Académique Documents
Professionnel Documents
Culture Documents
Biochemistry - Final Exam Biology 020.305 December 15, 2011 Instructions For Examination
Transféré par
Jenna ReynoldsDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Biochemistry - Final Exam Biology 020.305 December 15, 2011 Instructions For Examination
Transféré par
Jenna ReynoldsDroits d'auteur :
Formats disponibles
Biochemistry Final Exam 2011
name______________________________
Biochemistry Final Exam
Biology 020.305
December 15, 2011
Instructions for examination:
1. Print your full name clearly on each page. If there are any pages missing, obtain a new
exam. No excuses will be accepted for missing pages. There are a total of 14 pages including:
1 cover page 1
10 question pages 2-11
1 tally page 12
1 page with metabolic pathways page 13
1 page with codon table page 14
2. Write answers clearly and concisely in the space provided. Extra space for doodling etc. is
found on last page of the exam. If you desire partial credit for anything that is NOT in the answer
space provided, you must clearly indicate where we should look for that partial credit.
3. Exams not written in blue or black permanent ink will lose the privilege of regrading.
4. Calculators are not necessary and their use is not allowed. When arithmetic calculations
are requested, if you are unable to do the calculations manually, set up the solution as completely
as possible without actually solving for the final number.
5. This exam is closed note and closed book. Books, notes, etc., are to be placed out of sight
before the beginning of the exam and are not to be referred to during the exam.
6. If you have any questions, please raise your hand and a proctor will come to you. If you prefer
to address your question to a course instructor, please request that a proctor contact an instructor.
7. You will be allowed 2 hours to take this exam. Once time is called you MUST stop writing.
Any writing after time is called is considered an ethics violation and we take these very
seriously. Please write your name and JHED ID on the tally page. Failure to do so will cost 2
points.
8. When you are done with the exam, please show your ID to a proctor who will accept your
exam. Then sign out by marking your test number on the roster. You will not be allowed to leave
the room until you turn in your exam.
9. Ethics Pledge: Please make the following commitment by signing and dating underneath the
following pledge:
I agree to complete this exam without unauthorized assistance from any person, materials, or
device. I will not take more than the allotted time. I will not alter my answers in any way after
receipt of the graded exam.
Signature:
Date:
1. (15 points total) Circle True or False. If False, correct the statement to make it true.
a) True/False: ATP is kinetically stable but thermodynamically unstable. 3
1
Biochemistry Final Exam 2011
name______________________________
b) True/False: Protein folding is favored by the hydrophobic effect and hydrogen bonding, but
disfavored by the entropy of folding.3
c) True/False: Allosteric inhibitors bind at an enzyme active site. 3
d) True/False: Covalent modification is an example of an irreversible form of protein regulation.
e) True/False: Carbohydrate modifications on proteins are important for cell-cell signaling and
recognition. 3
2. 7 total. Evidence from electron microscopy shows that the complexes of the electron transport
chain form supercomplexes in living systems. The supercomplex contains Complex I, Complex
III, and Complex IV.
a) What is one advantage of using a supercomplex for electron transport instead of separate
complexes? 3
b) Complex II (succinate coenzyme Q oxidoreductase) has never been observed in the
supercomplex. How is Complex II different from the other complexes in the electron transport
chain? Circle all that apply. 4
i) it participates in the TCA cycle
ii) it does not contain electron carriers
iii) it does not transport protons across the mitochondrial inner membrane
iv) it is not associated with the mitochondrial inner membrane
3. 6 points The RNA world hypothesis states that life started with RNA molecules that could
both store information (like DNA) and catalyze chemical reactions (like enzymatic proteins).
Biochemistry Final Exam 2011
name______________________________
a) Which of the following are ribozymes that contain catalytic RNA? Circle all that apply. 2
i) tRNA synthetase
ii) spliceosome
iii) RNA polymerase
iv) poly A polymerase
v) ribosome
b) Why are proteins better catalysts for chemical reactions than RNA? 2
Synthetic ribozymes designed to bind and cleave specific mRNAs have been tested in clinical
trials as therapeutic drugs for cancer and other diseases, but none have been approved by the
FDA.
