Académique Documents
Professionnel Documents
Culture Documents
Sample Operations Narrative 01-Oct-03
Transféré par
rambabuCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Sample Operations Narrative 01-Oct-03
Transféré par
rambabuDroits d'auteur :
Formats disponibles
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
Concept of Operations
PERSONNEL
All employees entering the facility will use the Main Entrance.
Administrative staff who will not be entering the manufacturing areas can
proceed directly to their work places in street clothes. The remainder of the
personnel will use the following gowning procedures:
All personnel entering the manufacturing operations must change
from street clothes into plant supplied and laundered uniforms.
Lockers will be supplied for each employee. The uniforms must be
left in the facility when departing. Once an employee has a put on a
uniform they may not leave the facility wearing it. This level will be
considered Uniformed Personnel (UP).
Personnel entering Class 100k clean space must add Hair & Shoe
covers in the passthrough airlocks before entering the clean space.
This level will be Hair & Shoe Personnel (HSP).
Finally all personnel entering sterile and or virally contaminated
space must be fully gowned. This should include full Tyvek or
similar coverall (preferably with boots and hood attached), two layers
of latex or similar disposable gloves, micro-filtering mask, and full
goggles. No skin should be exposed to the room environment. This
will be fully gowned or Gowned Personnel (GP).
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
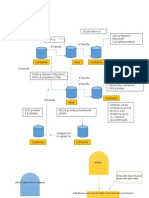
AIRLOCKS
There are a number of types Airlocks used in the facility. All are designed to
segregate one area from another for contamination control, for containment
or both. Each airlock or suite of airlocks will have electronic interlocks to
insure that only one door can be opened at a time.
In case of a fire
emergency the door locks will be automatically overridden. The follow
describes in general the uses for this interstitial space.
Airlock (A/L) Throughout the facility these rooms separate two
adjacent rooms with different air classifications. They are for
personnel or personnel carrying manually transportable items
such as samples for testing.
Equipment Lock (E/L) The equipment lock is used for the
transfer of equipment and materials between two adjacent rooms
with different air classifications.
Normal procedure is for a
Material Handler (MH) to deliver the material and place it in the
Equipment Lock.
If possible the MH should do so without
physically entering the E/L. When the door is closed a MH or an
operator will retrieve the item from the other room, if possible
without entering the E/L. Hydraulic pallet jacks or carts are
particularly useful here.
Gown In Suite All personnel entering the Sterile Core will first
enter an A/L then they will enter the Gown In suite. Within this
room they will put on the appropriate attire for the next level of
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
area classification. The final separation is the Step Over Bench.
Here personnel will don their final shoe cover and place the clean
booted foot on the opposite side of the bench.
Gown Out Suite All personnel leaving an area wherein viral
components are handled will leave through a Gown Out or DeGown Suite. The objective is to assure that contaminants, which
may be on the exterior garments, are controlled.
Personnel
leaving an operating area will follow a reverse procedure from
the one above, with the last step being removal of shoe covers as
they step over the bench.
HOUSEKEEPING
There are basically four levels of housekeeping in the facility. These are:
General all administrative, training, cafeteria and common
corridors. General areas will be cleaned by janitorial personnel,
using normal household cleaners and equipment.
Clean Space areas which are maintained as Class 100k space.
Primary concern for these areas is particulate contamination.
Clean space will require the personnel to vacuum and wipe-down
floors walls and ceilings.
Viral Space any area, which has the potential of being
contaminated by live virus.
The cleaning of Viral Space will
require the use of disinfectant cleaners to neutralize the active
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
virus. Rooms should be washed with a disinfectant cleaning
solution known to be effective on the particular viral strain. These
solutions will be changed regularly to avoid the virus becoming
resistant.
Sterile Space areas which are maintained to prevent
contamination of sterile products and components. These will be
Class 10k and Class 100 space. Sterile Space will be cleaned as
outlined above in Viral Space.
The intent is to remove any
contaminants, which can get into the product.
RECEIVING
Material Handlers (MH) (UP) in the receiving area will enter the receiving
area via the main corridor. Pallet loads of raw and packaging materials will
be delivered from the main warehouse in Building in daily quantities to
minimize staging requirements. The pallets will then be transferred into the
material airlock and picked up by a material handler on the clean side.
Materials going to the second level will be placed in the material lift.
