Académique Documents
Professionnel Documents
Culture Documents
Acid Base
Transféré par
docbin0 évaluation0% ont trouvé ce document utile (0 vote)
30 vues16 pagesacids
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentacids
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
30 vues16 pagesAcid Base
Transféré par
docbinacids
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 16
Acids and Bases
Brnsted-Lowry Acids and Bases
A Brnsted-Lowry acid is a proton donor.
A Brnsted-Lowry base is a proton acceptor.
H+ = proton
Acids and Bases
Brnsted-Lowry Acids and Bases
Some molecules contain both hydrogen atoms and lone
pairs and thus, can act either as acids or bases,
depending on the particular reaction. An example is the
addictive pain reliever morphine.
Acids and Bases
Reactions of Brnsted-Lowry Acids and Bases
A Brnsted-Lowry acid base reaction results in the transfer of
a proton from an acid to a base.
In an acid-base reaction, one bond is broken, and another
one is formed.
The electron pair of the base B: forms a new bond to the
proton of the acid.
The acid HA loses a proton, leaving the electron pair in the
HA bond on A.
Acids and Bases
Reactions of Brnsted-Lowry Acids and Bases
The movement of electrons in reactions can be illustrated using
curved arrow notation. Because two electron pairs are involved in
this reaction, two curved arrows are needed.
Loss of a proton from an acid forms its conjugate base.
Gain of a proton by a base forms its conjugate acid.
A double reaction arrow is used between starting materials and
products to indicate that the reaction can proceed in the forward
and reverse directions. These are equilibrium arrows.
Examples:
Acids and Bases
Acid Strength and pKa
Acid strength is the tendency of an acid to donate a proton.
The more readily a compound donates a proton, the stronger an acid
it is.
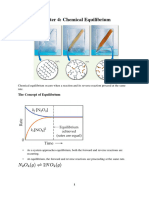
Acidity is measured by an equilibrium constant
When a Brnsted-Lowry acid HA is dissolved in water, an acidbase reaction occurs, and an equilibrium constant can be written for
the reaction.
Acids and Bases
Acid Strength and pKa
Because the concentration of the solvent H2O is essentially constant,
the equation can be rearranged and a new equilibrium constant, called
the acidity constant, Ka, can be defined.
It is generally more convenient when describing acid strength to use
pKa values than Ka values.
Acids and Bases
Acid Strength and pKa
Acids and Bases
Factors that Determine Acid Strength
Anything that stabilizes a conjugate base A: makes the
starting acid HA more acidic.
Four factors affect the acidity of HA. These are:
o Element effects
o Inductive effects
o Resonance effects
o Hybridization effects
No matter which factor is discussed, the same procedure is
always followed. To compare the acidity of any two acids:
o Always draw the conjugate bases.
o Determine which conjugate base is more stable.
o The more stable the conjugate base, the more acidic the
acid.
Acids and Bases
Factors that Determine Acid Strength
Element EffectsTrends in the Periodic Table.
Across a row of the periodic table, the acidity of HA
increases as the electronegativity of A increases.
Positive or negative charge is stabilized when it is spread
over a larger volume.
Acids and Bases
Factors that Determine Acid Strength
Element EffectsTrends in the Periodic Table.
Down a column of the periodic table, the acidity of HA
increases as the size of A increases.
Size, and not electronegativity, determines acidity down a
column.
The acidity of HA increases both left-to-right across a row
and down a column of the periodic table.
Although four factors determine the overall acidity of a
particular hydrogen atom, element effectsthe identity of
Ais the single most important factor in determining the
acidity of the HA bond.
Acids and Bases
Factors that Determine Acid StrengthInductive Effects
An inductive effect is the pull of electron density
through bonds caused by electronegativity
differences in atoms.
In the example below, when we compare the acidities
of ethanol and 2,2,2-trifluoroethanol, we note that the
latter is more acidic than the former.
Acids and Bases
Factors that Determine Acid StrengthInductive Effects
The reason for the increased acidity of 2,2,2trifluoroethanol is that the three electronegative
fluorine atoms stabilize the negatively charged
conjugate base.
Acids and Bases
Factors that Determine Acid StrengthInductive Effects
When electron density is pulled away from the negative
charge through bonds by very electronegative atoms, it is
referred to as an electron withdrawing inductive effect.
More electronegative atoms stabilize regions of high electron
density by an electron withdrawing inductive effect.
The more electronegative the atom and the closer it is to the
site of the negative charge, the greater the effect.
The acidity of HA increases with the presence of electron
withdrawing groups in A.
Acids and Bases
Factors that Determine Acid StrengthResonance Effects
Resonance is a third factor that influences acidity.
In the example below, when we compare the acidities of
ethanol and acetic acid, we note that the latter is more acidic
than the former.
