Académique Documents
Professionnel Documents
Culture Documents
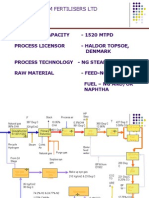
Ammonia Plant Design
Transféré par
lockas222Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Ammonia Plant Design
Transféré par
lockas222Droits d'auteur :
Formats disponibles
() www.final-yearproject.com | www.finalyearthesis.
com
PLANT DESIGN
FOR
AMMONIA PRODUCTION
(CAPACITY: 200 TPD)
A PROJECT REPORT
SUBMITTED TOWARDS PARTIAL FULFILMENT OF THE
REQUIREMENTS FOR THE AWARD OF DEGREE OF
BACHELOR OF TECHNOLOGY
IN
CHEMICAL ENGINEERING
Project Guide
Asst. Prof. Ashutosh
(H.O.D of Chemical Engg.
Neeraj Kumar
Sandeep Kumar
Yash Batra
(Final B.Tech. Chemical Engg.)
Submitted By
Mishra
Deptt.)
() www.final-yearproject.com | www.finalyearthesis.com
ACKNOWLEDGEMENT
We would like to convey our deepest gratitude to Asst. Prof. Ashutosh Mishra, who guided us
through this project. His keen interest, motivation and advice helped us immensely in
successfully completing this project. We would also like to thank him for allowing us to avail
all the facilities of the Department necessary for this project.
We also wish to express our gratefulness towards all the faculty members for their guidance
throughout the project.
Lastly, we cant forget the contribution of the Lab Attendants in giving wings to our efforts.
Neeraj Kumar
Sandeep Kumar
Yash Batra
(Final B.Tech. Chemical Engg.)
() www.final-yearproject.com | www.finalyearthesis.com
CERTIFICATE
This is to certify that the project entitled PLANT DESIGN FOR MANUFACTURE OF
AMMONIA (GAS BASED) submitted by Neeraj Kumar, Sandeep Kumar and Yash Batra to
the Department of Chemical Engineering, Dr. Ambedkar Institute of Technology for
Handicapped, Kanpur, as the final year undergraduate project is a bonafide piece of work
carried out by them under my guidance and supervision.
This work has not been submitted either in part or full in any other university for any purpose
whatsoever.
Asst. Prof. Ashutosh Mishra
(H.O.D . Of Chemical Engg. Deptt.)
() www.final-yearproject.com | www.finalyearthesis.com
ABSTRACT
Sincere efforts have been made to cover as comprehensively as possible the various aspects
of the stated problem. The report begins with an introduction of ammonia and its importance
as regards an Indian viewpoint. It gives a list of various properties and uses of ammonia,
which have been prepared after an extensive literature survey.
The report then describes the various processes that are available for its manufacture and
then deals with the basic raw materials required for the ammonia manufacture i.e natural gas.
It then covers the important aspects of process selection and the selected process is described
in detail along with the relevant equations and flow diagrams.
After this, report incorporates a detailed material and energy balance of the process selected
and also gives a list of the sizing of the equipment in the flow diagram according to the
capacity. This is followed by a detailed chemical and mechanical design of some important
equipment.
The report then gives some information on aspects such as PI & Control, safety, storage &
handling, utilities, plant location & layout, pollution control, etc.
Finally, the report deals with the most important aspect of the economic viability and techno
economic feasibility of the plant in the Indian environment.
() www.final-yearproject.com | www.finalyearthesis.com
INDEX
Introduction
Physical Properties
Chemical Properties
Flow Sheet
Material Balance
Energy Balance
Process Design
CO2 Absorber
Ammonia Converter
CO2 Stripping Column
Process Utility
Catalyst Used
Plant Safety
Hazard Identification
Effluent treatment
Storage handling and Transportation
Site Selection and Plant Layout
Cost Estimation
Conclusion
Bibliography
() www.final-yearproject.com | www.finalyearthesis.com
Introduction
Ammonia is a chemical consisting of one atom of nitrogen and three atoms of hydrogen. It is
designated in chemical notation as NH3.
Ammonia is extremely soluble in water and is frequently used as a water solution called aqua
ammonia. Ammonia chemically combines with water to form ammonium hydroxide.
Household ammonia is a diluted water solution containing 5 to 10 percent ammonia. On the
other hand, anhydrous ammonia is essentially pure (over 99 percent) ammonia. "Anhydrous"
is a Greek word meaning "without water;" therefore, anhydrous ammonia is the ammonia
without water.
Refrigerant grade anhydrous ammonia is a clear, colourless liquid or gas, free from visible
impurities. It is at least 99.95 percent pure ammonia. Water cannot have a content above 33
parts per million (ppm) and oil cannot have a content above 2 ppm. Preserving the purity of
the ammonia is essential to ensure proper function of the refrigeration system.
Physical Properties
Anhydrous ammonia is a clear liquid that boils at a temperature of -28F. In refrigeration
systems, the liquid is stored in closed containers under pressure. When the pressure is
released, the liquid evaporates rapidly, generally forming an invisible vapour or gas. The
rapid evaporation causes the temperature of the liquid to drop until it reaches the normal
boiling point of -28F, a similar effect occurs when water evaporates off the skin, thus
cooling it. This is why ammonia is used in refrigeration systems.
Liquid anhydrous ammonia weighs less than water. About eight gallons of ammonia weighs
the same as five gallon of water
Liquid and gas ammonia expand and contract with changes in pressure and temperature. For
example, if liquid anhydrous ammonia is in a partially filled, closed container it is heated
from 0F to 68F, the volume of the liquid will increase by about 10 percent. If the tank is 90
percent full at 0F, it will become 99 percent full at 68F. At the same time, the pressure in
the container will increase from 16 pounds per square inch (psi) to 110 psi.
Liquid ammonia will expand by 850 times when evaporating:
Anhydrous ammonia gas is considerably lighter than air and will rise in dry air. However,
because of ammonias tremendous affinity for water, it reacts immediately with the humidity
in the air and may remain close to the ground.
The odour threshold for ammonia is between 5 - 50 parts per million (ppm) of air. The
permissible exposure limit (PEL) is 50 ppm averaged over an 8 hour shift. It is recommended
that if an employee can smell it they ought to back off and determine if they need to be using
respiratory protection.
() www.final-yearproject.com | www.finalyearthesis.com
Chemical Properties
Ammonia, especially in the presence of moisture, reacts with and corrodes copper, zinc, and
many alloys. Only iron, steel, certain rubbers and plastics, and specific nonferrous alloys
resistant to ammonia should be used for fabrications of anhydrous ammonia containers,
fittings, and piping.
Ammonia will combine with mercury to form a fulminate which is an unstable explosive
compound.
Anhydrous ammonia is classified by the Department of Transportation as non-flammable.
However, ammonia vapour in high concentrations (16 to 25 percent by weight in air) will
burn. It is unlikely that such concentrations will occur except in confined spaces or in the
proximity of large spills. The fire hazard from ammonia is increased by the presence of oil or
other combustible substances.
Anhydrous ammonia is an alkali.