c) Why do you think ribozymes might not be effective as drugs? Circle all that apply. 2
i) ribozymes are single-stranded
ii) ribozymes are not sequence specific
iii) ribozymes contain uracil instead of thymine
iv) ribozymes are degraded by RNA interference
v) ribozymes are not very stable
4. (8 total) On the molecule below, please label the following:
Biochemistry Final Exam 2011
name______________________________
a) Label the polar head group by putting a box around it
b) Label the fatty acid chain(s) with a circle or circles
c) Name two properties of the fatty acid chains that will affect the melting temperature of this
molecule and describe how that property will affect the melting temperature. See the example as
a guide. 4
example
property
affect
color
more blue = higher melting temperature
1
2
d) What properties are shared by all lipids? Circle all that apply 2
i) largely non-polar
ii) non-polymeric
iii) small
iv) highly reactive
v) unstable at low temperatures
Biochemistry Final Exam 2011
name______________________________
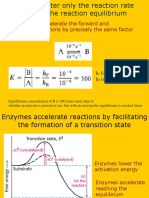
5. 32 total. Hexokinase catalyzes the first reaction of glycolysis.
a) Circle True or False: Hexokinase increases the rate of the reaction but does not change the
thermodynamics of the reaction. 2
b) Hexokinase performs a group transfer reaction to produce glucose-6-phosphate. Draw the
chemical structure of glucose-6-phosphate. 4
Arora et al. (1991) studied hexokinase function by making specific mutations in one of the HK
variants expressed in humans, HK-1. They created a plasmid that encoded the human HK-1 gene
and moved the plasmid to an E. coli bacterial expression system.
c) What must have been included in the plasmid for it to encode human HK-1 protein made in E.
coli? Circle all that apply. 3
i) a version of the gene exactly as it is encoded in the human genome
ii) a version of the gene that does not include introns
iii) a version of the gene that can be alternatively spliced
iv) a version of the gene that encodes polyA at the 3 end
v) a version of the gene that includes a Shine-Dalgarno sequence
vi) a version of the gene in which the first 3 bases transcribed make a start codon
vii) a version of the gene with a bacterial promoter upstream of the coding sequence
viii) a telomere
d) Based on the sequence of HK-1, the isoelectric point is estimated to be 6.51. If the researchers
used buffer of pH 8.5 during protein purification, which type of column would bind the HK-1?
Circle one. 2
i) cation exchange (negatively charged column)
ii) anion exchange (positively charged column)
iii) a non-polar column
Biochemistry Final Exam 2011
name______________________________
5 continued. Using purified HK-1, Arora et al. found the following:
area of mutation
mutation
KM(app) glucose
KM (app) ATP
Relative vmax
glucose binding site
wild-type
0.045
2.4
100
glucose binding site
Ser603-->Ala
0.0084
2.0
5.9
glucose binding site
Glu708-->Ala
2.28
2.7
9.6
glucose binding site
Glu742-->Ala
0.62
2.6
6.4
ATP binding site
wild-type
0.046
2.2
100
ATP binding site
Lys558-->Arg
0.05
2.2
70
ATP binding site
Lys558-->Met
0.047
2.1
29
e) The researchers chose to mutate Serine and Glutamate residues in the glucose binding site.
Which of the following properties do Serine and Glutamate share? Circle all that apply. 2
i) both are small
ii) both are bulky
iii) both are non-polar
iv) both are polar
v) both are mostly negatively charged at cellular pH
vi) both are mostly positively charged at cellular pH
f) Why are serine and glutamate good amino acids for the binding of glucose? 2
g) Based on the data above, which mutant version of HK has the lowest affinity for glucose?
Circle one. 2
i) Ser603 to Ala
ii) Glu708 to Ala
iii) Glu742 to Ala
iv) Lys558 to Met
v) impossible to tell from these data
5 continued.
