to $eter"ine the rate of heating of the lea$. &2'
f )ssu"ing that energy continues to !e supplie$ at the sa"e rate# calculate the
specific latent heat of fusion of lea$. &3'
)S an$ ) 5e.el 6hysics 7riginal "aterial 8 1a"!ri$ge 9ni.ersity 6ress 2010 2
21 Worksheet (A2)
12 *he $iagra" shows pipe$ water !eing heate$ !y an electrical heater.
*he water flows through the heater at a rate of 0.015 kg s
1
. *he heater war"s the water
fro" 15 01 to 42 01. )ssu"ing that all the energy fro" the heater is transferre$ to
heating the water# calculate the power of the heater. &5'
13 ) gas is hel$ in a cylin$er !y a friction<free piston. 2hen the force hol$ing the piston in
place is re"o.e$# the gas e-pan$s an$ pushes the piston outwar$s.
,-plain why the te"perature of the gas falls. &2'
14 ?ot water of "ass 300 g an$ at a te"perature of 40 01 is a$$e$ to 200 g of col$ water
at 10 01. 2hat is the final te"perature of the "i-ture@ Aou "ay assu"e there are no
losses to the en.iron"ent an$ all heat transfer takes place !etween the hot water an$
the col$ water. &5'
15 ) "etal cu!e of "ass ;5 g is heate$ in a nake$ fla"e until it is re$ hot. *he "etal !lock is
(uickly transferre$ to 200 g of col$ water. *he water is well stirre$. *he graph shows the
.ariation of the te"perature of the water recor$e$ !y a $atalogger $uring the e-peri"ent.
*he "etal has a specific heat capacity of 500 J kg
1
K
1
. 9se the a$$itional infor"ation
pro.i$e$ in the graph to $eter"ine the initial te"perature of the "etal cu!e. Aou "ay
assu"e there are no losses to the en.iron"ent an$ all heat transfer takes place !etween
the "etal !lock an$ the water. &5'
*otal%
;1
Score% B
)S an$ ) 5e.el 6hysics 7riginal "aterial 8 1a"!ri$ge 9ni.ersity 6ress 2010 3
Vous aimerez peut-être aussi
- Transformationsclassifieds PDFDocument35 pagesTransformationsclassifieds PDFVijay BhaskarPas encore d'évaluation
- Worksheet 32Document2 pagesWorksheet 32Pathmanathan NadesonPas encore d'évaluation
- Worksheet 33Document3 pagesWorksheet 33Pathmanathan NadesonPas encore d'évaluation
- Worksheet 31 PDFDocument3 pagesWorksheet 31 PDFVijay BhaskarPas encore d'évaluation
- Worksheet 31Document3 pagesWorksheet 31Vijay BhaskarPas encore d'évaluation
- Worksheet 33A LevelDocument3 pagesWorksheet 33A LevelVijay BhaskarPas encore d'évaluation
- Worksheet 32Document2 pagesWorksheet 32Vijay BhaskarPas encore d'évaluation
- Worksheet 30Document4 pagesWorksheet 30Vijay BhaskarPas encore d'évaluation
- Worksheet 26 PDFDocument3 pagesWorksheet 26 PDFVijay BhaskarPas encore d'évaluation
- Worksheet 29Document4 pagesWorksheet 29Vijay BhaskarPas encore d'évaluation
- Worksheet 30 PDFDocument4 pagesWorksheet 30 PDFVijay Bhaskar100% (3)
- Worksheet 27Document3 pagesWorksheet 27Vijay Bhaskar0% (1)
- Worksheet 28 PDFDocument2 pagesWorksheet 28 PDFVijay Bhaskar100% (3)
- Worksheet 29 PDFDocument4 pagesWorksheet 29 PDFVijay BhaskarPas encore d'évaluation
- Worksheet 28Document2 pagesWorksheet 28Vijay Bhaskar100% (1)
- Worksheet 24Document3 pagesWorksheet 24Vijay BhaskarPas encore d'évaluation
- 27 Worksheet (A2) : AS and A Level Physics Original Material © Cambridge University Press 2010Document3 pages27 Worksheet (A2) : AS and A Level Physics Original Material © Cambridge University Press 2010Syed Abdul Rehman ShahPas encore d'évaluation
- Worksheet 26Document3 pagesWorksheet 26Vijay BhaskarPas encore d'évaluation
- Worksheet 21 PDFDocument3 pagesWorksheet 21 PDFVijay Bhaskar0% (1)
- Worksheet 25 PDFDocument3 pagesWorksheet 25 PDFVijay Bhaskar100% (2)
- Worksheet 25Document3 pagesWorksheet 25Vijay Bhaskar100% (1)
- Worksheet 20Document3 pagesWorksheet 20Vijay BhaskarPas encore d'évaluation
- 22 Worksheet (A2) : AS and A Level Physics Original Material © Cambridge University Press 2010Document3 pages22 Worksheet (A2) : AS and A Level Physics Original Material © Cambridge University Press 2010Vijay BhaskarPas encore d'évaluation
- Worksheet 24 PDFDocument3 pagesWorksheet 24 PDFVijay BhaskarPas encore d'évaluation
- Worksheet 23 PDFDocument2 pagesWorksheet 23 PDFVijay Bhaskar100% (1)
- Worksheet 23Document2 pagesWorksheet 23Vijay BhaskarPas encore d'évaluation
- Worksheet 22Document3 pagesWorksheet 22Vijay BhaskarPas encore d'évaluation