All eggs will be received at a separate dock door. Pallet loads of boxed eggs
will be moved to the staging refrigerators using powered pallet jacks. Eggs
received on flats in racks will be moved manually. The egg racks can weigh
over 1,000 lb each and will require two handlers to move them. The eggs
will be placed in the staging refrigerators from one set of doors and will be
removed from the opposite end.
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
EGG WASH
Egg Preparation Operators (EPO) will be UP. They will enter the egg prep
area via the main corridor.
EPOs will remove boxes of eggs with a
hydraulic pallet jack or racks of eggs manually from the staging refrigerator.
Boxed eggs must be manually unpacked on an inspection table and
transferred to plastic flats using a vacuum assisted egg lift. The device will
lift all 36 eggs from the pressboard flat and place them in a clean plastic flat.
Clean flats will be delivered to the area via an airlock to the clean corridor.
Eggs will be inspected for breakage and broken eggs will be replaced to
maintain full flats. The EPO will place the flat on the conveyor, which
passes through the wall to the egg washer. Corrugate and pressboard flats
will be compacted for disposal.
PRE-INCUBATION
The MHs in this area are HSP. They will take dried racks of eggs and
manually transfer them to the input side of the incubators. The racks are
pushed into the incubators manually, and there are tracks in the floor to
guide the casters. There are doors at both ends of the incubators to preserve
First In First Out (FIFO) control. The operators will pre-start the units before
starting to place the racks in, and temperature and humidity will be
controlled by a PLC.
PRE-INOCULATION CANDLING
At the end of the defined incubation period, the egg racks are manually
removed by a MH in HSP from the incubator and transferred into the PreInoculation Candling room via the 100k plant corridor. In the candling suite
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
the egg flats are manually removed from the rack by a MH in HSP using a
spoon and set on the staging section of a conveyor for transport. The
conveyor is constantly running with three sections separated by stops. The
Candlers in HSP are seated along both sides of the conveyor with candling
lights to inspect each egg for embryo viability, air sack location, and
cracking. Unsatisfactory eggs are manually discarded into a lined container.
INOCULATION
The conveyor from candling passes through a wall, which represents
another change in state. The Inoculation room is considered hot in that
live virus is handled here. This requires an additional level of gowning for
the Egg Handlers (EH). EH will be GP and will enter from the Class 100k
corridor through a gowning air lock, where they will put on additional
protection.
Once the employee has entered the inoculation room they
cannot go back into the gowning suite and must exit through a prescribed
de-gowning area. The general area is Class 10k.
POST-INOCULATION INCUBATION
Incubators are provided to accommodate the post inoculation, incubation
step. MHs are GP in this area and must enter through a gowning suite and
depart through the de-gowning room. The MH will manually move racks
from the inoculation room to the input side of the incubators. The weight of
the egg-laden racks will require two operators. As with the pre-incubators,
these units will have tracks to guide the casters.
The incubators are
equipped with doors at both ends to accommodate FIFO operations.
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
POST INOCULATION CANDLING
After the required incubation time has elapsed, the racks are manually
removed from the incubators, by a MH, in the order they were installed.
The racks are moved the post inoculation candling room and placed at the
upstream end of the candling conveyor. This room will be maintained as
Class 10k.
The conveyor is constantly running with three sections separated by stops.
Candlers (GP) are seated along both sides of the conveyor with candling
lights to inspect each egg for embryo viability, air sack location, and
cracking. Unsatisfactory eggs are manually discarded into a lined container.
HARVEST FEED
Chilled egg racks are manually removed from the chiller and transferred to
the Class 10k Harvest Feed Room by MH (GP). EH (GP) will manually
place the flats of chilled eggs on the infeed conveyor to the egg de-capper.
When a rack is emptied it will be placed into the automatic rack washer.
When the cycle is complete the cleaned and dried rack will be removed by a
MH (HSP) and stored in clean staging. The unit will pass through wall
separating the viral area from clean staging.
The flats of decapped eggs pass through a wall on the conveyor to the
Harvesting Room. The two conveyors will merge into one double stacked
unit under a Class 100 HEPA filtered Laminar flow hood. The decappped
flats will move to the Harvesters (GP) on the top conveyor. The fluid is
removed from the eggs by vacuum with a wand.