6
Biochemistry Final Exam 2011
name______________________________
h) Mutations in the ATP binding site do not have a dramatic effect on ATP binding, but they have
significant impact on Vmax. How can this be explained? Circle all that apply. 2
i) Lys558 is more important to catalysis than to ATP binding
ii) Lys558 is important for glucose binding
iii) Vmax is estimated using high [substrate] so binding is not important in finding Vmax
iv) Vmax cannot be accurately estimated for enzymes with more than one substrate
v) a single amino acid change cannot affect both binding and activity
i) The KM for ATP was measured in the absence of glucose. When HK-1 binds glucose, a
conformational change occurs and the affinity for ATP increases. Why is this two-step binding
with a conformational change good for ensuring G6P is produced efficiently? 3
Naturally-occurring mutations in a version of HK found in red blood cells, HK-R, cause
haemolytic anemia which is anemia due to the death of red blood cells.
j) HK-R is transcribed from a different promoter than HK-2 or HK-3. HK-R, HK-2, and HK-3
are: Circle one. 2
i) splice-variants
ii) orthologs
iii) isozymes
iv) ribozymes
v) non-homologous
Other symptoms of haemolytic anemia are similar to symptoms of diabetes such as high blood
glucose levels. You decide to study HK-R mutations and find that HK-R mutant cells are
sensitive to insulin.
k) Which of the following would be evidence that insulin signaling is functional in these cells?
Circle all that apply. 2
i) dimerized insulin receptors
ii) phosphorylation of insulin receptors
iii) GTP binding by Galphai
iv) accumulation of insulin inside the cell
v) movement of the insulin receptor to the nucleus
5 continued.
Biochemistry Final Exam 2011
name______________________________
You decide to use the presence of GLUT4 on membranes as evidence of insulin signaling in HKmutant cells. You find that GLUT4 is induced on membranes in response to insulin, just like in
wild-type cells.
l) If GLUT4 is abundant on membranes, why do blood sugar levels remain high? 4
GLUT4 is a glucose transporter found only in eukaryotes. When you look at the amino acid
sequence, you do not see a consistent pattern of fully-hydrophobic alpha helices.
m) Why might this protein not contain fully-hydrophobic alpha helices? Circle all that apply. 2
i) residues where two helices interact may be polar
ii) GLUT4 is in the plasma membrane, which is a polar membrane
iii) side chains of alpha helices face the inside of the helix and can therefore be polar
even within a membrane
iv) alpha helices cannot pass through membranes
6. 20 total. Translation
Microarrays are useful to determine which genes are transcribed into mRNA, but mRNA levels
do not always correlate with the cellular levels of the encoded protein.
a) Why is this? Circle all that apply. 2
i) more than one copy of a protein can be synthesized from a single mRNA
ii) mRNAs can be stabilized by short complimentary RNAs
iii) mRNAs do not encode protein
iv) mRNAs can be degraded to prevent translation
One way to determine which mRNAs are translated is to isolate the mRNAs attached to
functional ribosomes, determine the sequence of those mRNAs, and match the mRNA sequence
to the gene sequence encoded in the DNA: a process called Ribosome Profiling.
b) If you performed Ribosome Profiling on mouse cells, what type of mRNA would you expect
to find associated with active ribosomes? Circle all that apply. 4
i) mRNA with a 5 cap
ii) mRNA with a 3 CCA extension
iii) mRNA that contains both introns and exons
iv) mRNA base-paired to snoRNAs
v) mRNA with a polyA tail
vi) mRNA bound by translation initiation factors (eIFs)
vii) mRNA bound by the RISC complex
c) Why are protein levels a better output for cell behavior than mRNA levels? 2
Biochemistry Final Exam 2011
name______________________________
6. continuted. Cycloheximide (shown in the figure below) can be used to inhibit translation in
eukaryotic systems like the mouse cells described in part b above.
Cycloheximide has been shown to bind in the E site of the 60S ribosomal subunit by hydrogen
bonding to a C in the 28S rRNA. Cycloheximide hydrogen-bonds to Cytosine in a way that
mimics the C-G base pair. When Cycloheximide is bound in the E site, empty tRNAs cannot exit
the ribosome.
d) On the CMP molecule below, circle the atom(s) involved in hydrogen bonding with guanine. 3
Cycloheximide doesnt act instantly. Studies using radioactive Phenylalanine and a polyU
template have shown that one peptide bond is formed after addition of Cycloheximide.
e) Why does Cycloheximide block elongation only after one peptide bond is formed? 3
Another inhibitor of eukaryotic translation is Harringtonine. Harringtonine also binds the 60S
subunit but only in the initiation complex.
f) What is unique about the initiation complex compared to any other complex in which the
ribosome is actively transcribing? Circle all that apply. 2
i) only the initiation complex includes the mRNA
ii) only the initiation complex includes empty E site
iii) the initiation complex includes a special initiator met-tRNA
iv) only the initiation complex includes GTP
Biochemistry Final Exam 2011
name______________________________
g) On the mRNA sequence below, circle the codon in the P site when a cell has been treated with
harringtonine. 2
h) Put a box around the codon that will be in the P site when a cell is treated with cyclohexamide.