When the conjugate bases of the two species are compared, it
is evident that the conjugate base of acetic acid enjoys
resonance stabilization, whereas that of ethanol does not.
Acids and Bases
Factors that Determine Acid StrengthResonance
Effects
Resonance delocalization makes CH3COO more stable
than CH3CH2O, so CH3COOH is a stronger acid than
CH3CH2OH.
The acidity of HA increases when the conjugate
base A: is resonance stabilized.
Acids and Bases
Factors that Determine Acid StrengthResonance
Effects
Electrostatic potential plots of CH3CH2O and CH3COO
below indicate that the negative charge is
concentrated on a single O in CH3CH2O, but
delocalized over both of the O atoms in CH3COO.
Acids and Bases
Factors that Determine Acid StrengthHybridization
Effects
The final factor affecting the acidity of HA is the
hybridization of A.
Let us consider the relative acidities of three different
compounds containing CH bonds.
The higher the percent of s-character of the hybrid
orbital, the closer the lone pair is held to the nucleus,
and the more stable the conjugate base.
Acids and Bases
Factors that Determine Acid StrengthHybridization
Effects
Acids and Bases
Factors that Determine Acid StrengthHybridization
Effects
10
Acids and Bases
Commonly Used Acids in Organic Chemistry
In addition to the familiar acids HCl, H2SO4 and HNO3, a
number of other acids are often used on organic
reactions. Two examples are acetic acid and p-toluenesulfonic acid (TsOH).
Acids and Bases
Commonly Used Acids in Organic Chemistry
Common strong bases used in organic reactions are
more varied in structure.
11
Acids and Bases
Commonly Used Acids in Organic Chemistry
It should be noted that:
Strong bases have weak conjugate acids with high pKa
values, usually > 12.
Strong bases have a net negative charge, but not all
negatively charged species are strong bases. For example,
none of the halides F, Cl, Br, or I, is a strong base.
Carbanions, negatively charged carbon atoms, are especially
strong bases. A common example is butyllithium.
Two other weaker organic bases are triethylamine and
pyridine.
Acids and Bases
Lewis Acids and Bases
The Lewis definition of acids and bases is more general
than the Brnsted-Lowry definition.
A Lewis acid is an electron pair acceptor.
A Lewis base is an electron pair donor.
Lewis bases are structurally the same as Brnsted-Lowry
bases. Both have an available electron paira lone pair or
an electron pair in a bond.
A Brnsted -Lowry base always donates this electron pair
to a proton, but a Lewis base donates this electron pair to
anything that is electron deficient.
12
Acids and Bases
Lewis Acids and Bases
A Lewis acid must be able to accept an electron pair, but
there are many ways for this to occur.
All Brnsted-Lowry acids are also Lewis acids, but the
reverse is not necessarily true.
o Any species that is electron deficient and capable of
accepting an electron pair is also a Lewis acid.
Common examples of Lewis acids (which are not BrnstedLowry acids) include BF3 and AlCl3. These compounds
contain elements in group 3A of the periodic table that can
accept an electron pair because they do not have filled
valence shells of electrons.
Acids and Bases
Lewis Acids and Bases
Any reaction in which one species donates an electron pair
to another species is a Lewis acid-base reaction.
In a Lewis acid-base reaction, a Lewis base donates an
electron pair to a Lewis acid.
Lewis acid-base reactions illustrate a general pattern in
organic chemistry. Electron-rich species react with electronpoor species.
In the simplest Lewis acid-base reaction one bond is formed
and no bonds are broken. This is illustrated in the reaction of
BF3 with H2O. H2O donates an electron pair to BF3 to form a
new bond.
13
Acids and Bases
Lewis Acids and Bases
A Lewis acid is also called an electrophile.
When a Lewis base reacts with an electrophile other
than a proton, the Lewis base is also called a
nucleophile. In this example, BF3 is the electrophile and
H2O is the nucleophile.
electrophile
nucleophile
Acids and Bases
Lewis Acids and Bases
Two other examples are shown below. Note that in each
reaction, the electron pair is not removed from the
Lewis base. Instead, it is donated to an atom of the
Lewis acid and one new covalent bond is formed.
14
Acids and Bases
Lewis Acids and Bases
In some Lewis acid-base reactions, one bond is formed
and one bond is broken. To draw the products of these
reactions, keep in mind the following steps:
o Always identify the Lewis acid and base first.
o Draw a curved arrow from the electron pair of the
base to the electron-deficient atom of the acid.
o Count electron pairs and break a bond when needed
to keep the correct number of valence electrons.
Acids and Bases
Lewis Acids and Bases
Consider the Lewis acid-base reaction between
cyclohexene and HCl. The Brnsted-Lowry acid HCl
is also a Lewis acid, and cyclohexene, having a bond,
is the Lewis base.