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
FIVE MICRON FILTRATION
FOs entering the Sterile Core will fully gown in the Gown In airlock. Once
inside the operation the FO cannot return to the gowning room. The FO
(GP) in the Five Micron Filtration room will manually connect a jacketed
vessel to the cooling system. They will take a bottle of thawed material and
using a parastaltic pump under a BSC transfer the contents to a holding
vessel.
CENTRIFUGATION
A Centrifuge Operator (CO) (GP) is responsible for the set up and startup of
the Centrifuge. COs entering the Sterile Core will fully gown in the Gown
In airlock. Once inside the operation the CO cannot return to the gowning
room.
The material will be manually transported to the Centrifuge room. The CO
will start the Centrifuge and add the gradient. A transport vessel with
filtered harvest fluid will be delivered to the Centrifuge Room from the
Filtration manually. The jacketed vessel will be connected to the cooling
system and the harvest fluid feed lines will be connected to the transfer
pump using a LAF hood. When the centrifugation is complete the CO will
collect the fractions in separate bottles using the LAF to insure no
contamination. The CO will manually transfer the dirty transfer vessel and
contaminated tubing to the dirty corridor equipment lock.
Sample Operations Narrative 01-Oct-03.doc
PHE 535 / Lecture 5 / Architectural Design Guidelines / Operations Narrative
POOLING AND STERILIZING OF FRACTIONS
The CO will manually deliver the selected, pooled fractions to the Sterilizing
Operator (SO) (GP).
As with the previous sterile suites doors will be
interlocked. The SO will have entered the sterile core through the gowning
suite. The SO will pump the vials into a mixing vessel under a BSC to
reduce the chance of contamination. Under a Class 100 LAF the SO will
pump the pooled fractions through the sterilizing filter into bulk bottles.
EQUIPMENT WASHING
Equipment Washers (EW) (HSP) will remove decontaminated equipment
from the pass through Autoclaves and place it in the staging area. Large
equipment items such as tanks and large waste containers are manually
moves on casters to the Tank Washer. Glassware and small containers are
manually removed from the decon carts and placed on the infeed conveyor
to the Glassware/Parts Washer. Additional small machine components
such as inoculator heads and decapper tooling are manually placed in the
Ultrasonic Sinks for cleaning.
Sample Operations Narrative 01-Oct-03.doc
Vous aimerez peut-être aussi
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- ఆంధ్రమహాభారతం ఆదిసభాపర్వాలుDocument957 pagesఆంధ్రమహాభారతం ఆదిసభాపర్వాలుrambabu80% (20)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- AntibioticsDocument4 pagesAntibioticsrambabuPas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- PrintDocument7 pagesPrintrambabuPas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Predictor PlatesDocument40 pagesPredictor PlatesrambabuPas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- For Your InformationDocument146 pagesFor Your InformationrambabuPas encore d'évaluation
- Air ClassificationDocument6 pagesAir ClassificationjithendrakumarbPas encore d'évaluation
- For Your InformationDocument146 pagesFor Your InformationrambabuPas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- 628 Q+examDocument133 pages628 Q+examrambabuPas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Bowie-Dick Parameters Re-Take Test With Set Parameters To Be Released by STERIS DART121 Is Labelled As BD132 and ReverseDocument1 pageBowie-Dick Parameters Re-Take Test With Set Parameters To Be Released by STERIS DART121 Is Labelled As BD132 and ReverserambabuPas encore d'évaluation
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Update On ISO Cleanroom Standards 14644 Amp 14698Document26 pagesUpdate On ISO Cleanroom Standards 14644 Amp 14698rambabuPas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Containe Containe Vess: AlcoholDocument2 pagesContaine Containe Vess: AlcoholrambabuPas encore d'évaluation
- Pizza Home Made: Pizza Making in A Kitchen OvenDocument1 pagePizza Home Made: Pizza Making in A Kitchen OvenrambabuPas encore d'évaluation
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- PME 535A Project ReportDocument6 pagesPME 535A Project ReportrambabuPas encore d'évaluation