2
5 cap-ACCUGAUGGACACGUACGAUCUGGAAAUAACAGCCUAAAAAAAAAAAAAA
7. 12 points total Phosphofructose kinase (PFK) catalyzes the conversion of fructose-6phosphate to fructose 1,6- bisphosphate during glycolysis. PFK is an important point of
metabolic regulation. Similar to hemoglobin, PFK binds substrates cooperatively and has two
stable conformational states, R and T.
a) Where is PFK located in cells? Circle one answer. 2
i) plasma membrane
ii) mitochrondrial matrix
iii) mitochondrial inner membrane
iv) cytoplasm
v) nucleus
The following graph shows the effects of ATP and AMP on PFK activity.
b) For the following statements, circle True or False. 1 point each, 6 points
i) True or False: PFK has multiple fructose-6-phosphate binding sites
ii) True or False: PFK is regulated by negative feedback inhibition
iii) True or False: ATP is an inhibitor of PFK
iv) True or False: ATP is a substrate for PFK
v) True or False: AMP preferentially binds the T-state
vi) True or False: ATP preferentially binds the T-state
10
Biochemistry Final Exam 2011
name______________________________
An increase in [H+] caused by lactic acid fermentation in muscles inhibits PFK activity. The
graph below shows PFK activity at two different pH values.
c) Which curve represents the lower pH? Circle one answer. 2
A
d) If a muscle creates a lot of force and uses ATP rapidly, which of the following would be true?
Circle one answer. 2
i) hemoglobin is highly saturated with O2 and PFK has high activity
ii) hemoglobin is less saturated with O2 and PFK has high activity
iii) hemoglobin is highly saturated with O2 and PFK has low activity
iv) hemoglobin is less saturated with O2 and PFK has low activity
11
Vous aimerez peut-être aussi
- Sample Exam 4 Fall 11Document14 pagesSample Exam 4 Fall 11janohxPas encore d'évaluation
- BIOC 307 Old Exam 1Document17 pagesBIOC 307 Old Exam 1Katie RosePas encore d'évaluation
- Biotechnology 2012 13Document54 pagesBiotechnology 2012 13Aziz KhanPas encore d'évaluation
- BIO307 Lecture 5 (Enzyme Kinetics I)Document11 pagesBIO307 Lecture 5 (Enzyme Kinetics I)Phenyo Mmereki100% (1)
- Answer HPLCDocument3 pagesAnswer HPLCMuhammad Firdaus100% (1)
- The Structure and Function of MacromoleculesDocument70 pagesThe Structure and Function of MacromoleculesLustried Nadyang100% (1)
- Plasma Protein Metabolism: Regulation of Synthesis, Distribution, and DegradationD'EverandPlasma Protein Metabolism: Regulation of Synthesis, Distribution, and DegradationMarcus RothschildÉvaluation : 5 sur 5 étoiles5/5 (1)
- Membrane Research: Classic Origins and Current ConceptsD'EverandMembrane Research: Classic Origins and Current ConceptsA. L. Muggleton-HarrisPas encore d'évaluation
- Enzymes Tutorial Part 1 and 2Document10 pagesEnzymes Tutorial Part 1 and 2Akeisha King100% (1)
- Channels, Carriers, and Pumps: An Introduction to Membrane TransportD'EverandChannels, Carriers, and Pumps: An Introduction to Membrane TransportPas encore d'évaluation
- A MLS Biochemistry Intro 2020 Lec 1Document25 pagesA MLS Biochemistry Intro 2020 Lec 1نجوي عبدالوهاب100% (1)
- Quantitative Human Physiology: An IntroductionD'EverandQuantitative Human Physiology: An IntroductionÉvaluation : 2 sur 5 étoiles2/5 (1)
- Nzymes: By: Mrs. Kalaivani Sathish. M. Pharm, Assistant Professor, Pims - PanipatDocument63 pagesNzymes: By: Mrs. Kalaivani Sathish. M. Pharm, Assistant Professor, Pims - Panipaturmila pandeyPas encore d'évaluation