Summary of properties:
Boiling Point
-28F
Weight per gallon of liquid at -28F
5.69 pounds
Weight per gallon of liquid at 60F
5.15 pounds
Specific gravity of the liquid (water=1)
0.619
Specific gravity of the gas (air=1)
0.588
Flammable limits in air
16-25%
Ignition temperature
1204F
Vapour pressure at 0F
16 psi
Vapour pressure at 68F
110 psi
Vapour pressure at 100F
198 psi
One cubic foot of liquid at 60F expands to
850 cubic foot of gas
Ammonia Molecular weight : 17.03 g/mol
() www.final-yearproject.com | www.finalyearthesis.com
Ammonia Melting point : -78oC
Ammonia Latent heat of fusion (1,013 bar, at triple point) : 331.37 kJ/kg
Ammonia Liquid Density (1.013 bar at boiling point) : 682 kg/m3 (250 K : 669 kg/m3)
(300 K : 600 kg/m3) (400 K : 346 kg/m3)
Ammonia Liquid Specific Heat Capacity (cp) (250 K : 4.52 kJ/kg.K) (300 K : 4.75
kJ/kg.K) (400 K : 6.91 kJ/kg.K)
Ammonia Liquid/gas equivalent (1.013 bar and 15oC (59oF)) : 947 vol/vol
Ammonia Liquid Dynamic Viscosity (250K : 245 106 Ns/m2) (300K : 141 106 Ns/m2)
(400K : 38 106 Ns/m2)
Ammonia Liquid Thermal Conductivity (250 K : 592 106 kW/m.K) (300 K : 477 106
kW/m.K) (400 K : 207 106 kW/m.K)
Ammonia Boiling point (1.013 bar) : -33.5oC
Ammonia Latent heat of vaporization (1.013 bar at boiling point) : 1371.2 kJ/kg
Ammonia Vapour pressure (at 21oC or 70oF) : 8.88 bar
Ammonia Critical point - Critical temperature : 132.4oC - Critical pressure : 112.8 bar
Ammonia Gas Density (1.013 bar at boiling point) : 0.86 kg/m3
Ammonia Gas Density (1.013 bar and 15oC (59oF)) : 0.73 kg/m3
Ammonia Gas Compressibility Factor (Z) (the ratio of the actual volume of the gas to
the volume determined according to the perfect gas law) (1.013 bar and 15oC (59oF)) :
0.9929
Ammonia Gas Specific Gravity (air = 1) (1.013 bar and 21oC (70oF)) : 0.597
Ammonia Gas Specific volume (1.013 bar and 21oC (70oF)) : 1.411 m3/kg
Ammonia Gas Specific Heat Capacity at constant pressure (cp) (1.013 bar and 15oC
(59oF)) : 0.037 kJ/(mol.K)
Ammonia Gas Specific Heat Capacity at constant volume (cv) (1.013 bar and 15oC
(59oF)) : 0.028 kJ/(mol.K)
() www.final-yearproject.com | www.finalyearthesis.com
Ammonia Gas Ratio of Specific Heats (Gamma: cp/cv) (1.013 bar and 15oC (59oF)) :
1.309623
Ammonia Gas Dynamic Viscosity (1.013 bar and 0oC (32oF)) : 0.000098 Poise
Ammonia Gas Thermal conductivity (1.013 bar and 0oC (32oF)) : 22.19 mW/(m.K)
Ammonia Gas Solubility in water (1.013 bar and 0oC (32oF)) : 862 vol/vol
Ammonia Gas Auto ignition temperature : 630oC
() www.final-yearproject.com | www.finalyearthesis.com
Desulphuriser
Steam
Natural gas
Primary Reformer
Secondary Reformer
Shift convertor
CO2
Air
CO2 removal system
Absorber & Stripper
Ammonia convertor
Compressor
Ammonia product
Methanator
Separator
PGR
Purge gas
() www.final-yearproject.com | www.finalyearthesis.com
MATERIAL BALANCE
Natural gas composition (as supplied by ONGC):
Component
Vol %
CH4
89.96
C2H6
4.09
C3H8
2.3
i-C4H10
0.46
n-C4H10
0.6
i-C5H12
0.2
n-C5H12
0.2
CO2
0.35
N2
1.84
Sulphur content : 5 ppm
(Data taken from IFFCO Kalol plant manual)
Calculating from above data
Mole fraction of carbon , xC : 0.219
Mole fraction of hydrogen , xH : 0.781
PRIMARY REFORMER:
() www.final-yearproject.com | www.finalyearthesis.com
Main reaction of primary reformer is
CH4 + H2O
CO + 3H2
H = +ve
CO + H2O
CO2 + H2
H = -ve
Inlet temperature of primary reformer: 500C
Outlet temperature of primary reformer: 800C
Let flow rate of inlet stream: F1 (mol/s)
Composition of primary reformer outlet:
xH2
0.337
xCO2
0.051
xH2O
0.489
xCO
0.087
xCH4
0.0357
Steam added = 3 xC F1 = 0.657F0
Let outlet flow rate = F2 mol/s
Balancing carbon at inlet and outlet of primary reformer
0.219F1 = (0.087+0.051+0.0357) F2
0.219F1 = 0.1737F2
F2 = 1.261F1
SECONDARY REFORMER:
. (1)
() www.final-yearproject.com | www.finalyearthesis.com
Inlet temperature: 800C
Reactions taking place here are:
CH4 + H2O
CO + 3H2
H = +ve
CH4 + O2
CO + 2H2
H = -ve
H2 + O2
H2O
H = -ve
CO+ O2
CO2
H = -ve
Air is added here so that complete conversion of CH4 takes place
Let air added = A mol/s
So,
0.21 A = 2 xCH4 F2
A = 0.34 F2 = 0.429F1
(2)
Composition at secondary reformer outlet
xH2
0.365
xCO2
0.055
xN2
0.1446
xCO
0.0735
xCH4
0.0012
xinert
0.0007
xsteam
0.36
Balancing carbon at inlet and outlet of secondary reformer:
(0.087+0.051+0.0357)F2
(0.0735+0.055+0.0012) F3
F3 = 1.339 F2 = 1.689F1
HIGH TEMPERATURE SHIFT CONVERTOR:
(3)
() www.final-yearproject.com | www.finalyearthesis.com
Inlet temperature: 400C
CO + H2O
CO2 + H2
Outlet composition of HTSC:
xH2
0.418
xCO2
0.108
xN2
0.145
xCO
0.02
xCH4
0.0012
xinert
0.0007
xsteam
0.3
Balancing carbon:
0.1297 F3 = (0.02+.108+.0012) F4
F4 = 1.004F3 = 1.689F1
. (4)
LOW TEMPERTURE SHIFT CONVERTOR:
Inlet temperature: 200C
CO + H2O
CO2 + H2
Outlet composition of LTSC:
xH2
0.436
xCO2
0.126
xN2
0.145
xCO
0.002
xCH4
0.0012
xinert
0.0012
xsteam
0.289
Balancing carbon,
We get .
F5 = F4 = 1.689F1
.. (5)
() www.final-yearproject.com | www.finalyearthesis.com
CONDENSER:
Outlet from LTSC goes to condenser, where steam gets condensed out and rest of the gases
go to CO2 absorber section.
At inlet of condenser:
xH2
0.436
0.736F1
xCO2
0.126
0.213 F1
xN2
0.145
0.245 F1
xCO
0.002
0.003 F1
xCH4
0.0012
0.0012 F1
xinert
0.0012
0.0012 F1
xsteam
0.289
0.488 F1
SYNTHESIS LOOP:
Fci
Make up gas, M
Recycle comp
Fco
Convertor
Separator
NH3
P.G.R
Recycle gas, R
Purge gas, Pg
Compositions: (mole fraction)
() www.final-yearproject.com | www.finalyearthesis.com
Component
Purge ( Pg)
Recycle (R)
At convertor inlet,
(Fci)
H2
0.721
0.618
0.645
N2
0.0898
0.205
0.215
NH3
0.0955
0.0257
0.019
Inerts
0.0932
0.151
0.1199
Applying mass balance for completer loop,
M (xinert)M =
Pg(xinert)Pg
M (xN2)M = Pg(xN2)Pg + (Pg(xNH3)Pg +136.2)
M (xH2)M = Pg(xH2)Pg + 3/2 (Pg(xNH3)Pg +136.2)
Adding all these,
M = 1.095Pg +272.4
.. (6)
Also at separator,
Fco = Pg + R + 136.2
.. (7)
At recycle point,
Fci = M+ R
.. (8)
Taking 14% conversion per pass for the reactor at 200 atm pressure and 500C,
Fco = Fci (xN2)Fci0.86 + Fci (xH2)Fci0.86 + Fci (xNH3)Fci + Fci (xN2)Fci0.28 + Fci (xinert)Fci
On solving,
Fco = 0.9387Fci
. (9)
Also at recycle,
R(xNH3)R = Fci(xNH3)Fci
Fci = 1.35R
Solving eqs. (6), (7), (8), (9) and (10)
.. (10)
() www.final-yearproject.com | www.finalyearthesis.com
Stream
Flow rate (Mol/s)
2136.12
747.64
Fci
2883.76
Pg
434.18
Fco
2706.98
Make up gas to synthesis loop comes from methanator.
