Académique Documents
Professionnel Documents
Culture Documents
Pedaleo
Transféré par
CristianLopezTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Pedaleo
Transféré par
CristianLopezDroits d'auteur :
Formats disponibles
Locomotor Strategy for Pedaling: Muscle Groups and Biomechanical
Functions
CHRISTINE C. RAASCH1,2 AND FELIX E. ZAJAC13
Rehabilitation Research and Development Center, Veterans Affairs Palo Alto Health Care System, Palo Alto 94304-1200;
and Departments of 2Mechanical Engineering (Biomechanical Engineering Division) and 3Functional Restoration, Stanford
University, Stanford, California 94305-3030
Raasch, Christine C. and Felix E. Zajac. Locomotor strategy for
pedaling: muscle groups and biomechanical functions. J. Neurophysiol. 82: 515525, 1999. A group of coexcited muscles alternating
with another group is a common element of motor control, including
locomotor pattern generation. This study used computer simulation to
investigate human pedaling with each muscle assigned at times to a
group. Simulations were generated by applying patterns of muscle
excitations to a musculoskeletal model that includes the dynamic
properties of the muscles, the limb segments, and the crank load.
Raasch et al. showed that electromyograms, pedal reaction forces, and
limb and crank kinematics recorded during maximum-speed start-up
pedaling could be replicated with two signals controlling the excitation of four muscle groups (1 group alternating with another to form
a pair). Here a four-muscle-group control also is shown to replicate
steady pedaling. However, simulations show that three signals controlling six muscle groups (i.e., 3 pairs) is much more biomechanically
robust, such that a wide variety of forward and backward pedaling
tasks can be executed well. We found the biomechanical functions
necessary for pedaling, and how these functions can be executed by
the muscle groups. Specifically, the phasing of two pairs with respect
to limb extension and flexion and the transitions between extension
and flexion do not change with pedaling direction. One pair of groups
(uniarticular hip and knee extensors alternating with their anatomic
antagonists) generates the energy required for limb and crank propulsion during limb extension and flexion, respectively. In the second
pair, the ankle plantarflexors transfer the energy from the limb inertia
to the crank during the latter part of limb extension and the subsequent
limb extension-to-flexion transition. The dorsiflexors alternate with
the plantarflexors. The phasing of the third pair (the biarticular thigh
muscles) reverses with pedaling direction. In forward pedaling, the
hamstring is excited during the extension-to-flexion transition and in
backward pedaling during the opposite transition. In both cases hamstrings propel the crank posteriorly through the transition. Rectus
femoris alternates with hamstrings and propels the crank anteriorly
through the transitions. With three control signals, one for each pair of
groups, different cadences (or power outputs) can be achieved by
adjusting the overall excitatory drive to the pattern generating elements, and different pedaling goals (e.g., smooth, or energy-efficient
pedaling; 1- or 2-legged pedaling) by adjusting the relative excitation
levels among the muscle groups. These six muscle groups are suggested to be elements of a general strategy for pedaling control, which
may be generally applicable to other human locomotor tasks.
INTRODUCTION
A common idea in motor control is the use of primitives to
control complex tasks. Bernstein (1967) posited that because of
The costs of publication of this article were defrayed in part by the payment
of page charges. The article must therefore be hereby marked advertisement
in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
the large number of degrees of freedom involved in multijoint
movement (including muscle states as well as kinematic
states), control must be hierarchical. Understanding how a
muscle-coordination pattern required to execute one task
changes to execute another may give insight into the way
existing motor patterns serve as initial guesses in the learning of new tasks (Greene 1982) and into the features common
among the patterns (the primitives) (Giszter et al. 1993;
Mussa-Ivaldi et al. 1994). Coexcitation of muscles (i.e., partitioning of muscles into a group, called a functional muscle
group) (Jacobs and MacPherson 1996; Raasch et al. 1997) is
one possible primitive and is a common concept in motor
control (McCollum 1993; Zajac and Gordon 1989).
Functional muscle groups have been identified in intact
animals during walking and postural balance (Engberg and
Lundberg 1969; Macpherson et al. 1986; Rasmussen et al.
1978). Two groups alternating in excitation with one another
(called a pair of alternating muscle groups) (Raasch et al. 1997)
are often anatomic antagonists (Bradley and Smith 1988; McCollum 1993) though not necessarily (Jacobs and MacPherson
1996).
Alternating muscle groups are a basic tenet in the concept of
pattern generation of rhythmic locomotor movements (Grillner
1975, 1981; Shik and Orlovsky 1976). Electromyographic
(EMG) recordings from animal locomotor preparations have
been shown to be compatible with the idea of grouped-muscle
control (Rossignol 1996). In fictive locomotion, the basic
alternation of neural output to the muscle groups even can be
generated in the absence of movement (Grillner 1988; Grillner
and Zangger 1979). To respond to disturbances and changing
task demands, however, movement and other feedback are
believed to be critical to locomotor pattern modulation (i.e.,
gain and phase modulation of muscle groups) (Pearson 1993;
Rossignol et al. 1988). For example, the cat locomotor pattern generator could produce a faster walking speed or a
higher limb power output at the same speed (e.g., to walk
upslope) by increasing the neural excitation to the muscle
groups without changing their phasing. This can be achieved
by increasing the overall tonic drive to the spinal cord through
supraspinal descending or unspecific afferent signals (Grillner
1975, 1981, 1988; Shik and Orlovsky 1976). Changing the gain
of one or more groups may be a sufficient control strategy for
responding to disturbances (Forssberg 1979). At other times,
changing muscle phasing is required as well, such as to switch
to a different gait (Grillner 1981; Shik and Orlovsky 1976).
In humans, elucidation of locomotor muscle groups from
515
516
C. C. RAASCH AND F. E. ZAJAC
studies of walking is difficult because of the complexity of the
task. Nevertheless studies of supported treadmill stepping in
spinal-cord-injured patients have suggested the existence of
pattern generators in the human spinal cord (Barbeau and
Rossignol 1994; Calancie et al. 1994; Dietz et al. 1995; Rossignol and Barbeau 1995; Rossignol et al. 1996). The mutability of human locomotor patterns has been examined in
split-treadmill walking, where different belt speeds cause ankle
flexors to be excited longer because a longer swing phase of the
leg on the slower belt is needed (Dietz et al. 1994). Also in
backward walking, uniarticular knee extensors remain excited
in the support phase (as in forward walking) (Winter et al.
1989), while bifunctional thigh muscles switch in phase relative to the two transitions between stance and swing (heelstrike and toe-off in forward walking; toe-strike and heel-off in
backward walking) (Thorstensson 1986). Thus it appears that
some parts of the locomotor pattern can be modified with some
muscles more dependent on task directionality than others.
Pedaling is a bipedal locomotor task well suited to the
identification of functional muscle groups and propulsion strategies in humans. Both pedaling and walking are cyclical at
about the same frequency (;1 Hz) (Coast and Welch 1985;
Winter 1983). Both tasks require the legs to alternate in flexion
and extension with most of the propulsive energy generated in
extension. Both demonstrate the same phase-dependent modulation of reflexes (Brooke et al. 1992; Brown and Kukulka
1993; Yang and Stein 1990). Ergometer pedaling is, however,
different from walking in other ways, some of which are
advantageous to the study of legged propulsion and muscle
coordination. Balance is not a factor in seated pedaling, so
muscle-coordination patterns applicable to propulsion per se
should dominate. In fact, experiments to elucidate the patterngenerating properties of locomotion in animals (Forssberg et
al. 1980; Grillner 1981; Grillner and Zangger 1979) and infants
(Forssberg 1985) also have emphasized propulsion coordination (cf. balance coordination). Because pedaling is a kinematically constrained task with fewer degrees of freedom than
walking, it is easier to simulate with a computer model and
more amenable to experimentation. Still, pedaling is sufficiently complex that muscle coordination is not trivial. For
example, because of dynamic interactions among the segments
(Zajac and Gordon 1989) and the constraints of the closed
linkage in pedaling (which also occurs in the double-support
phase of walking), the actions of individual muscles on the
system are not obvious; e.g., the biarticular hamstrings, anatomically classified as a hip extensor and a knee flexor, act to
accelerate the knee oppositely, into extension, in the crank
downstroke (Raasch et al. 1997). Therefore it is important to
account for the complex mechanics of muscle and segments
when hypothesizing neural control strategies or attempting to
deduce underlying strategies from experimental data.
We have used a computer model of the musculoskeletal
system, in conjunction with experimental work, to begin to
characterize the essential neural-control elements required for
pedaling propulsion based on the biomechanical requirements
of the task. In an experimental and theoretical study of maximum-speed start-up forward pedaling, Raasch et al. (1997)
have shown that a controller constrained by lower limb muscles organized into four groups performs as well (within 4% of
maximum speed during start-up) as an unconstrained controller
where each muscle is excited independently from the others
(Raasch et al. 1997). Nearly equal performance is achieved
because the biomechanics of the task, specifically, the individual muscle contributions to crank and limb propulsion, is
nearly replicated with control of the four muscle groups. In
fact, the control strategy can be simplified further because it
was found that each group alternates in phasing with one other
group. Thus the four groups can be arranged into two pairs,
with only one signal controlling each pair because the two
groups in each pair alternate due to their nearly opposing
biomechanical functions.