- Fractional Order Systems and Applications in EngineeringD'EverandFractional Order Systems and Applications in EngineeringPas encore d'évaluation
- Kinetic Vs Chemical MechanismDocument34 pagesKinetic Vs Chemical MechanismIgnacio Bascuñán OyarcePas encore d'évaluation
- Structure and Function of Biological MembranesD'EverandStructure and Function of Biological MembranesLawrence I. RothfieldPas encore d'évaluation
- BIO307 Lecture 3 (Introduction To Enzymes)Document13 pagesBIO307 Lecture 3 (Introduction To Enzymes)Phenyo MmerekiPas encore d'évaluation
- Enzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisD'EverandEnzymes: A Practical Introduction to Structure, Mechanism, and Data AnalysisÉvaluation : 4 sur 5 étoiles4/5 (2)
- Bioseparations Principles and Techniques Kindle Edition - CompressDocument279 pagesBioseparations Principles and Techniques Kindle Edition - Compresssoumya deep phadikar100% (1)
- Cell Biology LabManual Version 8.0 May 2012 1 TRUNCATEDDocument57 pagesCell Biology LabManual Version 8.0 May 2012 1 TRUNCATEDதுர்காஸ்ரீ கங்கா ராதிகாPas encore d'évaluation
- Lecture HPLC StudentDocument243 pagesLecture HPLC StudentNur Hariani100% (1)
- Isoelectric Focusing: Theory, Methodology and ApplicationD'EverandIsoelectric Focusing: Theory, Methodology and ApplicationPas encore d'évaluation
- BIOCHEMISTRY, CELL AND MOLECULAR BIOLOGY: Passbooks Study GuideD'EverandBIOCHEMISTRY, CELL AND MOLECULAR BIOLOGY: Passbooks Study GuidePas encore d'évaluation
- Chemical Modulators of Protein Misfolding and Neurodegenerative DiseaseD'EverandChemical Modulators of Protein Misfolding and Neurodegenerative DiseasePas encore d'évaluation
- Chapter 2 - Basics of EnzymesDocument40 pagesChapter 2 - Basics of EnzymesSakinah MuhamadPas encore d'évaluation
- Biomolecules and Cells:: Mr. Derrick Banda MSC, BSCDocument69 pagesBiomolecules and Cells:: Mr. Derrick Banda MSC, BSCAmon Sangulube100% (1)
- Avatics 2017 03-02-15!34!57protein Synthesis Worksheet PracticeDocument2 pagesAvatics 2017 03-02-15!34!57protein Synthesis Worksheet PracticeMiguel BernalPas encore d'évaluation
- Biotechnology of Microbial Enzymes: Production, Biocatalysis, and Industrial ApplicationsD'EverandBiotechnology of Microbial Enzymes: Production, Biocatalysis, and Industrial ApplicationsPas encore d'évaluation
- Selected Topics in the History of Biochemistry. Personal Recollections. Part IIID'EverandSelected Topics in the History of Biochemistry. Personal Recollections. Part IIIÉvaluation : 1 sur 5 étoiles1/5 (1)
- Medicinal Chemistry—III: Main Lectures Presented at the Third International Symposium on Medicinal ChemistryD'EverandMedicinal Chemistry—III: Main Lectures Presented at the Third International Symposium on Medicinal ChemistryP. PratesiPas encore d'évaluation
- The Plasma Proteins V5: Structure, Function, and Genetic ControlD'EverandThe Plasma Proteins V5: Structure, Function, and Genetic ControlFrank PutnamÉvaluation : 3 sur 5 étoiles3/5 (1)
- Capillary Electrophoresis: Theory and PracticeD'EverandCapillary Electrophoresis: Theory and PracticePaul D. GrossmanPas encore d'évaluation
- MicroRNA in Human MalignanciesD'EverandMicroRNA in Human MalignanciesMassimo NegriniPas encore d'évaluation
- Biotransfrmtns Prepartv Organic Chemistry: The Use of Isolated Enzymes and Whole Cell Systems in SynthesisD'EverandBiotransfrmtns Prepartv Organic Chemistry: The Use of Isolated Enzymes and Whole Cell Systems in SynthesisPas encore d'évaluation