METHANATOR
Outlet composition of this is same as that at inlet of synthesis loop.
Components
Mole
fraction
Flow rate
(Mol/s)
H2
0.737
552.88
N2
0.249
186.54
CH4
0.007
5.15
0.004
0.003
2.25
Inert
(CO, CO2, & Ar)
Steam
Inlet flow rate for methanator can be calculated using inlet composition for methanator and
balancing flow rate of nitrogen (as nitrogen remains unaffected in methanator).
Inlet flow rate and composition of methanator are:
() www.final-yearproject.com | www.finalyearthesis.com
Components
Mole
fraction
Flow rate
(Mol/s)
H2
0.745
562.54
N2
0.247
186.54
CH4
0.002
1.51
Inert
0.001
0.75
CO
0.004
3.02
CO2
0.001
0.75
CO2 ABSORBER:
In CO2 absorber, only Carbon dioxide is absorbed and rest of the gases remain unaffected.
Inlet and outlet compositions for CO2 absorber,
Components
Inlet mole
fraction
Outlet
mole
fraction
Outlet
flow rate
(Mol/s)
H2
0.687
0.745
562.54
N2
0.228
0.247
186.54
CH4
0.002
0.002
1.51
Inert
0.004
0.001
0.75
CO
0.078
0.004
3.02
CO2
0.001
0.001
0.75
Let inlet flow rate: Fa
Balancing hydrogen in CO2 absorber,
Fa 0.687 = 562.64
() www.final-yearproject.com | www.finalyearthesis.com
Fa =
818.98 mol/s
CO2 removed in CO2 absorber = 0.078818.98 - 0.75
= 63.13 mol/s
CONDENSOR:
Steam formed in LTSC & HTSC is removed in this unit. Outlet for condenser is inlet for the
absorber. As other gases do not get affected, so we can balance flow rate for any other gas.
From earlier calculations,
xH2
0.436
0.736F1
xCO2
0.126
0.213 F1
xN2
0.145
0.245 F1
xCO
0.002
0.003 F1
xCH4
0.0012
0.0012 F1
xinert
0.0012
0.0012 F1
xsteam
0.289
0.488 F1
Equating flow of hydrogen
0.736F1 = 562.64
F1 = 764.46 mol/s
Using relations (1), (2), (3), (4) and (5)
F2 = 963.98 mol/s
F3 = F4 = F5 = 1291.8 mol/s
Air added in secondary reformer, A = 327.95 mol/s
Steam added in primary reformer = 502.25 mol/s
ENERGY BALANCE
PRIMARY REFORMER:
() www.final-yearproject.com | www.finalyearthesis.com
Reactions taking place in primary reformer are:
CH4 + H2O
CO + 3H2
H298 = +206 kJ/mol
CO + H2O
CO2 + H2
H298 = - 41 kJ/mol
Inlet temperature : 500C
Outlet temperature : 800C
1073
xi Cpi
298
H1073 = H298 +
Thus ,
Heat required by first reaction : Flow rate of methane (H1073 )1
=
1.87 107 J/s
Heat liberated by second reaction : Flow rate of carbon monoxide (H1073 )2
=
7.55 105 J/s
Enthalpy content of outlet stream :
(F .x .Cp.1073) = 4.05 107 J/s
Enthalpy content of inlet stream :
(F .x .Cp.773)
= 3.81 107 J/s
Additional heat required,
4.05 107 + 1.87 107 - 7.55 105 - 3.81 107 J/s
= 2.03 107 J/s
Part of this heat is provided by the steam (at 30 atm)
Steam enthalpy = 2802.3 kJ/Kg = 50491.9 J/mol
Enthalpy content in steam = 2.53 106 J/s
Rest of heating is done in convection zone of primary reformer using natural gas,
Heat utility of convection zone: (2.03 0.253) 107 J/s
() www.final-yearproject.com | www.finalyearthesis.com
= 1.78 107 J/s
Calorific value of natural gas = 39383.2 kJ/m3
Amount of natural gas required
1.78
3.94
m3/s = 0.451 m3/s
SECONDARY REFORMER:
Air added in secondary reformer = 327.95 mol/s
This is pre-heated to 800C before being fed to secondary reformer.
Heat required for heating air from 30C to 800C = 7106 J/s
Again natural gas is used for heating purpose,
Amount of natural gas required : (7106) / (3.94107) = 0.178 m3/s
CH4 + H2O
CO + 3H2
H = +ve
H2 + O2
H2O
H = - ve
CO+ O2
CO2
H = - ve
Heat required by reaction 1 at 1073 K = 0.34 107 J/s
Heat liberated by reaction 2 at 1073 K = 0.49 107 J/s
Heat liberated by reaction 3 at 1073 K = 0.221 107 J/s
Heat released due to the 3 reactions = 0.371 107 J/s
This heat increases temperature of outlet gas. Let this temperature be T
0.371107 = (F .x .Cp.(T-1073) )
solving ,
T = 1151.7 K
Outlet temperature of secondary reformer = 1151.7 K
HEAT RECOVERY BETWEEN SECONDARY REFORMER & HTSC:
() www.final-yearproject.com | www.finalyearthesis.com
Effluent from secondary reformer is cooled to 693 K before being fed to HTSC. This heat is
utilized for producing steam at 373 K and 1 atm.
H
CpT s
Steam produced:
H = heat recovered = (F .x .Cp.(1151-693) ) = 2.22107 J/s
4.067 107 J/kmol
Steam produced = 0.547 kmol/s
HIGH TEMPERATURE SHIFT CONVERTOR:
Inlet temperature: 693 K
CO + H2O
CO2 + H2
H = -ve
Heat released by the reaction: 0.133 107 J/s
Let outlet temperature of HTSC = T
H = (F .x .Cp.(T-693) )
T = 723.38 K
Outlet temperature of HTSC = 723.38 K
HEAT RECOVERY BETWEEN HTSC & LTSC:
Inlet temperature of LTSC = 473 K
Heat removed = (F .x .Cp.(723.38-473)) = 1.093 107 J/s
This heat is utilized for producing steam at 373 K and 1 atm.
Amount of steam produced :
H
CpT s
= 269.33 mol/s
() www.final-yearproject.com | www.finalyearthesis.com
LOW TEMPERATURE SHIFT CONVERTOR:
Inlet temperature : 473 K
CO + H2O
CO2 + H2
H = -ve
Heat released by this reaction in LTSC = 0.0422 107 J/s
Let outlet temperature of LTSC = T
(F .x .Cp.(T-473)) = 0.0422 107
T = 483 K
CONDENSER:
Exit stream from LTSC contains steam which is an unwanted load on absorber. In condenser,
steam is condensed at 373 K ( this is inlet temperature of absorber).
Exit gases ( other than steam) are also cooled from 483 to 373 K through a heat exchanger
H = (F .x .Cp.(483-373))
= 4.66 107 J/s
METHANATOR:
Inlet temperature : 623 K
After absorber, gases are heated from 373 K to 623 K .