In this simple control scheme of pedaling, one pair of
alternating muscle groups produces the energy needed to propel the crank through limb extension (the uniarticular hip and
knee extensor muscles, EXT) and flexion (the uniarticular hip
and knee flexor muscles, FLEX), with some energy to accelerate the limb segments first. The other pair facilitates the
transfer of energy to the crank produced by the other muscles
and also produces energy to propel the crank directly, near the
end of extension and during the limb transition from extension
to flexion (the hamstring and ankle plantarflexor muscles,
HAM/TS) and near the end of flexion and during the flexionto-extension transition (the rectus femoris and ankle dorsiflexor
muscles, RF/TA). A muscle transfers energy by essentially
acting as a rigid link to constrain the acceleration of limb
segments (though it also may be simultaneously absorbing or
generating energy, and so may not be strictly isometric). For
example, plantarflexors can constrain ankle motion in downstroke so that acceleration of the shank by nonankle muscles
now also will accelerate the foot and, by intersegmental coupling (Zajac and Gordon 1989), the crank.
The biomechanical functions performed by the four muscle
groups (Raasch et al. 1997) (see preceding text) have been the
basis for analysis of EMG patterns generated by neurologically
healthy and impaired individuals during pedaling. Kautz and
Brown (1998) found that the degradation of pedaling performance measured in older adults with poststroke hemiparesis
was related to abnormalities in the execution of specific biomechanical functions, as evidenced by changes in EMG timing. In a study of forward pedaling at different cadences by
young healthy adults, Neptune et al. (1997) concluded that the
phasing of the functions remain unaltered, although a few
muscles under specific pedaling conditions may contribute to
more than one biomechanical function. These studies suggest,
therefore, that while the phasing of a biomechanical function
(e.g., leg extension) remains constant for a given pedaling task,
a muscles contributions to it may depend on the specific
pedaling condition. Nevertheless each muscle group, as defined
and studied here, can be associated with one primary biomechanical function. Thus coordination of biomechanical functions, which may serve as control primitives for pedaling (Ting
et al. 1999), can be revealed by studying functional musclegroup control of pedaling.
The objective of this study was to use computer simulations to
ascertain how adaptable the four muscle groups or, equivalently,
the biomechanical functions are to pedaling under a variety of
conditions. Pedaling at different cadences, with different workloads, and in different directions (forward and backward) was
studied because these conditions, when imposed on locomoting
animals, have led to the development of principles of locomotion
in animals (Buford and Smith 1990; Grillner 1981). Pedaling at a
constant crank angular velocity and at a steady cadence (i.e.,
FUNCTIONAL MUSCLE GROUPS USED IN PEDALING
517
smoothly) and with high metabolic energy efficiency, also was
studied to obtain insight into the role of the muscle groups and to
assess the ability of a simple controller to provide sufficient
richness for a range of pedaling goals. Finally, to assess how the
basic pattern could be adapted to a significantly altered loading
condition, pedaling with one leg was studied because this task is
similar kinematically but different biomechanically from a twolegged pedaling task (i.e., the contralateral nonpedaling leg no
longer provides crank propulsion when the pedaling leg is undergoing limb flexion).
This work is based on a PhD dissertation by C. C. Raasch
(1996).
METHODS
Musculoskeletal model and the four muscle groups
The dynamic musculoskeletal model was identical to the one used
to study coordination of muscles for maximum acceleration of the
crank from rest (Raasch et al. 1997). Briefly, it consists of two legs
rigidly connected by cranks to an inertial and frictional load emulating
a Monarch ergometer flywheel with a changeable frictional load. The
model includes a state-dependent load so that, like a normal ergometer, the crank can freewheel (decouple from the flywheel) when the
total cranking torque drops to approximately zero. When freewheeling
occurs, the inertia of the load is reduced ;500-fold (i.e., to that of the
crank and chain drive system only) and the frictional load to virtually
zero. During freewheeling, pedaling may continue although crank
rotation lags flywheel rotation. If enough propulsive force again is
created, the crank accelerates and impacts the inertia of the flywheel,
causing a sudden jerk during recoupling. Each leg of the model is
constrained to a parasagittal plane with hip center fixed, and feet
attached to the pedal to emulate fixation with cleats or clipless pedals.
The dynamic equations of motion of the crank and load, the limb
segments, and the muscles are of the general form
q 5 M 21 ~q!@R M z F M ~q, q , l M , a M ! 1 G~q! 1 V~q, q ! 1 D~q, q !#
(1)
a M 5 f~a M , u~t!!
a M 5
@u 2 a M # z @c 1 u 1 @c 2 c 2 # T #
@u 2 a M # z c 2
u $ aM
u , aM
(2)
where q is a vector of generalized coordinates (i.e., crank angle, qcrank;
right and left pedal angles); q,q are the angular velocity and acceleration vectors with, for example, qcrank (an element of q) being the
crank angular velocity; M(q) and RM are the system mass and moment
arm matrices, respectively; lM and aM are vectors of muscle fiber
length and activation, respectively; FM(q, q, lM, aM) is a vector of
muscle forces and includes the active and passive force-length and
velocity properties of muscle and accounts for compliance in tendon
and aponeurosis [note: lM is a vector of muscle fiber velocities and is
imbedded in FM(. . .) since lM 5 f(lM, q, q)]; G(q), V(q, q), and D(q,
q) are vectors of gravity, motion-dependent, and frictional terms,
respectively. Nine muscles (Fig. 1) provide the forces required to
move each leg. Excitation-contraction dynamics (Eq. 2), which describe the relation between neuromuscular excitation u(t) and muscle
activation a(t), include terms c1 and c2, which are related to muscle
21
activation/deactivation time constants, with c1 5 t21
act 2 tdeact and
c2 5 t21
.
Activation
and
deactivation
time
constants
were
50
and 66
deact
ms, respectively (Winters and Stark 1987).
The nine muscles (Fig. 1) of each leg were partitioned into four
muscle groups (Fig. 2A, black bars). The nominal ON/OFF phasing of
the groups were derived by averaging the ON (OFF) times of the
homologous muscle groups in the right and left legs of the simulated
FIG. 1. Nine muscles in the model (Raasch et al. 1997). Thirty-two
muscles from SIMM (MusculoGraphics , Evanston, IL) that contribute significantly to sagittal-plane motion were represented by 15 equivalent muscles,
with each modeled by Hill-type active and passive elements. These 15 muscles
were combined further into 9 muscle sets, with muscles in each set receiving
the same excitation signal. The 9 muscle sets (shown in figure) and the 15
muscles are: gluteus maximus and adductor magnus (GMAX); medial hamstrings and biceps femoris long head (HAM); rectus femoris (RF); three-part
vastus (VAS); gastrocnemius (GAS); soleus and a muscle representing other
uniarticular plantarflexors (SOL); tibialis anterior (TA); biceps femoris short
head (BFsh); iliacus and psoas (IL). The 9 muscle sets are the 9 muscles
referred to in the text. Inset: configuration of legs relative to pedal, crank, and
seat post.
first-cycle of maximum-speed start-up forward pedaling (Raasch et al.
1997). For submaximum speed pedaling, this corresponds to a simple
strategy of exciting muscles when they can contribute to crank propulsion. Such a strategy should be sufficient to achieve forward
pedaling but not necessarily optimal in any sense. The first cycle was
used because it was the closest to 60 rpm (the nominal pedaling
cadence). The four groups of each leg were assumed to alternate 180
out of phase with their contralateral counterparts. All muscles in a
group were excited at the same intensity level.
A study of the capability of such a four-group control was our
primary goal. Nevertheless unconstrained optimizations also were
run, with all nine muscles excited independently, as a check to see if
the groupings were producing unrealistic constraints on excitation
timing and excitation levels. Detailed performance figures for these
studies are not included; however, key similarities and differences are
noted in the following text.
Different cadences and workloads
Simulations of pedaling at different cadences (60 and 90 rpm) and
power outputs (120 and 180 W) in the forward direction were attempted by exciting the four groups with the nominal phasing pattern
(Fig. 2A). For any given cadence and power output, muscle groups
were excited at the same intensity level because such excitation of
groups is the strategy required in maximum-speed start-up pedaling
(i.e., excitation of a muscle is maximum during its burst) (Raasch et
al. 1997). Thus it was assumed that a reduction in excitation intensity
of the groups, each by the same amount, would achieve less-thanmaximum crank power output [crank power output 5 cadence (cycles/s) 3 workload (energy output per cycle)].
Different pedaling goals
A parameter optimization algorithm (Pandy et al. 1992) was used to
determine how the four-group control would need to be modified to
518
C. C. RAASCH AND F. E. ZAJAC
pedal more smoothly or efficiently. The algorithm found the phasing
and the level of excitation of each of the four groups that minimizes
either the amount of nonsmoothness or metabolic muscular energy
consumed. No constraint of alternation of one group with another was
imposed (however, see RESULTS). Muscle metabolic energy consumption EM was calculated from mechanical work and heat production as
follows (Schutte et al. 1993):
EM 5
E E
PM 5
@h ~l M , a M , l M , F M ! 2 F M l M #
(3)
J 1 5 1/T
J 2 5 1/T
uq crank 2 q ave u
~Nonsmoothness!
(4)
EM
~Average Metabolic Energy Rate!