- Group21 Bioinformatics Assignment6 .Primary-structure-Protein-localizationdocxDocument6 pagesGroup21 Bioinformatics Assignment6 .Primary-structure-Protein-localizationdocxHuỳnh NhưPas encore d'évaluation
- The Pyridine Nucleotide CoenzymesD'EverandThe Pyridine Nucleotide CoenzymesJohannes EversePas encore d'évaluation
- Laboratory Techniques in Biochemistry and Molecular BiologyD'EverandLaboratory Techniques in Biochemistry and Molecular BiologyÉvaluation : 3 sur 5 étoiles3/5 (1)
- Study GuideDocument3 pagesStudy GuideSam Khader100% (1)
- Drug Design: Medicinal Chemistry: A Series of Monographs, Vol. 2D'EverandDrug Design: Medicinal Chemistry: A Series of Monographs, Vol. 2E. J. AriënsPas encore d'évaluation
- Fundamentals of BiochemistryDocument37 pagesFundamentals of BiochemistryMuhammadRizkyRamadhan100% (1)
- Monoclonal Antibodies Against Bacteria: Volume IIID'EverandMonoclonal Antibodies Against Bacteria: Volume IIIAlberto J. L. MacarioPas encore d'évaluation
- L 17 Structure and Functions of ProteinsDocument43 pagesL 17 Structure and Functions of ProteinssPas encore d'évaluation
- Temperature-Responsive Polymers: Chemistry, Properties, and ApplicationsD'EverandTemperature-Responsive Polymers: Chemistry, Properties, and ApplicationsPas encore d'évaluation
- Bioinformatics: Applications: ZOO 4903 Fall 2006, MW 10:30-11:45 Sutton Hall, Room 312 Jonathan WrenDocument75 pagesBioinformatics: Applications: ZOO 4903 Fall 2006, MW 10:30-11:45 Sutton Hall, Room 312 Jonathan WrenlordniklausPas encore d'évaluation
- Analysis of Sterols and Other Biologically Significant SteroidsD'EverandAnalysis of Sterols and Other Biologically Significant SteroidsW. David NesPas encore d'évaluation
- Chapter 1 The Nature of Analytical ChemistryDocument35 pagesChapter 1 The Nature of Analytical ChemistryAnalie RavinaPas encore d'évaluation
- Dna Sequencing and Gene Clonning: Kalpana DaleiDocument31 pagesDna Sequencing and Gene Clonning: Kalpana DaleiBinod Sahu100% (1)
- Biosimilars of Monoclonal Antibodies: A Practical Guide to Manufacturing, Preclinical, and Clinical DevelopmentD'EverandBiosimilars of Monoclonal Antibodies: A Practical Guide to Manufacturing, Preclinical, and Clinical DevelopmentPas encore d'évaluation
- 02 Transactions 1Document61 pages02 Transactions 1Jenna ReynoldsPas encore d'évaluation
- Study Guide Lesson 7Document1 pageStudy Guide Lesson 7Jenna ReynoldsPas encore d'évaluation
- Biochem Exam 3 - 2013Document14 pagesBiochem Exam 3 - 2013Jenna ReynoldsPas encore d'évaluation
- JHU Biochem - Exam 2 - 2013Document11 pagesJHU Biochem - Exam 2 - 2013Jenna ReynoldsPas encore d'évaluation
- Matlab Mathworks Data AnalysisDocument167 pagesMatlab Mathworks Data Analysisbob_mirkPas encore d'évaluation
- MCQ 2 - PharmacodynamicsDocument5 pagesMCQ 2 - PharmacodynamicsHillary Nguyen100% (3)
- Transfer of Genetic InformationDocument13 pagesTransfer of Genetic InformationStevanus JonathanPas encore d'évaluation
- Protein Engineering-Methods and ProtocolsDocument347 pagesProtein Engineering-Methods and Protocolslzy100% (1)
- Microbiology Principles and Explorations 10Th Edition Black Test Bank Full Chapter PDFDocument59 pagesMicrobiology Principles and Explorations 10Th Edition Black Test Bank Full Chapter PDFlloydkieran71epfi100% (10)