15
Acids and Bases
Lewis Acids and Bases
To draw the product of this reaction, the electron pair in
the bond of the Lewis base forms a new bond to the
proton of the Lewis acid, generating a carbocation.
The HCl bond must break, giving its two electrons to
Cl, forming Cl.
Because two electron pairs are involved, two curved
arrows are needed.
16
Vous aimerez peut-être aussi
- 5 KOF Acid Bases PDFDocument27 pages5 KOF Acid Bases PDFIstiPas encore d'évaluation
- 3 Acid & Base PDFDocument5 pages3 Acid & Base PDFAppy ZombaaPas encore d'évaluation
- Organic Chemistry For Biology Students: Acids BasesDocument27 pagesOrganic Chemistry For Biology Students: Acids BasesTrần Minh HiếuPas encore d'évaluation
- Chapter02 Smith3e PPTDocument40 pagesChapter02 Smith3e PPTLuisa PalomoPas encore d'évaluation
- Acids BasesDocument20 pagesAcids BasesAsaf RavivPas encore d'évaluation
- Organic Chemistry,: Acids & BasesDocument30 pagesOrganic Chemistry,: Acids & BasesilhamfaturachmanagusPas encore d'évaluation
- Ch02 - Basics2Document44 pagesCh02 - Basics2Saguvij FopoPas encore d'évaluation
- Lecture 2 - Acids and BasesDocument34 pagesLecture 2 - Acids and Basesanasattiq078Pas encore d'évaluation
- Acids and Bases in Organic Chemistry: 1. An Arrhenius Acid Is A Source of HDocument13 pagesAcids and Bases in Organic Chemistry: 1. An Arrhenius Acid Is A Source of HAndile VeziPas encore d'évaluation
- Organic Chemistry, Second Edition Janice Gorzynski Smith, ch2Document16 pagesOrganic Chemistry, Second Edition Janice Gorzynski Smith, ch2sungyeon heoPas encore d'évaluation
- Acid StrengthDocument35 pagesAcid StrengthUmrotul MunaPas encore d'évaluation
- Acidity and BasicityDocument5 pagesAcidity and BasicityDenielle BaybayPas encore d'évaluation
- Acid-Base Chemistry: Relative Strengths of AcidsDocument4 pagesAcid-Base Chemistry: Relative Strengths of Acidslet2830Pas encore d'évaluation
- Introductory Chemistry II Acids and Bases Lecture 6Document21 pagesIntroductory Chemistry II Acids and Bases Lecture 6condoleeza smithPas encore d'évaluation
- Acidity, Basicity and PkaDocument39 pagesAcidity, Basicity and PkaZubaydah Abdullah100% (1)
- Intro To Organic Reactions Chm457Document52 pagesIntro To Organic Reactions Chm457PUTRI DAYANA BATRIESYA ABDUL HANIFPas encore d'évaluation
- Brønsted-Lowry Acids and BasesDocument39 pagesBrønsted-Lowry Acids and BasesEr Bipin VermaPas encore d'évaluation
- Effect of Acidity and Basicity of Organic CompoundsDocument34 pagesEffect of Acidity and Basicity of Organic Compoundsajibolaakorede20Pas encore d'évaluation
- Introduction To Organic Chemistry: 5.3 Acids and BasesDocument14 pagesIntroduction To Organic Chemistry: 5.3 Acids and BasesLemony SnickitPas encore d'évaluation
- 11 ChemistryDocument20 pages11 ChemistrykabhiPas encore d'évaluation
- Organic Chemistry 1-Chapter 2-Lecture NoteDocument7 pagesOrganic Chemistry 1-Chapter 2-Lecture Note남궁기택/학생/화학공학Pas encore d'évaluation
- Structure and Re Activity Acidity and BasicityDocument9 pagesStructure and Re Activity Acidity and BasicityMomer100% (2)
- 021020190L2 Biochemistry DR - JehadDocument24 pages021020190L2 Biochemistry DR - Jehadslmen1269Pas encore d'évaluation
- Organic Chemistry: Chapter 6: Lecture PowerpointDocument57 pagesOrganic Chemistry: Chapter 6: Lecture PowerpointAgung PratamaPas encore d'évaluation
- Water, H-Bonding, PH and Some Thermodynamics: NOT Section 1.4Document74 pagesWater, H-Bonding, PH and Some Thermodynamics: NOT Section 1.4Soji AdimulaPas encore d'évaluation
- 2-Acid & BasesDocument38 pages2-Acid & BasesRen H. RoxasPas encore d'évaluation
- Week7a - OrgChem1 - A-B Rxns in Org Chemistry - 2023Document18 pagesWeek7a - OrgChem1 - A-B Rxns in Org Chemistry - 2023happy cyenoPas encore d'évaluation
- Chapter 2 BIOCHEMDocument50 pagesChapter 2 BIOCHEMDentist_2011Pas encore d'évaluation