(5)
where P is metabolic power and h is a Hill-based model of muscle
heat production, which includes formulations for maintenance, shortening, and lengthening heat rate. In general, the heat rate increases
with activation, muscle force, and fiber velocity (except for very slow
lengthening, where it decreases slightly). An initial interval of pedaling allowed for settling of transients. Only the last pedaling cycle was
subjected to the steady-state constraints of fixed time per cycle and
equal initial and final crank velocity. For each simulation, the deviaM
tion of crank angular velocity from the desired cadence and the
metabolic muscular energy consumed, each averaged over the last
cycle, were calculated
where T is the last-cycle period and qave equals the desired cadence
(60 or 90 rpm). Notice from Eqs. 3 and 5 that the metabolic energy
consumed, when averaged over the last cycle, is the average metabolic
energy rate in the last cycle. Similarly, Eq. 4 yields the average
absolute difference from desired speed over the last cycle. Thus a
coordination pattern producing smoother pedaling reduces J1, and a
pattern causing higher energy efficiency reduces J2. Other measures of
nonsmoothness also could be used, such as functions of jerk (derivative of acceleration) or crank angle residuals (Fig. 2C). However,
because crank velocity is part of the state vector, it proved a straightforward quantity to monitor continuously and integrate during optimizations.
The effectiveness of the four muscle groups to achieve forward
pedaling with one leg was explored. The four groups were phased
with the nominal pattern (Fig. 2A) and excited at the same intensity
level. Improvement in performance was explored by changing the
relative levels of excitation among the four groups without altering
their phasing. In all simulations of one-legged pedaling, the model
was modified. One leg and a pedal were removed, and the frictional
load was halved from the nominal. Thus the mechanical work performed by the leg over the crank cycle in one-legged pedaling was
identical to the work performed by the leg in two-legged pedaling.
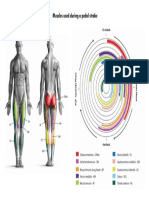
FIG. 2. Four muscle groups and their phasing (A), comparison of the
nominal pedaling coordination pattern with electromyographic (EMG) data
(B), and comparison of model generated and observed pedal and crank kinematics (C). A: pattern derived from a simulation of maximum-speed start-up
forward pedaling (Raasch et al. 1997) (see METHODS). Uniarticular hip extensors (GMAX) are coexcited with uniarticular knee extensors (VAS) (i.e., the
EXT group, or E group) and alternate with coexcitation of the uniarticular hip
flexors (IL) and the knee flexor (BFsh) (i.e., the FLEX group, or F group).
These groups form the EXT-FLEX pair. Other pair consists of the RF/TA
group [i.e., biarticular anterior thigh muscle (RF) and dorsiflexor muscles
(TA)], which alternates with the HAM/TS group [i.e., biarticular posterior
thigh muscles (HAM) and ankle plantarflexors (TS)]. TS includes both uniarticular plantarflexors (SOL) and gastrocnemius (GAS). Diagram shows phasing of the muscle groups with respect to the crank and its position relative to
the seatpost. Crank is vertical at TDC (top dead center) and BDC (bottom
dead center). Downstroke (leg extension) occurs as the crank proceeds from
TDC to BDC; upstroke (leg flexion) from BDC to TDC. B: phasing of the
groups (black straight bars) compared with EMG phasing (gray bars and lines,
mean 6 1 SD) (Neptune et al. 1997, by permission). Height of the bar shows
the excitation level of the group or muscle, with the peak EMG level normalized by the peak value from maximum voluntary contractions. Nominally, all
muscle groups in the simulation are excited at the same intensity level (27% of
maximum), with maximum equal to 1 (see scale, right). EMGs were measured
at a power output of 250 W; hence the higher level of VAS and SOL. EMG
levels for 120 W in Ericson (1986) (case with toe clips) are lower, particularly
VAS and GMAX. Averaging the magnitude for the 8 muscles yields a value
of 24% for 120 W (Ericson 1986), 31% for 250 W (Neptune et al. 1997). C:
comparison of simulation (black line) to experimental kinematic data (gray
regions, mean 6 1 SD) (Fregly 1993), measured with identical cadence, power
output, and inertial load. Similar data also are seen for 75 rpm in Fregly et al.
(1996). Top: crank angle residuals, defined as deviation from linearly increasing crank angle (constant velocity). Crank velocity is rarely if ever reported.
Crank residuals closely correspond to our nonsmoothness cost. Bottom: pedal
angles, with 0 defined as horizontal and negative as toe downward.
FUNCTIONAL MUSCLE GROUPS USED IN PEDALING
519
Backward pedaling
The feasibility of using the nominal forward-pedaling muscleexcitation pattern (Fig. 2A) to pedal backward was explored. That is,
the four muscle groups were phased (Fig. 6A) with respect to limb
extension and flexion and the transitions between extension and
flexion according to the nominal pattern (cf. Fig. 6A, left: phasing
diagram, with Fig. 2A). Muscle groups were excited at the same
intensity at a level compatible with the achievement of forward
pedaling (see RESULTS). Other attempts to pedal backward used excitation patterns where either the levels of excitation differed among the
groups or the phasing of the groups or muscles within a group differed
from the nominal pattern.
RESULTS
Nominal cadence and workload
The simulation was able to pedal forward at a steady 60rpm, 120-W power level with the excitation level of the fourgroup control set at 0.27 of maximum. The nonsmoothness cost
was 0.685 rpm and the metabolic cost 611 W. The oxygen
consumption rate for the nominal 60-rpm simulation, computed by assuming an aerobic metabolism of 20.1 kJ/L O2
O2 of 0.3 L
(Astrand and Rodahl 1977) and a baseline/resting V
O2/min (no-load pedaling) (Croisant and Boileau 1984), was
within 5% of measured oxygen uptake rate (Croisant and
Boileau 1984; Swain et al. 1987). The pedaling simulation was
very realistic, as judged by graphic display of the motion of the
body segments and crank relative to one another. The crank
angular velocity was maintained to within 1.2 rpm (1.8%) of
the desired crank angular velocity, so that cadence was constant to within 6 0.1%.
The crank and pedal kinematics were similar to those recorded from subjects (Fig. 2C, gray regions) (Fregly et al.
1996). The phasing pattern of the four groups corresponded
generally well to the EMG pattern recorded during pedaling
(Neptune et al. 1997) (Fig. 2B, gray bars). However, subtle
differences between simulation and experimental data exist
(see DISCUSSION). Nevertheless if muscles are allowed to be
excited independently instead of being constrained into four
groups, our musculoskeletal model can replicate the experimental data even better (Neptune and Hull 1998).
Different cadences and workloads
Continuous pedaling in the forward direction at the higher
cadence (90 rpm) and power output (180 W) was achieved with
the nominal phasing pattern (Fig. 2A). Pedaling at the faster
cadence or higher power output required, however, a higher
excitation intensity (0.349 and 0.365 of maximum, respectively) and cost more metabolic energy J2 (913 and 823 W).
The nonsmoothness cost increased for higher power (1.157
rpm) but decreased for higher cadence (0.538 rpm). Pedaling at
other cadence/workload combinations, though not thoroughly
studied, also were achieved with the nominal pattern.
Different pedaling goals
To maximize smoothness, the intensity of excitation of the
four muscle groups had to be changed much more than the
phasing of the excitations (Fig. 3A). The excitation levels of the
RF/TA and HAM/TS groups had to be increased substantially
(50 and 44%, respectively) and the excitation of the EXT group
FIG. 3. Optimal 4-group coordination pattern for maximizing smoothness
in crank angular velocity when pedaling forward. A: nominal (n) and optimal
(u) patterns, with bars indicating the excitation level and phasing of the groups.
B: simulated crank angular velocities (qcrank) for the nominal pattern (z z z), and
the optimal pattern ().
decreased (225%). During limb flexion, the excitation level of
the FLEX group had to be increased (36%). Nonsmoothness
cost was reduced from 0.685 to only 0.247 rpm. These changes
decreased the crank deceleration at the limb transitions and
reduced the peak-to-peak crank velocity variations (from 2 to
1 rpm; Fig. 3B, compare with z z z), but metabolic energy
consumption increased (from 611 to 648 W, 15%). Unconstrained optimization with all nine muscles excited independently produced very similar results. Excitation of some muscles in the RF/TA, HAM/TS, and FLEX groups had to be
increased (i.e., RF, TA, HAM, IL, BFsh) and excitation of
muscles in the EXT group decreased [i.e., VAS, GMAX]. Only
very small changes in phasing occurred.
To maximize energetic efficiency, excitation of the four muscle
groups also had to be changed much more than their phasing (Fig.
4A). Propulsion was provided almost exclusively by the EXT
group through an increase in its excitation level (185%), although
its burst duration was shorter. The FLEX group was not excited
anywhere in the cycle, and the excitation levels of the RF/TA and
HAM/TS groups decreased substantially (285 and 237%, respectively). The metabolic energy cost decreased from 611 to 518
W. Consistent with the decreased use of the RF/TA and HAM/TS
groups, peak-to-peak crank velocity variations increased (to 5
rpm) although freewheeling did not occur. With muscles excited
independently, a similar emphasis on the use of extensors was
seen, with VAS recruited first and then GMAX at higher workloads. Some excitation of SOL and TA still was required. However, HAM and RF were not excited at all and freewheeling
occurred.
Smooth one-legged pedaling in the forward direction was
found to be possible with the four groups, though the excitation
levels among the groups had to be quite different (Fig. 5A). The
EXT group was not excited, even during limb extension, but
520
C. C. RAASCH AND F. E. ZAJAC
a lower intensity of excitation of the groups, pedaling through
limb flexion was impossible (not shown).