Heat required = (F .x .Cp.(623-373))
= 0.554 107 J/s
Steam is used for heating,
Amount of steam utilized:
H
s
= 133 mol/s
Reaction taking place in methanator is :
CO2 + 3H2
CH4 + H2O
Heat released by this reaction in the methanator = 5.4 107 J/s
Let outlet temperature = T
5.4 107 = (F .x .Cp.(T-623))
T = 626 K
() www.final-yearproject.com | www.finalyearthesis.com
() www.final-yearproject.com | www.finalyearthesis.com
PROCESS DESIGNCO
Gas flow rate at bottom Gb = 818.98 mol/s = 7.2 Kg/s
ABSORBER
Gas out
MEA 0.001 CO2
Gas flow rate at the top Gt = 755.19 mol/s = 4.43 Kg/s
Yt =0.001
Yb =0.0845
PROPERTIES
Gas density g = 0.487 kg/m3
Liquid density l = 934.4 kg/m3`
Gas viscosity g= 0.0175 cP
Gas in
Liquid viscosity l = 0.299 cP
Gas diffusivity Dg = 1.65X10-5m2/s
Liquid diffusivity Dl = 1.96X10-5m2/s
Gas heat capacity Cpg = 2.094 kJ/kg K
Liquid heat capacity Cpl = 4.145 kJ/kg K
Equilibrium data for absorption of CO2 in MEA
X mol CO2 /mol inerts
Y mol CO2/mol MEA sol
0.1
0.2
0.002
0.25
0.004
0.3
0.008
0.35
0.016
0.4
0.03
0.45
0.052
0.48
0.08
CO2 rich MEA to stripper
() www.final-yearproject.com | www.finalyearthesis.com
0.49
0.09
From Graph
(Lin/Gin)min = 0.217
(Lin/Gin)actual = 1.50.217= 0.3255
Lin
= 0.325755.19
= 266.578 mol/s
Top Section
Lt = Ls (1+Xt)
= 266.578 mol/s
Bottom Section
Lb = 266.578(1+Xb)
= 361.4798 mol/s
(as Xb = 0.356 , from graph)
14.5 wt% MEA souliton has molecular wt.= 20.08 kg/kmol
Lt = 5.35 kg/s
Lb = 7.258 kg/s
Thus amount of MEA required for absorber = 5.35 kg/s with 0.1% of CO 2 coming from
stripper.
CALCULATION OF COLOUMN DIAMETER
Choosing 38 mm pall rings (metal)
Void fraction = 0.95
Packing factor Fp = 28
Surface area a = 128 m2/m3
At the bottom (L/G)(gl) = 0 .023
At the top (L/G)(g/l) = 0.0275
() www.final-yearproject.com | www.finalyearthesis.com
Hence choosing the larger value of 0.027 (at top)
From Graph
Gf2Fpl0.2gl) = 0.01
Where
Gf = gas superficial velocity
Fp = packing factor = 28
= correction factor for density = 1
l = viscosity of liquid in cp = 0.299
g = density of gas = 0.487 kg/m3
l = density of liquid = 934.4 kg/m3
g = acceleration due to gravity
On substituting we obtain
Gf = 6.047 kg/m2 s
Operating G = 0.85Gf
= 5.1399 kg/m2s
Ac = Gt/G
Ac = 4.43/5.375
Ac = 0.86 m2
Di = 1.047 m
Design dia =1.05 m
Evaluation of tower height
Z = HOGNOG
HOG = Hg + m(Gm/Lm)Hl
For ring type of packings
(for 200 N/m2 per m pressure)
() www.final-yearproject.com | www.finalyearthesis.com
Hg = 0.017D1.24Z0.33Scg0.5/(Lf1f2f3)0.5
= parameter for a given packing = 75
D = column diameter
Scg = Schmidt no for gas phase =2.1778
L = liquid rate = 6.2 kg/m2s
f1 = (l/w) = 0.8243
f2 = (l/w) =1.0885
f3 = (w/l) = 1.0611
Substituting we get
Hg = 0.822Z0.33
Liquid phase transfer unit
Hl = (C/3.28)Scl0.5(Z/3.05)0.15
= correlation parameter for a given packing = 0.11
C = correlation parameter for high gas flow rates = 0.5
Scl = Schmidt no for liquid phase = .00163
Z = tower height
On substitution
Hl= .000573Z0.15
m = slope of the equilibrium curve =0.27
Lm = 266.578 mol/s
Gm = 755.19 mol/s
HOG = 0.822Z0.33 + 0.00044Z0.15
0.0845
dy
y y
0.001
NOG =
- ln[(1-yb)/(1-yt))
() www.final-yearproject.com | www.finalyearthesis.com
y*
1/(y-y*)
.001
1000
.01
0.0005
105.283
.02
.001
52.63
.03
.0015
35.08
.04
.0025
26.26
.05
.004
21.74
.06
.006
18.5
.07
.009
16.4
.08
.0125
14.8
.084
.0145
14.38
NOG = 4.78
Z = 3.93Z0.33 + 0.0021Z0.15
By iterations,
Z = 7.70 m
Height of bed in absorber = 7.7 m
Pressure drop = 1540 N/m2
() www.final-yearproject.com | www.finalyearthesis.com
AMMONIA CONVERTOR
Let fraction of feed goes to first bed and rest of 1- fraction mixes with effluent from 1st
bed as quench gas to maintain temperature of gas at 500C.
Also it is assumed that conversion in first bed is from 0 to 8 % , whereas in second converter
conversion of 14 % is achieved.