- Worksheet 19Document2 pagesWorksheet 19etud3cl100% (1)
- Worksheet 20 PDFDocument3 pagesWorksheet 20 PDFVijay BhaskarPas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- CE Certification Split Package Type Central Air Conditioning UnitDocument12 pagesCE Certification Split Package Type Central Air Conditioning UnitpogisimpatikoPas encore d'évaluation
- Pioro 2005Document25 pagesPioro 2005Thamil ThedalPas encore d'évaluation
- Boilere Solare ELBI COMBIDocument4 pagesBoilere Solare ELBI COMBICsiszer RobertPas encore d'évaluation
- Heat Exchanger DesignDocument27 pagesHeat Exchanger DesignTri Vicca WibisonoPas encore d'évaluation
- Project: Techcalc-Projekt: Boundary ConditionsDocument5 pagesProject: Techcalc-Projekt: Boundary ConditionsBogdan LucianPas encore d'évaluation
- Catalogo Split Standar Lennox 10 Seer PDFDocument4 pagesCatalogo Split Standar Lennox 10 Seer PDFZecarlos Santos DávilaPas encore d'évaluation
- Heat & Thermodynamics Complete SankalpDocument58 pagesHeat & Thermodynamics Complete SankalpASHOK KUMAR RANAPas encore d'évaluation
- The electromagnetic spectrum and uses of ultraviolet wavesDocument3 pagesThe electromagnetic spectrum and uses of ultraviolet wavesJhade Kianne Mendoza BarzaPas encore d'évaluation
- 4 ModellingDocument91 pages4 Modellingمصطفى العباديPas encore d'évaluation
- 2.1. Fundamentals of Heat Transfer and Thermodynamics 2.1.1. ThermodynamicsDocument5 pages2.1. Fundamentals of Heat Transfer and Thermodynamics 2.1.1. ThermodynamicsLeslie CatindigPas encore d'évaluation
- Thermodynamics Laboratory: TITLE: Heat ConductionDocument6 pagesThermodynamics Laboratory: TITLE: Heat ConductionHaziq HanifahPas encore d'évaluation
- Temperature and Heat 1Document27 pagesTemperature and Heat 1Jonaluz PagalpalanPas encore d'évaluation
- Heat Transfer and Exergy Analysis of A Spiral Heat ExchangerDocument16 pagesHeat Transfer and Exergy Analysis of A Spiral Heat ExchangerManuela AguirrePas encore d'évaluation
- Corrugated Plate Heat Exchanger ReviewDocument9 pagesCorrugated Plate Heat Exchanger ReviewElvis AnsuPas encore d'évaluation
- Comprehensive HVAC project report with technical specificationsDocument2 pagesComprehensive HVAC project report with technical specificationsBalaji JenarthananPas encore d'évaluation
- Heat Exchanger Question - ANSDocument7 pagesHeat Exchanger Question - ANSBikas SahaPas encore d'évaluation
- Warm Italian-style solar water heaters for stylish living spacesDocument8 pagesWarm Italian-style solar water heaters for stylish living spacesdiribaPas encore d'évaluation
- Experiment No.01: Study of Domestic RefrigeratorDocument3 pagesExperiment No.01: Study of Domestic RefrigeratorPrathamesh DhulapkarPas encore d'évaluation
- PERPINDAHAN KALORDocument3 pagesPERPINDAHAN KALORSheilaPas encore d'évaluation
- Inst TFC6Document2 pagesInst TFC6Steve WuPas encore d'évaluation
- Che310 2Document27 pagesChe310 2Enendu BlessingPas encore d'évaluation
- Dimensionless NumberDocument14 pagesDimensionless NumberSarang KumarPas encore d'évaluation
- ns5 Cam Heat Light Study PresentationDocument15 pagesns5 Cam Heat Light Study Presentationapi-281736360Pas encore d'évaluation
- Heat Transfer Chapter 3Document45 pagesHeat Transfer Chapter 3Gregory Simmon100% (1)
- Seal Aftermarket Products: An Easy Fix For A Self-Inflicted FailureDocument69 pagesSeal Aftermarket Products: An Easy Fix For A Self-Inflicted Failurechoco84Pas encore d'évaluation
- Solutions To Home Work # 1: 3-14C Convection Heat Transfer Through The Wall Is Expressed AsDocument6 pagesSolutions To Home Work # 1: 3-14C Convection Heat Transfer Through The Wall Is Expressed Aspriyadarshini212007Pas encore d'évaluation
- Chapter 3 ConvectionDocument123 pagesChapter 3 ConvectionPHƯƠNG ĐẶNG YẾNPas encore d'évaluation
- Department of Thermal Power Engineering: Visvesvaraya Technological UniversityDocument26 pagesDepartment of Thermal Power Engineering: Visvesvaraya Technological UniversityRaju SubediPas encore d'évaluation
- PHE SelectionsDocument67 pagesPHE SelectionsSelva Kumar Selva KumarPas encore d'évaluation
- Cooling SystemsDocument45 pagesCooling SystemsSapari VelPas encore d'évaluation