- No. of Courses Term 1 Spring-2015 2 Spring-2015 3 Fall-2015 4 Spring-2015 5 Summer-2015 6 Fall-2015 7 Fall-2015Document2 pagesNo. of Courses Term 1 Spring-2015 2 Spring-2015 3 Fall-2015 4 Spring-2015 5 Summer-2015 6 Fall-2015 7 Fall-2015rambabuPas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Recipe - Bromhexine SyrupDocument1 pageRecipe - Bromhexine SyruprambabuPas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Packing List For GermanyDocument2 pagesPacking List For GermanyarjungangadharPas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Dressmaking 7 ExamDocument2 pagesDressmaking 7 ExamMelchie RamosPas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The History of Your FaceDocument18 pagesThe History of Your Facep_om_chopPas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- CollaborativewritingDocument2 pagesCollaborativewritingapi-309370966Pas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- A7LDon DoffDocument16 pagesA7LDon DoffDainXBPas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Impact of Covid To Textile Industry of Sri LankaDocument15 pagesImpact of Covid To Textile Industry of Sri LankaSajaniPas encore d'évaluation
- The Brake SystemDocument15 pagesThe Brake SystemweldsaidiPas encore d'évaluation
- Remember:: Before Caring For Patients With Confirmed or Suspected COVID-19, Healthcare Personnel (HCP) MustDocument4 pagesRemember:: Before Caring For Patients With Confirmed or Suspected COVID-19, Healthcare Personnel (HCP) MustJhann OdiracPas encore d'évaluation
- Warriors (1979) HandbookDocument35 pagesWarriors (1979) HandbookBrian SmithPas encore d'évaluation
- Anthropologicalsl02thomuoft BWDocument160 pagesAnthropologicalsl02thomuoft BWChristine H WhytePas encore d'évaluation
- The King Buzzard: Bano Qudsia's Raja GidhDocument18 pagesThe King Buzzard: Bano Qudsia's Raja GidhMasood Ashraf RajaPas encore d'évaluation
- Alternatives & RecommendationDocument14 pagesAlternatives & RecommendationRezwana Newaz SuroviPas encore d'évaluation
- 19 20 - AP Lit Summer Reading Short Story SetDocument39 pages19 20 - AP Lit Summer Reading Short Story SetSophie PhiloPas encore d'évaluation
- SculptureDocument31 pagesSculptureDexter AlvarinaPas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- AntrakuinonDocument5 pagesAntrakuinonIlhamPas encore d'évaluation
- Nice Guy To Alpha Male GuideDocument24 pagesNice Guy To Alpha Male GuideBrito Kevin57% (74)
- Apparel TestingDocument9 pagesApparel TestingAl AminPas encore d'évaluation
- Bed and Bathroom Linen - 29.12.2020Document2 pagesBed and Bathroom Linen - 29.12.2020Eden Led LightingPas encore d'évaluation
- Hasina: Through My Eyes by Michelle Aung Thin, Edited by Lyn White ExtractDocument28 pagesHasina: Through My Eyes by Michelle Aung Thin, Edited by Lyn White ExtractAllen & Unwin100% (1)
- "A Study On Customer Prefrences Towards Sports Wear Brand": A Summer Training Project ReportDocument74 pages"A Study On Customer Prefrences Towards Sports Wear Brand": A Summer Training Project Reportkaran wasanPas encore d'évaluation
- Manual Spectrum 375 CutMateDocument36 pagesManual Spectrum 375 CutMateAnonymous SB69nITw100% (2)
- VW Golf AccessoriesDocument32 pagesVW Golf AccessoriesSvoura Svouras50% (2)
- Từ Vựng + Ngữ Pháp Tiếng Anh 9Document19 pagesTừ Vựng + Ngữ Pháp Tiếng Anh 9Nguyễn Ngọc LinhPas encore d'évaluation
- Gipe Electricity IndiaDocument98 pagesGipe Electricity IndiaNitin VarmaPas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- 745 Handloom Bed SheetDocument12 pages745 Handloom Bed SheetMangal CorporationPas encore d'évaluation
- Jones, D. F. - Colossus (English, 287p)Document287 pagesJones, D. F. - Colossus (English, 287p)argentronicPas encore d'évaluation
- Mhlanga 23 PDFDocument486 pagesMhlanga 23 PDFParasmento0% (1)
- Twenty-One Days in India - Aberigh-Mackay, George Robert, 1848-1881Document94 pagesTwenty-One Days in India - Aberigh-Mackay, George Robert, 1848-1881pradipkumarpatilPas encore d'évaluation
- NEU180 Series User ManualDocument16 pagesNEU180 Series User ManualalbertoalejPas encore d'évaluation
- Sampling, Pattern Making and Marker MakingDocument9 pagesSampling, Pattern Making and Marker MakingSamikhya SharmaPas encore d'évaluation