Backward pedaling
FIG. 4. Optimal 4-group coordination pattern for minimizing metabolic
energy consumption when pedaling forwards. u, optimal pattern; n, nominal
pattern (see Fig. 3).
the FLEX and RF/TA groups had to be excited with more
intensity. The FLEX group, which produces three times less
energy than the EXT group in two-legged pedaling (Raasch et
al. 1997), was required in one-legged pedaling to generate
more power in the upstroke than the EXT group does in
downstroke because the weight of the leg decelerates the crank
in upstroke and accelerates the crank in downstroke. Because
the weight of the limb opposes the initial part of the flexionto-extension transition (Fig. 5A, diagram), the RF/TA group
also must contribute more energy than the HAM/TS group in
one-legged pedaling. With all four groups excited at the same
(high) intensity, pedaling with one leg was feasible but it was
very nonsmooth (nonsmoothness cost of 22 vs. 0.8 rpm for
unequal excitation) and freewheeling occurred (Fig. 5B). With
FIG. 5. One-legged forward pedaling. A: muscle groups excited at different
intensities but phased identically as in 2-legged pedaling (Fig. 2). Left: circular-bar width shows excitation level: HAM/TS group excited at 45%, somewhat higher than the nominal amount, FLEX and RF/TA groups maximally
excited, and EXT group not excited. Right: crank angular velocities (qcrank) for
1- and 2-legged pedaling. B: muscle groups in 1-legged pedaling excited as in
nominal 2-legged pedaling (i.e., phasing the same as in Fig. 2A and all groups
excited at 77% of maximum). Wide region of freewheeling (n at top; see text)
is associated with extreme variations in qcrank (compare with - - -). Note that
scales in A and B differ.
Continuous backward pedaling was found to be impossible
with the four groups excited at the same intensity and phasing
as in forward pedaling, where phasing is relative to limb
extension, flexion, and the extension/flexion transitions (Fig.
6A). The resulting freewheeling and reversals in crank direction at the limb transitions were due to the high deceleration of
the crank caused by ipsilateral HAM and contralateral RF
forces near 180, and ipsilateral RF and contralateral HAM
forces near 360. This retardation became evident by analyzing
FIG. 6. Backward pedaling with 2 legs. A, left: 4 muscle groups excited at
the same intensity and phased according to limb extension and flexion and their
transitions as in forward 2-legged pedaling (Fig. 2). Right: crank speed for
backward pedaling () and forward pedaling (- - -). Positive speed indicates
the crank is moving in the desired direction. Backward progressing crank ()
freewheels (n) and slows at the 1st transition (2180), and then cannot
progress backward through the 2nd (2360), which contrasts sharply with the
steady progression in forward pedaling (- - -). B, left: all muscles in the 4
muscle groups still phased according to limb extension and flexion and their
transitions, as in forward pedaling, except HAM and RF muscles are not
excited. Right: crank still freewheels at the transitions, resulting in highly
nonsmooth pedaling, but backward progression is possible. C, left: all muscles
phased as in forward pedaling except HAM and RF muscles are phase reversed
(i.e., excited at the opposite limb transition). All muscles excited at the same
equal intensity as in forward pedaling (i.e., 27%). Right: freewheeling is absent
and backward progression is smooth as in forward pedaling. Note the lesssensitive crank-speed scale in A.
FUNCTIONAL MUSCLE GROUPS USED IN PEDALING
the contributions of the HAM/TS group and the RF/TA group
to the two propulsive components essential to cranking the
limb through its transitions (i.e., through 180 and 360), which
are to accelerate the crank and to transfer energy from the limb
inertia to the crank (Raasch et al. 1997). Continuous backward
pedaling was, nevertheless, possible as long as HAM and RF
muscles were not excited anywhere in the crank cycle and the
other muscles were excited as in forward pedaling, although
freewheeling occurred at transitions (Fig. 6B). In this case, TS
and TA muscles were able to transfer to the crank the energy
delivered previously to the limb inertia by the EXT and FLEX
groups, respectively, as in forward pedaling (Raasch et al.
1997).
Very smooth backward pedaling was achievable with the
groups excited at the same intensity, however, if the RF/TAHAM/TS pair was subdivided into two pairs (RF-HAM pair
and TA-TS pair; Fig. 6C). Importantly, the phasing of the
HAMS and RF groups had to be reversed from that required in
forward pedaling, relative to the limb transitions, so they could
propel the crank through the limb transitions (cf. Fig. 6C with
Fig. 2A near 180 and 360). Decoupled from HAM and RF, the
TS and TA groups were able to transfer energy from limb
inertia to crank as in forward pedaling. With the RF and HAM
groups now able to provide crank propulsion at the limb
transitions rather than retardation, excitation of all six groups
could be at the same intensity.
521
in a given direction. For example, continuation of forward
cranking is impossible when the biarticular thigh and the ankle
muscles are combined with either the FLEX or the EXT group
(Fig. 7A, left). The leg oscillates forward/backward at the limb
extension/flexion transitions (Fig. 7A, right). Changing the
phasing of the groups of the one pair, or the grouping of
muscles, does not improve performance because the EXT and
FLEX muscles then decelerate the crank during their relaxation. Also the improperly phased biarticular thigh muscles and
ankle muscles are then unable to provide propulsion at the limb
transitions. Shortening the burst durations of the two groups of
the one pair alleviates the otherwise high deceleration during
limb extension and flexion, but freewheeling still occurs at the
limb transitions (Fig. 7B). For smooth pedaling, the biarticular
thigh and ankle muscles must be excited during the limb
transitions (Raasch et al. 1997). Of course, any strategy that
delivers sufficient energy to overcome the load and propel the
limb will suffice to turn the crank, although not necessarily
smoothly (e.g., the metabolically efficient use of EXT, SOL,
and TA in the unconstrained optimization). If the inertia of the
ergometer is very high (e.g., to emulate bicycle riding) (Fregly
et al. 1996), such a strategy might even produce acceptable
smoothness. Hard-coupling of the crank and flywheel would
simplify further control of smooth pedaling because the inertia
of the flywheel would assist the limb in coasting through
transitions.
The ability to control six muscle groups to execute six
DISCUSSION
Continuous, smooth, efficient pedaling in the forward direction at different cadences and workloads was found to be
possible with just four muscle groups of coexcited muscles.
The successful pattern is simpler than four independent controls because each group alternates with another (i.e., 2 pairs,
with each pair consisting of 1 muscle group alternating with
another). In fact, a control strategy that is constrained further to
excite all muscles at the same intensity also suffices well.
However, the excitation levels of the groups must be different
to accomplish different pedaling goals, such as to pedal
smoothly with one or two legs or to pedal with the highest
energetic efficiency.
Robust pedaling strategy: six muscle groups arranged into
three pairs
We believe that a strategy employing fewer than six muscle
groups is not biomechanically adaptable to be considered a
general pedaling strategy because pedaling forward and backward imposes different biomechanical requirements. Nevertheless, it is possible, at times, to turn the crank forward and
backward by controlling less than six groups (e.g., 4 groups,
Figs. 2A and 6B). In this case, smooth crank progression when
pedaling in reverse is impossible because no four-group excitation pattern that smoothly pedals, say, forward can accelerate
the crank backward at the limb transitions adequately (e.g., cf.
Fig. 6B with Fig. 2). In fact, if the frictional load is sufficiently
high, continuous progression in reverse is not possible at all,
and the demand for six muscle groups to be controlled becomes
even more evident.
Of course, pedaling with fewer than four groups is even less
adaptable, not only with respect to pedaling direction but even
FIG. 7. Forward pedaling with 2 groups. All muscles are grouped into 2
groups (E* group alternates with F* group). A, left: phasing of the 2 groups is
constrained so that at all times 1 of the 2 groups is excited. Right: notice that
freewheeling (n) is so severe that the crank enters into a limit cycle at the
transition between limb extension and flexion (i.e., the crank oscillates around
0 speed and does not advance). B, left: phasing of the 2 groups is constrained
so that at the switch points neither group is excited for awhile. Right: notice the
crank pedals continuously but still freewheels at the limb transitions. Note the
less-sensitive velocity scale in A.
522
C. C. RAASCH AND F. E. ZAJAC
biomechanical functions is required, therefore, to be able to
fulfill a variety of pedaling goals, including forward and backward pedaling. Four groups are insufficient because the phasing of the HAM and RF groups are directionally sensitive and
the phasing of the TS and TA groups are not. Thus HAM
control must be separated from TS control and RF from TA
control. Nevertheless HAM and RF still can be paired because
they still alternate with one another, and TS still can be paired
with TA. Similar uncoupling of muscles is seen in posture,
where the ankle strategy (HAM/TS alternating with RF/TA)
is in some situations replaced with a mixed strategy(Horak and
Nashner 1986). The coupling of HAM/TS and RF/TA in the
four-group control may be more accurately described as coincidence because the groups actually serve different biomechanical functions in pedaling.