Heat liberated after first bed = F 0.08 0.215 2 46 103
=1582.4 F
After first Bed:
H2
(0.6450.92) F
0.5934 F
N2
(0.2150.92) F
0.1972 F
(20.2150.08+0.02 ) F =
0.0544 F
NH3
Inert
0.12 F
Total = 0.9656 F
Mole fraction
Cp (J/mol-K )
H2
0.614
29.572
N2
0.205
31.258
NH3
0.056
50.431
Inert
0.1242
20.796
For the gas mixture,
Cp = 30.66 J/mol-K
0.9656 F Cp T = 1582.4 F
T = 53C
(T1)out = 480+53 = 533C
0.9656 F Cp806 +(1- ) F Cp626 = F(0.9656 +1- ) Cp753
= 0.71
() www.final-yearproject.com | www.finalyearthesis.com
2nd bed inlet:
H2
0.5934 F + (1- ) F0.645 = 0.608 F
N2
0.1978 F + (1- ) F 0.215 = 0.208 F
NH3
0.0544 F + (1- ) F 0.02 = 0.0444 F
Inert
0.12 F
= 0.12 F
Flow rate = 0.9752 F
Inlet mole fraction
H2
0.623
N2
0.208
NH3
0.045
Inert
0.0123
= [( F NH3)out (F NH3)in] 46103
Heat liberated
= (2706.980.087 - 0.04442883.76) 46103
= 4162.54103 J/s
Out flow rate
= 2706.98 mol/s
Cp
= 30.5 J/mol-K
2706.98 30.5 T
= 4162.54103
T = 50.4 0C
Final Temp = 530.4 0C
Heat Exchanger
0.71F(753-626)
= 2706.98(803.5-T)
812.87- T
= (0.712883.76127)/2706.98
= 707.44 K
() www.final-yearproject.com | www.finalyearthesis.com
Volume of catalyst bed:
V1CAo / FAo =
Space velocity = 15000 hr-1 ( at standard conditions )
Space time = 0.0747 s
Space time at operating condition =( / CAo) CAo
CAo = (10.215)/(0.0821273)
= 9.6 mol/m3
CAo = (200.215)/ (.0821773)
= 678.4 mol/m3
Space time at operating conditions = 0.0747678.4/ 9.6
= 5.28 s
V1CAo / FAo = 5.28
Volume of first catalyst bed:
V1 =
=
(5.280.2152883.760.71)/(678.4)
3.375 m3
Volume of second catalyst bed :
V2 =
=
(5.280.2050.97522883.76)/(646.85)
4.695 m3
Volume of first bed:
3.375 m3
Volume of second bed:
4.695 m3
() www.final-yearproject.com | www.finalyearthesis.com
CO2 STRIPPING COLUMN
Liquid flow rate into stripping column = 266.578 mol/s
Molecular weight of liquid (15.3% MEA in H2O) = 20.08
X2 = .356 kmol
CO2/kmol MEA
CO2 + steam
Steam
X1 =
0.001 kmol CO2
kmol CO2
kmol steam
Y1=0
Kmol MEA
Equilibrium data:
X
0.001
0.003167
0.002
0.006347
0.05
0.17713
0.1
0.403061
0.15
0.701183
0.2
1.112676
0.25
1.717391
0.3
2.693182
0.35
4.532787
0.356
4.869114
() www.final-yearproject.com | www.finalyearthesis.com
For minimum steam rate from graphY2 = 0.75
kmol CO2
kmol steam
Minimum Gs = Ls(X2-X1)/(Y2-Y1)
= 266.578*(.356-.001)/(1.1-0)
= 86 mol steam/sec
for 1.5 times minimum, steam flow rate = 126.2 mole/sec
therefore Y2 = 1.1
also, x1 = 0.001, y1 = 0
x2 = 0.356, y2 = 1.1
we make a y-x plot to determine total number of stages which are found to be 4.8
TOWER DIAMETER
L
G
G
L
= 0.045
= 0.0744*t + 0.01173
= 0.0304*t + 0.015
reference: Page 169 Mass transfer operations by R E Treybal
let t = 0.75 m
= 0.06753
= 0.0378
CF = [0.06753 log
1
0.04 0.2
0.0378](
)
0.556
0.02
0.04
() www.final-yearproject.com | www.finalyearthesis.com
= 0.1339
L G 0.5
)
G
V F = CF
= 4.1554 m/s
we use 80% of floding velocity
V = 3.32 m/s
An = Q/V = 2.33/3.32 = 0.7 m2
tray area used by one downspout = 8.8%
At = 0.7/(1-0.088)
= 0.7685 m2
D = 0.989 m
Diameter = 1.00 m
height of tower = 5 * 0.75
= 3.75 m
Hieght of tower = 3.75 + 2
= 5.75 m
() www.final-yearproject.com | www.finalyearthesis.com
PROCESS UTILITY
Feed Gas Compression:
Natural gas fuel heater
Feed gas Compressor
Start-up Compressor
Natural gas knock-out drum
Reforming:
Process air saturator
Primary Reformer
Stem super heater
Reactant preheater
Combustion air heater
Boiler
Feed heater
Process air heater
First make gas boiler
Second gas make boiler
Make gas steam super heater
Saturator DMW heater
Saturator effluent cooler
Flue gas fan
Sulphur catch
Secondary reformer
Flue gas stack
Steam drum
() www.final-yearproject.com | www.finalyearthesis.com
Continuous blow down drum
Intermittent blow down drum
NOX reduction unit.
CO Shift:
Make gas BFW heater
HT shift MG BFW heater
HT shift steam super heater
Naphtha Vaporiser
Feed gas Heater
Saturator circuit heater
LP boiler
HT sift converter
LT shift converter.
CO2 Removal:
CO2 absorber
MEA regenerator
Flash column
MEA filter
MEA make up filter
Activated carbon filter
Deaerator feed heater
Lean MEA cooler
Rich/lean exchanger
Syngas trim cooler
Semi-lean pump
MEA make up pump
MEA filter pump
Condensate pump
Process condensate pump
Lean solution pump
() www.final-yearproject.com | www.finalyearthesis.com
Semi rich solution pump
Water make up pump
Hydraulic turbine
MEA solution storage tank
Methanation and Syngas Drying:
Methanator exchanger
Methanator start-up heater
Syngas cooler
Adsorber regeneration heater
Methanator
Syngas adsorber
Ammonia Synthesis:
NH3 converter start up heater
NH3 loop BFW heater
NH3 loop hot interchanger
NH3 loop cooler
NH3 loop cold interchanger
NH3 loop chillers
Ammonia product heater
Product ammonia pump
Absorber bottoms pump
NH3 converter
NH3 converter cartridge
NH3 flash vessels
LP NH3 absorber
Purge Gas Treatment:
NH3 absorber
NH3 still
NH3 absorber feed water cooler
NH3 solution interchanger
NH3 still condenser
Purge gas H2 recovery unit
() www.final-yearproject.com | www.finalyearthesis.com
Synthesis Gas Compression:
Synthesis gas compressor
NH3 loop circulator
Process Air Compression:
Process air compressor
Refrigeration:
Refrigeration condenser
Refrigeration compressor
Refrigeration receiver
() www.final-yearproject.com | www.finalyearthesis.com
CATALYST USED
1.
Desulphurization
Supported cobalt-molybdenum (Comox) or nickel-molybdenum (Nimox) catalyst is
used for hydrogenation of organic sulphur compounds in hydrocarbon feedstock to
H2S which is then removed.
H2S is absorbed in a ZnO bed.
Temperature:
350-400 oC
Pressure:
40 Kg/cm2
Catalyst name:
ZnO
Shape and size:
Extruded rods or globules 3-5 mm dia.
Bulk density:
1.05-1.15 Kg/lit
Typical chemical composition (% by wt):
ZnO
95
R2O3
Fe2O3 + Al2O3
Special features:
Low attrition loss.
Gains strength in use.
High H2S absorption efficiency.
2.
Primary reformer
Temperature:
700-800 oC
Pressure:
30-40 Kg/cm2
Catalyst name:
supported nickel catalyst
Shape and size:
Raschig rings (16616 or 18816 mm)
Bulk density:
.05-1.15 Kg/lit
() www.final-yearproject.com | www.finalyearthesis.com
Typical chemical composition (% by wt):
NiO
18
CaO
18
Al2O3 74
Special features:
Highly pure and refractory.
Al2O3 supports high activity and stability and long life
3.
Secondary reformer
Temperature:
950 oC
Pressure:
30-40 Kg/cm2
Catalyst name:
supported nickel catalyst
Shape and size:
Raschig rings (16616 or 18816 mm)
Bulk density:
1.1-1.2 Kg/lit
Typical chemical composition (% by wt):
NiO
12
CaO
12
Al2O3 76
Special features:
4.
High thermal shock resistance free of carry over problem
High activity
Excellent mechanical stability
Long life
High temperature shift converter
Temperature:
340-450 oC
Pressure:
30-40 Kg/cm2
Catalyst name:
iron chromium catalyst
() www.final-yearproject.com | www.finalyearthesis.com
Shape and size:
solid cylindrical tablets (66 or 106 or 1010 mm)
Bulk density:
1.0-1.1 Kg/lit
Typical chemical composition (% by wt):
Fe2O3 90
Cr2O3 10
S
0.015
Special features:
Extremely low Sulphur content with very low desulphurization time
High and stable activity and strength
5.
Low temperature shift converter
Temperature:
200-250 oC
Pressure:
30-40 Kg/cm2
Catalyst name:
supported copper catalyst
Shape and size:
solid cylindrical tablets (64 mm)
Bulk density:
1.25-1.3 Kg/lit
Typical chemical composition (% by wt):
CuO
25
ZnO
25
Al2O3
35
Fe2O3 + TiO2 5
Special features:
High and stable activity and strength
High resistance to poisons and steam condensation
6.