- Types of Acids and BasesDocument3 pagesTypes of Acids and BasesJadie Barringer IIIPas encore d'évaluation
- 1.15 How Structure Affects Acid StrengthDocument35 pages1.15 How Structure Affects Acid StrengthKmilo OspinaPas encore d'évaluation
- Physical Pharmacy 4Document11 pagesPhysical Pharmacy 4husseinPas encore d'évaluation
- Acidos Bases Duros y Blandos1 24772Document7 pagesAcidos Bases Duros y Blandos1 24772fitriana dewi kurniawatiPas encore d'évaluation
- Acids and Bases & Oxides and HydroxidesDocument37 pagesAcids and Bases & Oxides and HydroxidesPrince HasanPas encore d'évaluation
- CBSE Class 11 Chemistry Notes: Ionic Equilibrium: by - October 22, 2014Document16 pagesCBSE Class 11 Chemistry Notes: Ionic Equilibrium: by - October 22, 2014scsa31619Pas encore d'évaluation
- Acidic & Basic StrengthDocument39 pagesAcidic & Basic Strengthchessguy2847Pas encore d'évaluation
- Scientific Method: Jayson Nota Maria Angelica BalicocoDocument21 pagesScientific Method: Jayson Nota Maria Angelica BalicocoGrace Ann ArimadoPas encore d'évaluation
- Acids, Bases and BuffersDocument62 pagesAcids, Bases and BufferskolangyefrankbenlePas encore d'évaluation
- Chapter 5.3 Acids and BasesDocument14 pagesChapter 5.3 Acids and Basesanon_292808201Pas encore d'évaluation
- Module 2: Acids and BasesDocument15 pagesModule 2: Acids and BasesDana MikaelaPas encore d'évaluation
- Lesson 14.2 Acid and Base StrengthsDocument9 pagesLesson 14.2 Acid and Base Strengthszelalem getachewPas encore d'évaluation
- Ch02-Acids and BasesDocument31 pagesCh02-Acids and BasesSİNEM GÜVENPas encore d'évaluation
- Chb401a L 1 SL1 21 1Document43 pagesChb401a L 1 SL1 21 1PrityyyPas encore d'évaluation
- Protons or The Most Basic Site in A Molecule. These Facts Can Be Important For DeterminingDocument3 pagesProtons or The Most Basic Site in A Molecule. These Facts Can Be Important For DeterminingizallllPas encore d'évaluation
- Ilovepdf Merged 2Document41 pagesIlovepdf Merged 2api-533764142Pas encore d'évaluation
- Jack Westin MCAT Content General ChemistryDocument25 pagesJack Westin MCAT Content General ChemistryLora100% (1)
- Important Topics of ChemistryDocument7 pagesImportant Topics of ChemistrystrangeankitPas encore d'évaluation
- Water PropertiesDocument52 pagesWater Propertiesapi-321453350Pas encore d'évaluation
- Basic Pharmaceutical Chemistry 15Document108 pagesBasic Pharmaceutical Chemistry 15Gideon AntwiPas encore d'évaluation
- Structure and Reactivity HandoutDocument14 pagesStructure and Reactivity HandoutAsmunirwan UsugPas encore d'évaluation
- Acid - Base Chemistry - ShortDocument27 pagesAcid - Base Chemistry - ShortSachin PatilPas encore d'évaluation
- LAcids and BasesDocument37 pagesLAcids and BasesAnonymous rFIshYy100% (1)
- Types of ElectrolytesDocument24 pagesTypes of ElectrolytesPranoy Baishya100% (1)
- Chapter 2 - Bronsted-Lowry TheoryDocument19 pagesChapter 2 - Bronsted-Lowry TheoryAcidri AbdulkarimPas encore d'évaluation
- Non-Aqueous TitrationsDocument40 pagesNon-Aqueous TitrationsAhmed Imran100% (2)
- Chapter 15Document4 pagesChapter 15Ayesha MohamudPas encore d'évaluation
- Unit 4 Chemical Equilibrium PDFDocument27 pagesUnit 4 Chemical Equilibrium PDFmisganamarcos10Pas encore d'évaluation
- محاضرة 6 (ن)Document22 pagesمحاضرة 6 (ن)انمي العراقPas encore d'évaluation
- Organic Chemistry Study Guide: Key Concepts, Problems, and SolutionsD'EverandOrganic Chemistry Study Guide: Key Concepts, Problems, and SolutionsÉvaluation : 3.5 sur 5 étoiles3.5/5 (10)
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsD'EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsPas encore d'évaluation
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersD'EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersPas encore d'évaluation