The ankle muscles (TS-TA pair, particularly TS) must position the feet properly at the limb transitions to transfer energy
from the limb to the crank. This transfer redirects energy that
would otherwise go toward accelerating the limb segments,
prevents extreme ankle dorsiflexion and knee hyperextension
at the end of limb extension, and avoids excessive ankle
plantarflexion at the end of limb flexion (Raasch 1996; Raasch
et al. 1997). Indeed, other backward-pedaling simulations by
us with TS-TA switched in phase do indeed hyperextend the
knee. Thus the phasing of TS should be near the end of limb
extension (i.e., following/overlapping activation of the EXT
group), and TA near the end of flexion, irrespective of pedaling
direction (Figs. 2A and 6C). The TS-TA pair therefore is
associated closely with the EXT-FLEX pair with a phase delay
between the pairs to properly configure the limb segments for
energy transfer (cf. alternative strategies, see following text).
In contrast, HAM and RF act primarily to accelerate the
crank when the leg is moving posteriorly (HAM) or anteriorly
(RF), also regardless of pedaling direction. Their phasing,
however, reverses with pedaling direction (Figs. 2A and 6C).
Other simulations by us also support the decoupling of
HAM-RF from TS-TA and the different biomechanical functions executed by these two pairs. For example, with the two
pairs separated, maximum smoothness is obtained by exciting
HAM-RF more than TS-TA because more crank power is
desired at the transitions and HAM and RF can produce such
power. On the other hand, maximum metabolic efficiency is
obtained by exciting TS more because the ankle must be
stabilized to ensure more complete transfer of the EXT-produced energy to the crank.
A strict association between each of the six functional muscle groups and a specific biomechanical function, as emphasized earlier for clarity, however, should be viewed cautiously
because recent studies suggest that a muscle can contribute to
more than one biomechanical function in pedaling (Neptune et
al. 1997; Ting et al. 1999). For example, a backward-pedaling
experimental study showed that the transition muscles HAM
and RF change their phasing, as compared with forward pedaling (Ting et al. 1999). However, the change in RF phasing
was much less than 180, probably because RF contributes to
limb extension as well as to the flexion-to-extension transition
function. Nevertheless all these studies agree that six biomechanical functions must be performed to pedal smoothly. The
association of a functional muscle group with a biomechanical
function, initially proposed by Raasch et al. (1997), thus provides a foundation for interpreting pedaling EMG patterns.
Alternative ankle-control strategies
Analysis of the differences between simulated pedaling with
the four muscle groups and the kinematics and EMGs recorded
from pedaling subjects (Fig. 2B) suggests that a variety of
ankle strategies are possible. EMG amplitudes are unequal
from one another, probably due to their dependence on the
specific pedaling task (e.g., pedaling both smoothly and efficiently; cf. Figs. 3 and 4). The earlier onset of TA and SOL in
pedaling subjects is probably due to subjects employing a
different ankle strategy than assumed in the simulation. Simulations show that some latitude is possible in controlling the
ankle (i.e., TA and SOL) as long as the essential biomechanical
requirement of transfer of energy from the hip and knee extensors to the crank is accomplished. SOL and TA may be
excited earlier because of their participation in the execution of
the EXT and FLEX biomechanical functions (i.e., extension/
flexion of the limb). In this case, SOL and TA still stiffen the
ankle to facilitate energy transfer from the limb to the crank,
but the stiffening is earlier. Consequently less kinetic energy is
stored temporarily in the limb segments. Pedal kinematics then
more closely match the subjects (e.g., peaks in pedal angles
are reduced) and ankle motion is reduced. This explanation is
compatible with the suggested secondary contribution of TA to
flex the limb in pedaling (Ting et al. 1999) and the suggested
contribution of SOL to extend the limb as well as plantarflex
the foot (Neptune et al. 1997). Another feasible ankle strategy
is to maintain the foot near-horizontal throughout the cycle (a
strategy more comfortable for many subjects). In this case,
SOL and TA must be excited slightly through much of downor up-stroke, even though this does not contribute significantly
to crank acceleration (and hence is not selected by an optimization that does not consider comfort). Still other strategies are
possible; for example, locking the ankle in a plantarflexed
position for much of the cycle with prolonged TS excitation
(Raasch 1996). Such a strategy effectively removes the ankle
degree of freedom and prevents knee hyperextension. However, this ankle strategy requires more metabolic energy than
the default strategy, and the resulting kinematics may be undesirable for subjects.
Phase control of motor output
Timing of the excitation of the muscle groups within the
pedaling cycle could be regulated by afferent feedback from
the limbs as in locomoting animals (Pearson 1993; Rossignol et
al. 1988). An estimate of the position of the feet relative to the
hips through neural processing of afferent information could be
the trigger signal for limb extension and flexion, as in animal
locomotion (Andersson and Grillner 1983; Grillner and Rossignol 1978). Phasing of the TS-TA pair could be time delayed
from EXT-FLEX phasing to assure appropriate transfer of
energy from the limb inertia to the crank. Although a continuous estimate of feet location also could be used to precisely
time (i.e., phase-control) the excitation of all six groups within
the cycle, motion-dependent cues (e.g., anterior/posterior motion of the ankles) also may participate. For example, if RF was
to be excited when the ankle is moving anteriorly, and HAM
when moving posteriorly, then the HAM-RF pair would be
phased properly in both forward and backward pedaling. If the
phasing of the biarticular thigh muscles is set primarily by
FUNCTIONAL MUSCLE GROUPS USED IN PEDALING
motion-dependent feedback, similar to the mutable synergies postulated by Smith (1987) in the restructuring of the cat
locomotor pattern, simple rule-based logic networks acting on
sensory information might be sufficient to implement the required phase control (Cruse et al. 1995a,b). Such control of
locomotion is being employed in the development of Functional Electrical Stimulation controllers to restore gait in paraplegics (Popovic 1991, 1993).
Gain control of motor output
Pedaling at a faster cadence or a higher workload can be
achieved by increasing the excitation of the muscle groups
equally. This regulation of cadence and power output could be
accomplished by a common gain control of the excitations of
the muscle groups. Common gain control could be effected by
neural control and neuromodulator concepts derived from animal locomotion studies (Grillner 1981, 1988; Pearson 1993;
Rossignol et al. 1988). For example, a descending tonic drive
from supraspinal structures onto pattern generator elements
could set the common scaling factor for excitation of the
muscle groups compatible with the desired limb power output
(equivalent to a certain cadence with a specific energy output
from the limb per cycle), similar to the setting achieved by
descending pathways from brain stem locomotor regions
(Grillner 1988; Shik et al. 1966).
Regulation of the intensity of motor output could be accomplished by afferent feedback. Neural processing of load-sensing feedback from the limb in pedaling (Raasch 1996) or
walking (Duysens and Loeb 1980; Duysens and Pearson 1976,
1980; Pearson et al. 1992), should be especially effective
(Raasch et al. 1997).
Relative excitation levels of the muscle groups
Some pedaling tasks require the excitation levels of the
muscle groups to be different, such as to pedal with one leg or
to pedal with ultrasmoothness or the highest energetic efficiency. Processing of afferent feedback to ensure that the
specific goals of the pedaling task are fulfilled could be attained
through high-level control of parameters utilized by a lowerlevel controller (Loeb et al. 1990; Prochazka 1989). In this
scheme, a specific pedaling task would be accommodated by
the setting of parameters, or schedules (Prochazka 1989),
which may be part of the initialization of the pedaling motor
program. This could allow for a more complex recruitment
scheme for muscles within a group, as conditions require, as
seen in the selective activation of individual extensor muscles
with increased load when muscles were allowed to be excited
independently (Raasch 1996). After initial parameter selection
by the high-level controller, pedaling could proceed by activating the low-level controller. Feedback would be used by the
high-level controller only to check periodically (e.g., once per
half-cycle) the appropriateness of the parameter set (e.g., the
phasing and excitation-level parameters of each muscle group).
In conjunction with the parameter set, feedback would be
employed by the low-level controller to assure continuous task
execution in the presence of reasonable environmental (and
internal) uncertainties; e.g., muscle and joint receptors could be
used to modulate TS-TA to prevent excessive ankle motion and
fine-tune the control of the pedal trajectory.
523
Locomotor strategies in animals and humans may be similar
The locomotor features outlined in the preceding text based
on pedaling, with muscles organized into alternating functional
groups and phase- and amplitude-controlled (perhaps through
high- and low-level neural controllers), have features common
to animal locomotion. First, spinal neuronal elements exciting
motor pools alternately through (perhaps) other interneurons
have long been the basis of locomotor concepts (Grillner
1975), and now their cellular, molecular, and pharmacological
properties are being elucidated in even vertebrates (Grillner
1988; Rossignol and Dubuc 1994). Second, phase-control of
these elements through neural processing of peripheral proprioceptive information is critical to pattern generator execution
of locomotion (Pearson 1993; Rossignol et al. 1988). For
example, load-bearing feedback in animal locomotion is important to flexor initiation and excitation of the extensors
(Pearson and Duysens 1976), and to the relative excitation of
flexors to extensors (Smith and Carlson-Kuhta 1995). In pedaling, because cranking force (and the reaction force felt on the
foot) is primarily a function of the contributing muscle forces
(Kautz and Hull 1993), force feedback could be used to balance the excitation between the EXT and FLEX groups (e.g.,
as required to achieve 1-legged pedaling, Fig. 5). Also, the
position of the limb relative to the hip is critical to phase
selection in cat locomotion (Andersson and Grillner 1983;
Grillner and Rossignol 1978) and could be in human pedaling
as well (see preceding text).