Methanation
() www.final-yearproject.com | www.finalyearthesis.com
Temperature:
300-400 oC
Pressure:
30-40 Kg/cm2
Catalyst name:
nickel supported on alumina catalyst
Shape and size:
solid cylindrical tablets (64 mm)
Bulk density:
1.25-1.3 Kg/lit
Typical chemical composition (% by wt):
NiO
20
R2O3
80
Special features:
Excellent thermal and mechanical stability
High activity and long life.
7.
Ammonia synthesis
Temperature:
400-500 oC
Pressure:
upto 1000 atm
Catalyst name:
doubly promoted iron catalyst
Shape and size:
solid cylindrical tablets (1.5-12 mm)
Bulk density:
2.1-2.3 Kg/lit
Typical chemical composition (% by wt):
Fe2O3
91
Al2O3
CaO
MgO
K2O
SiO2
0.5
Special features:
() www.final-yearproject.com | www.finalyearthesis.com
Product of Indian magnetite with high activity and mechanical strength
and stability
8.
Catalyst poisoning:
The active surface is sensitive to poisons.
Two classes of poisons are recognized: a) Permanent
b) Temporary
The permanent poison contains sulphur, phosphorus, arsenic, and chlorine and is represented
by such compounds as H2S and HCl. The temporary poisons contain oxygen and are
represented by such compounds as CO, CO2, O2, and H2O.
If exposure to temporary poisons does not last more than three to six days, the catalyst can
usually be brought back to normal activity simply by exposing it to pure synthesis gas. In the
presence of high concentration of hydrogen, the oxygenated compounds that make up the
temporary poisons are converted to H2O, which effectively blankets the active surfaces of the
catalyst. Thus, temporary poisons are all about equivalent on an oxygen basis, with 100 ppm
O2 having same effect as 100 ppm CO2 or 200 ppm H2O or CO.
() www.final-yearproject.com | www.finalyearthesis.com
PLANT SAFETY
Historical data show that the major accidents in ammonia plants are explosions and fires. In
addition there is also a potential of toxic hazard due to the handling and storage of liquid
ammonia.
The following credible major hazards events are identified in an ammonia production plant:
1. Fire/explosion hazard due to leaks from the hydrocarbon feed system.
2. Fire/explosion hazard due to leaks of synthesis gas in the CO removal/synthesis gas
compression areas (75% hydrogen).
3. Toxic hazard from the release of liquid ammonia from the synthesis loop.
In ammonia storage the release of liquid ammonia (by sabotage) is a credible major hazard
event.
Confined explosions in ammonia plants appear to be limited to explosions equivalent to a few
hundred kg TNT. Such explosions are normally not fatal for humans at 50-60m distance, and
thus in most cases not severe for people outside the plant fence. The same is true for fireballs
equivalent to 500kg hydrogen. Fires and explosions are usually not a hazard or only a minor
hazard to the local population although potentially most severe for the plant operators.
Appropriate precautions to protect both the operators and the local population are taken in the
design and operation of the plants.
The toxic hazard of a potential large release of liquid ammonia (ie. from a storage tank) may
be much more serious for the local population. An emergency plan for this event, covering
the operators and the local population must be maintained.
HAZARDS IDENTIFICATION
() www.final-yearproject.com | www.finalyearthesis.com
Human health:
Ammonia is toxic by inhalation, corrosive to all parts of the body and liquid splashes
can cause severe cold burns.
Skin Contact:
Liquid ammonia splashes may produce severe cold burns to skin. Vapour in presence
of moisture is an irritant to the skin.
Eye Contact:
Liquid ammonia splashes may cause permanent damage to eyes with the full effects
not being apparent for several days. Vapours can cause irritation and watering of eyes
and at high concentrations can cause severe damage.
Ingestion:
Will immediately cause severe corrosion and damage to the gastro-intestinal tract.
Inhalation:
Ammonia odour threshold 5-25ppm. Concentrations in the range 50-100ppm may
cause slight irritation following prolonged exposure. Immediate eye, nose and throat
irritation may occur with ammonia levels between 400-700ppm with symptoms of
slight upper respiratory tract irritation persisting beyond the period of exposure. At
higher concentrations, above 1000ppm, severe eye and upper respiratory tract
irritation can develop following a short period of exposure. Exposure to ammonia in
excess of 2000ppm for even short periods may result in severe lung damage and could
be fatal. Fluid build up on the lung (pulmonary dema) may occur up to 48 hours
after exposure and could prove fatal. Exposure to concentrations grossly in excess of
the occupational exposure limit may lead to permanent respiratory impairment.
Long term effects:
No evidence of adverse effects at exposure below occupational exposure limits.
Environment:
Free (non-ionised) ammonia in surface water is toxic to aquatic life, however the
ammonium ion which predominates in most waters is not toxic. In the event of water
contamination with ammonia, ammonium salts which may be formed will not present
a toxic hazard. Increases in pH above 7.5 leads to an increased level of non-ionised
ammonia.
Studies in fish have shown that repeated exposures produce adverse effects on growth
rate at concentrations greater than 0.0024mg/l.
() www.final-yearproject.com | www.finalyearthesis.com
Other:
Fire, heating and explosion
Flammable but difficult to ignite in open air. In enclosed space ammonia air mixtures
may be flammable/explosive.
Danger of tank or cylinder bursting when heated.
Large leaks of liquid ammonia may produce a dense cloud, restricting visibility.
FIRST-AID MEASURES
Speed is essential. Remove affected person from further exposure. Give immediate
first aid and obtain medical attention.
Skin Contact
Drench with large quantities of water. In case of frost bite (freeze burns) clothing may
adhere to the skin. Defrost with care using comfortable warm water. Remove clothing
and wash affected parts. Obtain immediate medical attention.
Eye Contact
Immediately irrigate the eyes with eyewash solution or clean water for at least 10
minutes. Continue irrigation until medical attention can be obtained. Hold eyelids
open during flushing.
Ingestion
Do not induce vomiting. If the person is conscious, wash out mouth with water and
give 2 or 3 glasses of water to drink. Obtain immediate medical attention.
Inhalation
Move the injured person to fresh air at once. Keep the patient warm and at rest.
Administer oxygen if competent person is available. Apply artificial respiration, if
breathing has stopped or shows sign of failing. Obtain immediate medical attention.
Further medical advice
Keep under medical review for possibility of rapid or delayed tracheal, bronchial and
pulmonary edema. Progressive ocular damage may arise.
FIRE-FIGHTING MEASURES
() www.final-yearproject.com | www.finalyearthesis.com
Ammonia vapour and liquid spills are difficult to ignite, particularly in the open air. In an
enclosed space, mixtures of ammonia and air within the limits (16-27%), might cause
explosion if ignited. Cold, dense cloud of ammonia may impair visibility.
Attempt to isolate source of leak.
Use foam, dry powder or CO2.
Use water sprays to cool fire-exposed containers and structures, to disperse vapours
and to protect personnel. Do not spray water into liquid ammonia.
Wear self-contained breathing apparatus and full protective clothing.
ACCIDENTAL RELEASE MEASURES
Those dealing with major releases should wear full protective clothing including
respiratory protection.
Evacuate the area down-wind of the release, if it is safe to do so. If not, then stay
indoors, close all windows and switch off any extraction fans or electrical fires.
Isolate source of leak as quickly as possible by trained personnel.
Ventilate area of spill or leak to disperse vapours.
Remove ignition sources.
Consider covering with foam to reduce evaporation.
Contain spillages if possible.
Use water sprays to combat gas clouds. Do not apply water directly into large
ammonia spills.
Take care to avoid the contamination of watercourses.
Inform appropriate authority in case of accidental contamination of watercourses or
drains.