- Folleto - Epmotion 5075t - Epmotion 5075m - Changing To NGS BaseDocument4 pagesFolleto - Epmotion 5075t - Epmotion 5075m - Changing To NGS Basepunctt.Pas encore d'évaluation
- Neurotransmitter TepiDocument32 pagesNeurotransmitter TepiAmallia Nuggetsiana SetyawatiPas encore d'évaluation
- (Sách) Edexcel a Level Biology Student Book 2 - Sách Gáy XoắnDocument306 pages(Sách) Edexcel a Level Biology Student Book 2 - Sách Gáy XoắnSACH TP HA NOI100% (2)
- Lect. 12 PL Path 111 - Variability in Plant PathogensDocument25 pagesLect. 12 PL Path 111 - Variability in Plant Pathogensdawit gPas encore d'évaluation
- Policies: Attendance - ID & Uniform - Quizzes - Grading System - 40% Quizzes - 30% Manual - 10% Exam - 60%Document67 pagesPolicies: Attendance - ID & Uniform - Quizzes - Grading System - 40% Quizzes - 30% Manual - 10% Exam - 60%kamiya008100% (1)
- Cell Cycle Cell Division PDFDocument13 pagesCell Cycle Cell Division PDFmuzamil shabir100% (1)
- JasmonatesDocument38 pagesJasmonatesSpinu LiliaPas encore d'évaluation
- 11 Biology Notes Ch08 Cell Structure and FunctionsDocument8 pages11 Biology Notes Ch08 Cell Structure and FunctionsVansh Mehta100% (1)
- WJEC Markscheme Gce Ms Biology Jun09legacy eDocument34 pagesWJEC Markscheme Gce Ms Biology Jun09legacy eEmi JiHyeon KimPas encore d'évaluation
- Glencoe Biology Textbook Glossary 2010Document84 pagesGlencoe Biology Textbook Glossary 2010Bradford McCallPas encore d'évaluation
- The Nature Milestones in DNA TechnologiesDocument16 pagesThe Nature Milestones in DNA TechnologiesJacoSkinfield0% (1)
- Biology Unit 1 Edexcel NotesDocument15 pagesBiology Unit 1 Edexcel NotesKibz Ahmed80% (5)
- Pulsed-Field Gel Electrophoresis (PFGE) - PulseNet Methods - PulseNet - CDCDocument2 pagesPulsed-Field Gel Electrophoresis (PFGE) - PulseNet Methods - PulseNet - CDCChaitanya KPas encore d'évaluation
- BSN - DNA Extraction ProtocolDocument3 pagesBSN - DNA Extraction ProtocolAnnica LozanoPas encore d'évaluation
- Physical Science Q1 Module 4Document22 pagesPhysical Science Q1 Module 4Alfred RodellasPas encore d'évaluation
- Protein Chemistry Section ExamDocument9 pagesProtein Chemistry Section ExamJoelPas encore d'évaluation
- 2.protein Primary StructureDocument83 pages2.protein Primary Structuremitchellpearson97Pas encore d'évaluation
- Module 4 - PharmacologyDocument53 pagesModule 4 - PharmacologyDonzzkie Don100% (3)
- Gel Electrophoresis PDFDocument15 pagesGel Electrophoresis PDFTeflon Slim100% (1)
- DNA Extraction and CharacterizationDocument4 pagesDNA Extraction and CharacterizationEricka Galang100% (1)
- A GCE Biology 2804 01 January 2008 Question PaperDocument24 pagesA GCE Biology 2804 01 January 2008 Question PaperVeer Ramloghun0% (1)
- The-Cell-Cycle-Worksheet With AnswersDocument5 pagesThe-Cell-Cycle-Worksheet With AnswersNicolai MarquezPas encore d'évaluation
- Chapter 2.0 Cell Signalling and Endocrine RegulationDocument93 pagesChapter 2.0 Cell Signalling and Endocrine RegulationNurarief AffendyPas encore d'évaluation
- Handbook of Shanti Swarup Bhatnagar Prize Winners (1958 - 1998)Document118 pagesHandbook of Shanti Swarup Bhatnagar Prize Winners (1958 - 1998)Hazari ChauhanPas encore d'évaluation
- IB BIOLOGY TOPIC 1 QuestionsDocument3 pagesIB BIOLOGY TOPIC 1 QuestionsSarah Mason100% (1)
- Cell JunctionsDocument1 pageCell JunctionsJoshua Mari De OcampoPas encore d'évaluation