The features used by humans to provide propulsion to walk
and pedal also may be similar (see INTRODUCTION). For example,
the switch in phasing of the thigh bifunctional muscles (e.g.,
HAM and RF) with walking direction (Thorstensson 1986) and
to anterior/posterior postural balance disturbances (Nashner
and McCollum 1985) is similar to the switch occurring in
HAM and RF in pedaling. We believe, therefore, that humans
pedal by using some of the sensorimotor control mechanisms
employed to walk (and stand).
Conclusion
When muscles are assigned into groups, our simulations
show that six groups are the minimal set to pedal in different
directions under a variety of conditions. Phasing of four groups
(uniarticular hip and knee extensors in alternation with hip and
knee flexors; ankle plantarflexors in alternation with dorsiflexors) do not change with direction. The biarticular thigh muscles
(anterior alternating with posterior muscles) do change with
direction (e.g., rectus femoris is excited during the transition
from flexion to extension in forward pedaling and during the
opposite transition from extension to flexion in backward pedaling). Different cadences and limb power output can be
achieved by controlling the overall excitatory drive to the
pattern generating elements, and different pedaling goals (e.g.,
1- versus 2-legged pedaling) can be achieved by controlling the
relative excitation levels among the muscle groups. We suggest
that these six muscle groups form a basic strategy for pedaling
propulsion and also may be a subset of the elements of a
general strategy for human locomotor propulsion. We believe
that pedaling affords a rich milieu for performing not only
detailed theoretical studies, such as those here, but also for
conducting controlled locomotor experiments in healthy and
524
C. C. RAASCH AND F. E. ZAJAC
neurologically impaired subjects (Brooke et al. 1987, 1992;
Brown and Kautz 1998; Brown and Kukulka 1993; Brown et
al. 1996, 1997; Kautz and Brown 1998; Kautz and Hull 1993;
Pierson-Carey et al. 1997; Ting et al. 1998, 1999), which
together may reveal motor control mechanisms of human locomotion.
We thank D. Brown and L. Ting for very helpful comments on an earlier
version of the manuscript, S. Kautz for insightful suggestions for improving
the different drafts of the manuscript, and D. Denney for assistance with figure
preparation. Special thanks to R. Neptune for providing us with experimental
pedaling data (Neptune et al. 1997).
The work was supported by National Institute of Neurological Disorders and
Stroke Grant NS-17662 and the Rehabilitation R&D Service of the Department
of Veterans Affairs (VA).
Present address of C. C. Raasch: 1108 W. Oraibi Dr., Phoenix, AZ 850274651.
Address for reprint requests: F. E. Zajac, Rehab R&D Center (153) (Bldg.
51), VA Palo Alto Health Care System, 3801 Miranda Ave., Palo Alto, CA,
94304-1200.
Received 26 May 1998; accepted in final form 22 March 1999.
REFERENCES
ANDERSSON, O. AND GRILLNER, S. Peripheral control of the cats step cycle. II.
Entrainment of the central pattern generators for locomotion by sinusoidal
hip movements during fictive locomotion. Acta Physiol. Scand. 118:
229 239, 1983.
ASTRAND, P.-O. AND RODAHL, K. Textbook of Work Physiology: Physiological
Bases of Exercise. New York: McGraw-Hill, 1977.
BARBEAU, H. AND ROSSIGNOL, S. Enhancement of locomotor recovery following spinal cord injury. Curr. Opin. Neurol. 7: 517524, 1994.
BERNSTEIN, N. The Coordination and Regulation of Movements. New York:
Pergamon, 1967.
BRADLEY, N. AND SMITH, J. Neuromuscular patterns of stereotypic hindlimb
behaviors in the first two postnatal months. I. Stepping in normal kittens.
Dev. Brain Res. 38: 3752, 1988.
BROOKE, J. D., BOYCE, D. E., MCILROY, W. E., ROBINSON, T., AND WAGAR, D.
Modulation of phase relations of myoelectric activity and propulsive force in
human bipedality at different speeds and resistances. Electromyogr. Clin.
Neurophysiol. 27: 419 425, 1987.
BROOKE, J., MCILROY, W., AND COLLINS, D. Movement features and H-reflex
modulation. I. Pedalling versus matched controls. Brain Res. 582: 78 84,
1992.
BROWN, D. A. AND KAUTZ, S. A. Increased workload enhances force output
during pedaling excercise in persons with poststroke hemiplegia. Stroke 29:
598 606, 1998.
BROWN, D. A., KAUTZ, S. A., AND DAIRAGHI, C. A. Muscle activity patterns
altered during pedaling at different body orientations. J. Biomech. 29:
1349 1356, 1996.
BROWN, D. A., KAUTZ, S. A., AND DAIRAGHI, C. A. Muscle activity adapts to
anti-gravity posture during pedalling in persons with post-stroke hemiplegia.
Brain 120: 825 837, 1997.
BROWN, D. A. AND KUKULKA, C. G. Human flexor reflex modulation during
cycling. J. Neurophysiol. 69: 12121224, 1993.
BUFORD, J. A. AND SMITH, J. L. Adaptive control for backward quadrupedal
walking. II. Hindlimb muscle synergies. J. Neurophysiol. 64: 756 766,
1990.
CALANCIE, B., NEEDHAM-SHROPSHIRE, B., JACOBS, P., WILLER, K., ZYCH, G.,
AND GREEN, B. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain 117:
11431159, 1994.
COAST, J. AND WELCH, H. Linear increase in optimal pedal rate with increased
power output in cycle ergometry. Eur. J. Appl. Physiol. 53: 339 342, 1985.
CROISANT, P. AND BOILEAU, R. Effect of pedal rate, brake load and power on
metabolic responses to bicycle ergometer work. Ergonomics 27: 691700,
1984.
CRUSE, H., BARTLING, C., CYMBALYUK, G., DEAN, J., AND DREIFERT, M. A
modular artificial neural network for controlling a six-legged walking system. Biol. Cybern. 72: 421 430, 1995a.
CRUSE, H., BRUNN, D. E., BARTLING, C., DEAN, J., DREIFERT, M., KINDERMANN,
T., AND SCHMITZ, J. Walking: a complex behavior controlled by simple
networks. Adapt. Behav. 3: 385 418, 1995b.
DIETZ, V., COLOMBO, G., JENSEN, L., AND BAUMGARTNER, L. Locomotor
capacity of spinal cord in paraplegic patients. Ann. Neurol. 37: 574 582,
1995.
DIETZ, V., ZIJLSTRA, W., AND DUYSENS, J. Human neuronal interlimb coordination during split-belt locomotion. Exp. Brain Res. 101: 513520, 1994.
DUYSENS, J. AND LOEB, G. E. Modulation of ipsi- and contralateral reflex
responses in unrestrained walking cats. J. Neurophysiol. 44: 1024 1037,
1980.
DUYSENS, J. AND PEARSON, K. G. The role of cutaneous afferents from the
distal hindlimb in the regulation of the step cycle of thalamic cats. Exp.
Brain Res. 24: 245255, 1976.
DUYSENS, J. D. AND PEARSON, K. G. Inhibition of flexor burst generation by
loading ankle extensor muscles in walking cats. Brain Res. 187: 321332,
1980.
ENGBERG, I. AND LUNDBERG, A. An electromyographic analysis of muscular
activity in the hindlimb of the cat during unrestrained locomotion. Acta
Physiol. Scand. 75: 614 630, 1969.
ERICSON, M. O. On the biomechanics of cycling. A study of joint and muscle
load during exercise on the bicycle ergometer. Scand. J. Rehab. Med., Suppl.
16: 1 43, 1986.
FORSSBERG, H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J. Neurophysiol. 42: 936 953, 1979.
FORSSBERG, H. Ontogeny of human locomotor control. I. Infant stepping,
supported locomotion and transition to independent locomotion. Exp. Brain
Res. 57: 480 493, 1985.
FORSSBERG, H., GRILLNER, S., AND HALBERTSMA, J. The locomotion of the low
spinal cat. I. Coordination within a hindlimb. Acta Physiol. Scand. 108:
269 281, 1980.
FREGLY, B. J. The Significance of Crank Load Dynamics to Steady-State
Pedaling Biomechanics: An Experimental and Computer Modeling Study
(PhD thesis). Stanford, CA: Stanford University, 1993.
FREGLY, B. J., ZAJAC, F. E., AND DAIRAGHI, C. A. Crank inertial load has little
effect on steady-state pedaling coordination. J. Biomech. 29: 1559 1567,
1996.
GISZTER, S. F., MUSSA-IVALDI, F. A., AND BIZZI, E. Convergent force fields
organized in the frogs spinal cord. J. Neurosci. 13: 467 491, 1993.
GREENE, P. Why is it easy to control your arms? J. Mot. Behav. 14: 260 286,
1982.
GRILLNER, S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol. Rev. 55: 247304, 1975.
GRILLNER, S. Control of locomotion in bipeds, tetrapods, and fish. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD:
Am. Physiol. Soc., 1981, sect. 1, vol. II, p. 1179 1236.
GRILLNER, S. Control of locomotion in vertebrates: spinal and supraspinal
mechanisms. Adv. Neurol. 47: 425 453, 1988.
GRILLNER, S. AND ROSSIGNOL, S. On the initiation of the swing phase of
locomotion in chronic spinal cats. Brain Res. 146: 269 277, 1978.
GRILLNER, S. AND ZANGGER, P. On the central generation of locomotion in the
low spinal cat. Exp. Brain Res. 34: 241261, 1979.