HANDLING AND STORAGE
Handling:
Avoid skin and eye contact and inhalation of vapours.
Provide adequate ventilation.
Control atmospheric levels in compliance with occupational exposure limits.
Wear full protective equipment where there is a risk of leaks or splashes.
Storage:
() www.final-yearproject.com | www.finalyearthesis.com
Store containers tightly closed in a cool, well ventilated area.
Keep away from heat, ignition sources and incompatible substances.
Do not permit smoking in the storage area.
Follow appropriate Industry or National codes for bulk and container (cylinder)
storage.
EXPOSURE CONTROL / PERSONAL PROTECTION
Recommended occupational exposure limits:
ACGIH [4] occupational exposure limits for ammonia and other components associated with
ammonia production are given in the table below. All the figures are ppmv:-
Component
TLV-TWA (8hr)
TLV-STEL (15min)
NH3
25
35
NO2
SO2
H2S
10
15
CO
50
400
CO2
5000
30000
The figures are subject to updating and may vary between countries.
Precautionary and engineering measures
Provide local exhaust ventilation where appropriate.
Provide safety showers and eye washing facility at any location where skin or eye
contact can occur.
Personal Protection:
Wear suitable breathing apparatus if exposure levels exceed the recommended limits.
Wear cold insulating PVC gloves, rubber boots, PVC suit.
() www.final-yearproject.com | www.finalyearthesis.com
EFFLUENT TREATMENT
EMISSIONS TO AIR
From steam reforming plants with a fired primary reformer emissions to air come from the
following sources:
Flue-gas from the primary reformer
Vent gas from CO2 removal
Breathing gas from oil buffers (seals/compressors)
Fugitive emissions (from flanges, stuffing boxes etc.)
Purge and flash gases from the synthesis section (usually added to the primary
reformer fuel)
Non-continuous emissions (venting and flaring)
1. Flue-gas from the primary reformer:
The flue-gas volume, at 3% (dry gas base) oxygen, for a gas-based conventional steam
reforming plant producing 200t/d, is approximately 26,667 Nm 3/h, containing about 8%
CO2 (dry gas base), corresponding to 500kg CO2/t NH3. The flue-gas volume from excess
air reforming may be lower. The other pollutants in the flue-gas are:
NOx: 200-400mg/Nm3, (98-195ppmv), or 0.6-1.3kg/t NH3 expressed as NO2
SO2: 0.1-2mg/Nm3, (<0.01kg/t), depending on fuel
CO: <10mg/Nm3, (<0.03kg/t)
The NOx emission depends on several factors and the following features reduce the
emission:
Low combustion air and fuel gas preheat
Steam/inert injection
Low ammonia content in injected purge-gas
Low excess oxygen
Low NOx burners
Post-combustion measures
The SO2 emission comes from the sulphur in the fuel gas and can be calculated by a
simple mass balance.
() www.final-yearproject.com | www.finalyearthesis.com
2. Vent gases from CO2 removal:
More or less of the CO 2 product may have to be vented, depending on the CO 2
requirements of other production facilities on the site. In some cases, high purity CO 2 is
used, while an air- CO2 mixture from a stripping column is vented. The CO2 contains
small traces of synthesis gas and absorption solvent vapour.
3. Breathing gas from oil buffers:
This contains traces of NH3, synthesis gas and lube oil.
4. Fugitive emissions:
The diffuse emissions from flanges, stuffing boxes etc. should be minimized.
5. Purge and flash gases:
The purge and flash gases from the synthesis section are usually washed with water to
remove/recover ammonia, and the purge gas may be treated in a recovery unit, before
routing the off-gases to the primary reformer fuel gas system. The off-gases are thus
combusted and end up as part of the flue-gas. It is important to remove the ammonia as
far as possible, as it will contribute considerably to the flue-gas NOx emission.
6. Non-continuous emissions:
Emission of NOx during flaring synthesis gas at start-up or trip situations is estimated to
be 10-20kg/hour as NO2 [1]. Some plants without a flare, vent to the atmosphere.
EMISSIONS TO WATER
Pollution problems related to water, during normal operation, may occur due to process
condensates or due to scrubbing of waste gases containing ammonia.
Process condensate is found primarily in the condensation section prior to the CO 2 removal,
in the order of 1m3 per ton NH3 produced. Without treatment this condensate can contain up
to 1kg of ammonia and 1kg methanol per m3. More than 95% of the dissolved gases can be
recovered by stripping with process steam and are recycled to the process. The stripped
condensate can be re-used as boiler feed water make-up after treatment by ion exchange.
Total recycle is obtained in this way. In some cases the process condensate is used for feedgas saturation and thus recycled to the process.
Usually the ammonia absorbed from purge and flash gases is recovered in a closed loop so
that no aqueous ammonia emissions occur. Emissions to water from the production plant
during normal operation can thus be fully avoided.
() www.final-yearproject.com | www.finalyearthesis.com
SOLID WASTES
The ammonia processes do not normally produce solid wastes. Spent catalysts and sieves
have to be removed and valuable metals are recovered from them.
Environmental Hazards Associated with Emissions and Wastes:
The production of ammonia is relatively clean compared to many other chemical processes.
During the normal operation of a reforming plant, only the NOx and CO2 emissions have to be
considered. In partial oxidation plants with oil-fired auxiliary boilers the reduction of SO 2
emissions can be achieved by using low sulphur fuel oil. Generally the emissions from
modern ammonia plants have little environmental impact.
EMISSION MONITORING
The following emissions to air should be monitored as part of a proper supervision:
a) NOx in flue-gases.
b) SO2 in flue-gases (may be calculated by mass balance instead of monitoring emission,
if S input is known).
The other emissions to air need not be monitored. CO 2 emission can be calculated from fuel
specification and energy consumption, CO emission is fixed by the operating conditions and
usually stable and low. Non-continuous and fugitive emissions are difficult to measure.
The frequency of monitoring depends on local circumstances and the operating stability of
the actual plant. Under normal operating conditions, measurements once a month are usually
sufficient.
Methods for discontinuous and continuous measurements of NOx, SO2 and H2S are available
and are to a large extent standardized at national level. Chemiluminescence or photometry are
the most widely used methods for NO x. SO2 is determined by InfraRed (IR) absorption
techniques. Traces of H2S are measured by lead acetate.
Emissions to water from new plants are virtually zero as process condensates are recycled
and monitoring is not normally required. In existing plants without recycle of process
condensate, the ammonia and methanol content should be monitored.
() www.final-yearproject.com | www.finalyearthesis.com
Emissions to Air
An extractive gas sampling system for continuous gas monitoring will typically comprise:
A coarse filter (heated if necessary) which may be in the stack or duct or outside.
A heated line to convey the sample gas from the stack but this may not be necessary if
probe dilution is used.
A cooler may be used to reduce moisture.
A further drier installed before the analyser.
A pump, situated before or after the analyser, as appropriate, to pull the gas from the
stack or duct.
A fine filter may be put immediately before the analyser.
A description of available methods for monitoring emissions is given:
ONLINE METHODS
Infra red spectrometry:
In the simplest form of IR spectrometry, the equipment consists of an optical filter, the
sample cell and a detector. When the wavelength of the radiation is not selected using
a prism or diffraction grating, the instrument is known as a non-dispersive infra red
gas analyzer (NDIR). In a single-beam instrument a filter selects the part of the
spectral range most characteristic of the substance. In a twin-beam instrument, (the
most commonly used for on-line analysis) the radiation from the source is split and a
comparison is made of the two beams after one has passed through a reference cell
and the other through the sample gas.