HORAK, F. AND NASHNER, L. Central programming of postural movements:
adaptation to altered support-surface configurations. J. Neurophysiol. 55:
1369 1381, 1986.
JACOBS, R. AND MACPHERSON, J. M. Two functional muscle groupings during
postural equilibrium tasks in standing cats. J. Neurophysiol. 76: 24022411,
1996.
KAUTZ, S. A. AND BROWN, D. A. Relationships between timing of muscle
excitation and impaired motor performance during cyclical lower extremity
movement in post-stroke hemiplegia. Brain 121: 515526, 1998.
KAUTZ, S. A. AND HULL, M. L. A theoretical basis for interpreting the force
applied to the pedal in cycling. J. Biomech. 26: 155165, 1993.
LOEB, G. E., LEVINE, W. S., AND HE, J. Understanding sensorimotor feedback
through optimal control. Cold Spring Harbor Symp. Quant. Biol. : 791 803,
1990.
MACPHERSON, J. M., RUSHMER, D. S., AND DUNBAR, D. C. Postural responses
in the cat to unexpected rotations of the supporting surface evidence for a
centrally generated synergic organization. Exp. Brain Res. 62: 152160,
1986.
MCCOLLUM, G. Reciprocal inhibition, synergies, and movements. J. Theor.
Biol. 165: 291311, 1993.
MUSSA-IVALDI, F. A., GISZTER, S. F., AND BIZZI, E. Linear combinations of
primitives in vertebrate motor control. Proc. Natl. Acad. Sci. USA 91:
7534 7538, 1994.
FUNCTIONAL MUSCLE GROUPS USED IN PEDALING
NASHNER, L. M. AND MCCOLLUM, G. The organization of human postural
movements: a formal basis and experimental synthesis. Behav. Brain Sci. 8:
135172, 1985.
NEPTUNE, R. R. AND HULL, M. L. Evaluation of performance criteria for
simulation of submaximal steady-state cycling using a forward dynamic
model. J. Biomech. Eng. 120: 334 341, 1999.
NEPTUNE, R. R., KAUTZ, S. A., AND HULL, M. L. The effect of pedaling rate on
coordination in cycling. J. Biomech. 30: 10511058, 1997.
PANDY, M. G., ANDERSON, F. C., AND HULL, D. G. A parameter optimization
approach for the optimal control of large-scale musculoskeletal systems.
J. Biomech. Eng. 114: 450 460, 1992.
PEARSON, K. G. Common principles of motor control in vertebrates and
invertebrates. Annu. Rev. Neurosci. 16: 265297, 1993.
PEARSON, K. AND DUYSENS, J. Function of segmental reflexes in the control of
stepping in cockroaches and cats. In: Neural Control of Locomotion, edited
by R. Herman, S. Grillner, P. Stein, and D. Stuart. New York: Plenum, 1976,
vol. 18, p. 519 538.
PEARSON, K. G., RAMIREZ, J. M., AND JIANG, W. Entrainment of the locomotor
rhythmn by group Ib afferents from ankle extensor muscles in spinal cats.
Exp. Brain Res. 90: 557566, 1992.
PIERSON-CAREY, C. D., BROWN, D. A., AND DAIRAGHI, C. A. Changes in
resultant pedal reaction forces due to ankle immobilization during pedaling.
J. Appl. Biomech. 13: 113, 1997.
POPOVIC, D. B. Strategies for functional electrical stimulation: implications for
control. In: Adaptability of Human Gait, edited by A. E. Patla. Amsterdam:
Elsevier, 1991, p. 413 438.
POPOVIC, D. Finite state model of locomotion for functional electrical stimulation systems. Prog. Brain Res. 97: 397 407, 1993.
PROCHAZKA, A. Sensorimotor gain control: a basic strategy of motor systems?
Prog. Neurobiol. 33: 281307, 1989.
RAASCH, C. C. Coordination of Pedaling: Functional Muscle Groups and Locomotor Strategies (PhD thesis). Stanford, CA: Stanford University, 1996.
RAASCH, C. C., ZAJAC, F. E., MA, B., AND LEVINE, W. S. Muscle coordination
of maximum-speed pedaling. J. Biomech. 30: 595 602, 1997.
RASMUSSEN, K., CHAN, A. K., AND GOSLOW, G.E.J. The cat step cycle:
electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J. Morphol. 155: 253270, 1978.
ROSSIGNOL, S. Neural control of stereotypic limb movements. In: Handbook of
Physiology. Exercise: Regulation and Integration of Multiple Systems. New
York: Oxford, 1996, sect. 12, p. 173216.
ROSSIGNOL, S. AND BARBEAU, H. New approaches to locomotor rehabilitation
in spinal cord injury. Ann. Neurol. 37: 555556, 1995.
ROSSIGNOL, S., CHAU, C., BRUSTEIN, E., BELANGER, M., BARBEAU, H., AND
DREW, T. Locomotor capacities after complete and partial lesions of the
spinal cord. Acta Neurobiol. Exp. 56: 449 463, 1996.
525
ROSSIGNOL, S. AND DUBUC, R. Spinal pattern generation. Curr. Opin. Neurobiol. 4: 894 902, 1994.
ROSSIGNOL, S., LUND, J. P., AND DREW, T. The role of sensory inputs in
regulating patterns of rhythmical movements in higher vertebrates. In:
Neural Control of Rhythmic Movements in Vertebrates, edited by A. H.
Cohen, S. Rossignol, and S. Grillner. New York: Wiley, 1988, p. 201297.
SCHUTTE, L. M., RODGERS, M. M., ZAJAC, F. E., AND GLASER, R. M. Improving
the efficacy of electrical stimulation-induced leg cycle ergometry: an analysis based on a dynamic musculoskeletal model. IEEE Trans. Rehab. Eng.
1: 109 125, 1993.
SHIK, M. L. AND ORLOVSKY, G. N. Neurophysiology of locomotor automatism.
Physiol. Rev. 56: 465501, 1976.
SHIK, M. L., SEVERIN, F. V., AND ORLOVSKY, G. N. Control of walking and
running by means of electrical stimulation of the mid-brain. Biofizika 11:
659 666, 1966.
SMITH, J. L. Hindlimb locomotion of the spinal cat: synergistic patterns, limb
dynamics and novel blends. In: Neurobiology of Vertebrate Locomotion,
edited by S. Grillner, P.S.G. Stein, D. G. Stuart, H. Forssberg, and R. M.
Herman. London: MacMillan, 1987, p. 185200.
SMITH, J. L. AND CARLSON-KUHTA, P. Unexpected motor patterns for hindlimb
muscles during slope walking in the cat. J. Neurophysiol. 74: 22112215,
1995.
SWAIN, D., COAST, J., CLIFFORD, P., MILLIKEN, M., AND STRAY-GUNDERSEN, J.
Influence of body size on oxygen consumption during bicycling. J. Appl.
Physiol. 62: 668 672, 1987.
THORSTENSSON, A. How is the normal locomotor program modified to produce
backward walking? Exp. Brain Res. 61: 664 668, 1986.
TING, L. H., KAUTZ, S. A., BROWN, D. A., AND ZAJAC, F. E. Phase reversal of
biomechanical functions and muscle activity in backward pedaling. J. Neurophysiol. 81: 544 551, 1999.
TING, L. H., RAASCH, C. C., BROWN, D. A., KAUTZ, S. A., AND ZAJAC, F. E.
Sensorimotor state of the contralateral leg affects ipsilateral muscle coordination of pedaling. J. Neurophysiol. 80: 13411351, 1998.
WINTER, D. Biomechanical motor patterns in normal walking. J. Mot. Behav.
15: 302330, 1983.
WINTER, D. A., PLUCK, N., AND YANG, J. F. Backward walking: a simple
reversal of forward walking? J. Mot. Behav. 21: 291305, 1989.
WINTERS, J. M. AND STARK, L. Muscle models: what is gained and what is lost
by varying model complexity. Biol. Cybern. 55: 403 420, 1987.
YANG, J. AND STEIN, R. Phase-dependent reflex reversal in human leg muscles
during walking. J. Neurophysiol. 63: 1109 1117, 1990.
ZAJAC, F. E. AND GORDON, M. E. Determining muscles force and action in
multi-articular movement. Exerc. Sport Sci. Rev. 17: 187230, 1989.
Vous aimerez peut-être aussi
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Effect of Wheel Diameter On Vertical and Horizontal Mountain Bike PositionDocument1 pageThe Effect of Wheel Diameter On Vertical and Horizontal Mountain Bike PositionCristianLopezPas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Power Pedal Muscles BlogReadyDocument1 pagePower Pedal Muscles BlogReadyCristianLopezPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Ayudas Ergogénicas y Suplementos en DeportesDocument21 pagesAyudas Ergogénicas y Suplementos en DeportesCristianLopezPas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Heart Rate Variability During High-Intensity ExerciseDocument13 pagesHeart Rate Variability During High-Intensity ExerciseCristianLopezPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (894)
- Perine y PartoDocument13 pagesPerine y PartoCristianLopezPas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- 41 - 2WCSCDocument1 page41 - 2WCSCCristianLopezPas encore d'évaluation
- Comparison of Power Output Demands For A Top-10 Ranking Between Tour de France and Vuelta A EspañaDocument1 pageComparison of Power Output Demands For A Top-10 Ranking Between Tour de France and Vuelta A EspañaCristianLopezPas encore d'évaluation
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- 44 - 2WCSCDocument1 page44 - 2WCSCCristianLopezPas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Comparison of Power Output Demands For A Top-10 Ranking Between Tour de France and Vuelta A EspañaDocument1 pageComparison of Power Output Demands For A Top-10 Ranking Between Tour de France and Vuelta A EspañaCristianLopezPas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- 35 - 2WCSCDocument1 page35 - 2WCSCCristianLopezPas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Effect of Wheel Diameter On Vertical and Horizontal Mountain Bike PositionDocument1 pageThe Effect of Wheel Diameter On Vertical and Horizontal Mountain Bike PositionCristianLopezPas encore d'évaluation
- 41 - 2WCSCDocument1 page41 - 2WCSCCristianLopezPas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Effects of A Seven Day Period of High-Intensity Training On Performance and Physiology of Competitive CyclistsDocument1 pageEffects of A Seven Day Period of High-Intensity Training On Performance and Physiology of Competitive CyclistsCristianLopezPas encore d'évaluation
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Comparing Time-Trial and Time To Exhaustion Performance: Book of AbstractsDocument1 pageComparing Time-Trial and Time To Exhaustion Performance: Book of AbstractsCristianLopezPas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Effect of Environmental Temperature On Pacing During A Simulated 16 KM Cycling Time TrialDocument1 pageEffect of Environmental Temperature On Pacing During A Simulated 16 KM Cycling Time TrialCristianLopezPas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Comparing Time-Trial and Time To Exhaustion Performance: Book of AbstractsDocument1 pageComparing Time-Trial and Time To Exhaustion Performance: Book of AbstractsCristianLopezPas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- 36-37 - 2WCSCDocument2 pages36-37 - 2WCSCCristianLopezPas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- 25 - 2WCSCDocument1 page25 - 2WCSCCristianLopezPas encore d'évaluation
- Do 3-Min All-Out Test Parameters Accurately Predict Competitive Cyclist Performance in The Severe Intensity Domain?Document1 pageDo 3-Min All-Out Test Parameters Accurately Predict Competitive Cyclist Performance in The Severe Intensity Domain?CristianLopezPas encore d'évaluation
- Relation Between Lactic Acid Steady-State and Muscle Oxygenation in Elite CyclistsDocument3 pagesRelation Between Lactic Acid Steady-State and Muscle Oxygenation in Elite CyclistsCristianLopezPas encore d'évaluation
- 32 - Congress of Science and CyclingDocument1 page32 - Congress of Science and CyclingCristianLopezPas encore d'évaluation
- Potentiation of Sprint Cycling Performance: The Effects of A High-Inertia Ergometer Warm-UpDocument1 pagePotentiation of Sprint Cycling Performance: The Effects of A High-Inertia Ergometer Warm-UpCristianLopezPas encore d'évaluation
- Multisensor Monitoring Cycle Ergometer: Book of AbstractsDocument1 pageMultisensor Monitoring Cycle Ergometer: Book of AbstractsCristianLopezPas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- 28 - 2WCSCDocument1 page28 - 2WCSCCristianLopezPas encore d'évaluation
- 26 - 2WCSCDocument1 page26 - 2WCSCCristianLopezPas encore d'évaluation
- Modelling of Critical Power From Road DataDocument2 pagesModelling of Critical Power From Road DataCristianLopezPas encore d'évaluation
- Multisensor Monitoring Cycle Ergometer: Book of AbstractsDocument1 pageMultisensor Monitoring Cycle Ergometer: Book of AbstractsCristianLopezPas encore d'évaluation
- Differences in Static and Dynamic Bike Fit With 3d Motion CaptureDocument1 pageDifferences in Static and Dynamic Bike Fit With 3d Motion CaptureCristianLopezPas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- 20 - 2WCSCDocument1 page20 - 2WCSCCristianLopezPas encore d'évaluation
- The Relationship Between Vertical Leg Stiffness and Gross Mechanical Efficiency in CyclistsDocument1 pageThe Relationship Between Vertical Leg Stiffness and Gross Mechanical Efficiency in CyclistsCristianLopezPas encore d'évaluation
- Reservoir Characterization 3 LoggingDocument47 pagesReservoir Characterization 3 LoggingMohamed AbdallahiPas encore d'évaluation
- Critical Thinking Portfolio AssignmentDocument8 pagesCritical Thinking Portfolio Assignmentapi-334295481Pas encore d'évaluation
- Journal of Statistical Planning and Inference: Akanksha S. KashikarDocument12 pagesJournal of Statistical Planning and Inference: Akanksha S. KashikarAkanksha KashikarPas encore d'évaluation
- Controversial Aquatic HarvestingDocument4 pagesControversial Aquatic HarvestingValentina RuidiasPas encore d'évaluation
- Inami, Problem Other MindsDocument19 pagesInami, Problem Other MindsfortyrrPas encore d'évaluation
- Digital Citizenship RubricDocument1 pageDigital Citizenship Rubricapi-300964436Pas encore d'évaluation
- Wave Hydro Dynamics Prof. V. Sundar Department of Ocean Engineering Indian Institute of Technology, MadrasDocument32 pagesWave Hydro Dynamics Prof. V. Sundar Department of Ocean Engineering Indian Institute of Technology, MadrasMuralidhar YarakalaPas encore d'évaluation
- Eps-07 Eng PDFDocument54 pagesEps-07 Eng PDFPrashant PatilPas encore d'évaluation
- I-K Bus Codes v6Document41 pagesI-K Bus Codes v6Dobrescu CristianPas encore d'évaluation
- 7949 37085 3 PBDocument11 pages7949 37085 3 PBAman ChaudharyPas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Compiled XiiDocument34 pagesCompiled XiiMridul VermaPas encore d'évaluation
- Abdul Azim Resume NewDocument9 pagesAbdul Azim Resume NewSayed WafiPas encore d'évaluation
- Loads Dead Loads Imposed Loads Floor Roof Determining Load Per M and m2 WindDocument58 pagesLoads Dead Loads Imposed Loads Floor Roof Determining Load Per M and m2 Windwaheedopple3998Pas encore d'évaluation
- Cricket Bat Thesis - 2006 SymesDocument297 pagesCricket Bat Thesis - 2006 SymesAnonymous unj3NHW82vPas encore d'évaluation
- ASVAB Arithmetic Reasoning Practice Test 1Document7 pagesASVAB Arithmetic Reasoning Practice Test 1ASVABTestBank100% (2)
- ME2142E Feedback Control Systems-CheatsheetDocument2 pagesME2142E Feedback Control Systems-CheatsheetPhyo Wai Aung67% (9)
- Leader 2Document13 pagesLeader 2Abid100% (1)
- RSBACDocument166 pagesRSBACtradersanPas encore d'évaluation
- Struts HTML Checkbox enDocument4 pagesStruts HTML Checkbox enjudesahayarajPas encore d'évaluation
- SPM Literature in English Tips + AdviseDocument2 pagesSPM Literature in English Tips + AdviseJessica NgPas encore d'évaluation
- RoboServer Users Guide PDFDocument25 pagesRoboServer Users Guide PDFdavid0young_2Pas encore d'évaluation
- Space Systems and Space Subsystems Fundamentals Course Sampler 140211082630 Phpapp02Document42 pagesSpace Systems and Space Subsystems Fundamentals Course Sampler 140211082630 Phpapp02danielPas encore d'évaluation
- Manage Faisalabad WasteDocument2 pagesManage Faisalabad WasteUsama SanaullahPas encore d'évaluation
- Difference Between Defect, Error, Bug, Failure and FaultDocument28 pagesDifference Between Defect, Error, Bug, Failure and FaultbhojanPas encore d'évaluation
- Lesson Plan-Brainstorming Session For The Group Occupational Report-Jmeck Wfed495c-2 V4a7Document2 pagesLesson Plan-Brainstorming Session For The Group Occupational Report-Jmeck Wfed495c-2 V4a7api-312884329Pas encore d'évaluation
- Propagating Trees and Fruit Trees: Sonny V. Matias TLE - EA - TeacherDocument20 pagesPropagating Trees and Fruit Trees: Sonny V. Matias TLE - EA - TeacherSonny MatiasPas encore d'évaluation
- Cost-effective laboratory thermostats from -25 to 100°CDocument6 pagesCost-effective laboratory thermostats from -25 to 100°CCynthia MahlPas encore d'évaluation
- Ch10 Mendelian GeneticsDocument42 pagesCh10 Mendelian GeneticseubacteriaPas encore d'évaluation
- Geo HK Guide To Retaining WallDocument259 pagesGeo HK Guide To Retaining WallZaireen AzmeePas encore d'évaluation
- What Is The Zeitgeist - PART IDocument13 pagesWhat Is The Zeitgeist - PART IMalcolm Armstrong100% (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionD'EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionÉvaluation : 4 sur 5 étoiles4/5 (402)
- The Age of Magical Overthinking: Notes on Modern IrrationalityD'EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityÉvaluation : 4 sur 5 étoiles4/5 (13)
- The Comfort of Crows: A Backyard YearD'EverandThe Comfort of Crows: A Backyard YearÉvaluation : 4.5 sur 5 étoiles4.5/5 (23)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsD'EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsÉvaluation : 3.5 sur 5 étoiles3.5/5 (3)
- Why We Die: The New Science of Aging and the Quest for ImmortalityD'EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedD'EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedÉvaluation : 5 sur 5 étoiles5/5 (78)