The two beams are brought together onto a half-silvered mirror or rotating chopper
which alternately allows each beam to reach a detector cell which compares the heat
received, by capacitance or resistance measurements. The twin-beam method is
preferred in an on-line system as it overcomes some of the problems associated with
drift due to small changes in detector sensitivity and in the optical and spectral
properties of the optical filter. However, regular zeroing and calibration are needed to
correct zero and range drift.
() www.final-yearproject.com | www.finalyearthesis.com
Chemiluminescence:
These instruments use the property of fluorescence, which can take place with some
chemical reactions. By selecting two gases to react under carefully controlled
conditions, the chemiluminescence can be measured to determine the concentration of
reacting gases.
NOx measurement makes use of the reaction:
NO + O3 -> NO2 + O2 + kv
The sample gas is passed through a catalytic converter to change any nitrogen dioxide
to nitric oxide and is then reduced in pressure and reacted with ozone. The
chemiluminescence (kv) is measured by a photomultiplier tube after passing through
an appropriate band pass filter.
MANUAL METHODS
Sulphur dioxide:
Standard methods rely on the solubility and acidic nature of the gas. An oxidizing
agent is generally used to convert the SO 2 to SO3. National standard methods include
the use of hydrogen peroxide solution as the oxidizing agent with titration against
standard alkali or gravimetric analysis using barium chloride and hydrochloric acid to
precipitate the sulphate. An alternative to this uses a sample collected in hydrogen
peroxide solution and titration against barium perchlorate with thorin as indicator. A
method which draws the filtered gas through a standard solution of iodine in
potassium iodide is also used. The unreacted iodine is determined by titration with
sodium thiosulphate and the SO2 calculated from the amount of iodine used to oxidise
the SO2.
All the methods have errors associated with the interferences and the user should be
knowledgeable about the method and its suitability.
Oxides of nitrogen
The instrumental methods given under online methods are preferred.
() www.final-yearproject.com | www.finalyearthesis.com
Carbon monoxide/carbon dioxide:
These gases are generally measured by solution absorption into liquid reagents
specific for each gas.
Emissions to Water:
Whilst emissions to water are likely to be intermittent and of a low level, it is likely that any
site operating an ammonia plant will have at least one overall consent for emissions to water
and a requirement for plant monitoring. Typical monitoring methods may rely on flow
proportioned sample collection or high frequency spot sampling and flow measurement. In
either case the samples obtained may be analyzed as follows:
Ammonia/Ammoniacal N :
The spectrophotometric method for ammonia relies on
the reaction in which monochloramine is reacted with phenol to form an indophenol
blue compound. This method is particularly suitable for the determination of ammonia
in cooling waters derived from saline sources (dock, estuarine or sea water) and may
be used in continuous flow colorimetry.
Ion selective electrodes can also be used and are suitable for saline applications as
well as pure water.
Note that free ammonia exists in equilibrium with NH4+ as follows:
NH4+ + H2O is in equilibrium with NH3 + H3O+
and that the equilibrium depends on pH. The above method determines the NH4+
ammonia. Free ammonia is particularly toxic to fish and should an incident occur, it
may be more important to relate the NH4+ result to free ammonia. Any suitable pH
determination may be used and the free ammonia estimated as given in Hampson B
L, J Cons Int Explor, Mer, 1977,37. 11 and Whitfield M, J Mar Biol. Ass UK,
1974,54, 562.
Manual laboratory based methods may be used for spot checks using Kjeldahl
methods for the determination of organic and ammoniacal nitrogen in a mineralized
sample.
() www.final-yearproject.com | www.finalyearthesis.com
Methanol:
The spectrophotometric method for methanol relies on the oxidation
reactions of potassium permanganate under acid conditions to give formaldehyde. The
formaldehyde is reacted with acetylacetone in the presence of excess ammonium salt,
to form diacetyldihydrolutidine. The method may be used in continuous flow
colorimetry or gas chromatography.
Achievable Emission Levels for New Plants :
The following emission levels can be achieved for new ammonia plants. These levels
relate to steady-state production, not accounting for peaks which can occur during the
unsteady transient conditions of start-up and shut-down and during emergencies.
Emissions to air
NOx (as NO2 at 3% O2)
ppmv
mg/Nm3
kg/t of product
75
150
0.45
Emissions to water
NH3 or NH4 (as N)
0.1kg/t of product
Waste material
Spent catalysts etc.
<0.2kg/t of product
In new reforming plants the total energy consumption should not exceed 32.5 GJ
(HHV)/t and process condensate recycle should be a pre-requisite.
COST OF POLLUTION CONTROL MEASURES
The costs of pollution control measures in the fertilizer industry are difficult to generalize as
they depend on a number of factors such as:
The emission target or standard to be met
The type of process, the degree of integration with other processes on site, production
volumes, type of raw materials being used etc.
whether the plant is new so that the design can be optimised with respect to pollution
abatement, or whether the plant is an existing one requiring revamping or end-of-pipe
pollution abatement equipment
Generally, it is more economical to incorporate the pollution abatement system at the process
design stage rather than revamping or adding-on equipment at later stage.
() www.final-yearproject.com | www.finalyearthesis.com
STORAGE, HANDLING AND TRANSOPRTATION OF AMMONIA
Storage:
Ammonia is stored in :
Cylindrical pressure vessels with dished ends called Bulets.
Spherical pressure vessels Horton Sphere.
Cylindrical, flat bottom Atmospheric storage tanks.
The choice of type of storage depends upon the quantity of ammonia to be stored.
Choice of Storage:
Large quantities of ammonia in storage constitute a potential hazard. Storage under
pressure involves considerable amounts of potential energy and because any escaping
liquid is above its atmospheric boiling point, large quantities of gaseous ammonia will be
released immediately into the atmosphere. Refrigerated storage, however, involves little
potential energy and if the ammonia is cooled to its atmospheric boiling point, there will
be no spontaneous production of gas from spilled liquid. Gas will, of course, still be
released partly due to the difference in partial pressure between the ammonia and the
atmosphere, and partly due to the heat gain from the surroundings. However, the amount
of gas liberated in this manner can be minimized by good binding.
For these reasons, a spill from an atmospheric storage tank constitutes lesser hazard than
a spill from a pressure tank. All ammonia storage installations are required to be
intrinsically safe. Nevertheless, the possibility of a spill cannot be excluded when
selecting a storage facility, and on a congested site, or on a site close to public roads,
hospitals, schools, or private dwellings, it would be advisable to choose potentially less
dangerous atmospheric storage.
It is unlikely that spheres greater than 3,000 tons capacity and working at pressure in the
order of 3.3kg/cm2 gauge will ever be built even if the building of such spheres were
economically attractive.
Handling and Transportation of Ammonia:
Anhydrous ammonia is transported in liquid form under pressure either in small cylinders, or
in road and rail tankers. A number of small ships are also in service capable of carrying
quantities upto 1,000 tons in pressure tanks. Recently, a number of marine carriers have
commenced shipping large quantities (upto 8,000 tons) of ammonia at atmospheric pressure
in refrigerated tanks specially built for the purpose. This practice is likely to grow.
The transfer of liquid between storage and carrier presents the greatest safety hazard. All
known serious accidents in the past few years have occurred during transfer operations.
Stringent safety precautions must be enforced at filling points.
() www.final-yearproject.com | www.finalyearthesis.com
SITE SELECTION AND PLANT LAYOUT
SITE SELECTION
The location of the plant can have a turning effect on the overall viability of a process plant,
and the scope for future expansion. Many factors must be considered when selecting a
suitable plant site. The most important factors are as follows:
1.
2.
3.
4.
5.
6.
7.
8.
9.
Location, with respect to the marketing area
Raw material supply
Transport facilities
Availability of labour
Availability of suitable land
Environmental impact and effluent disposal
Local community consideration
Climate
Political and strategic consideration
Location with Respect to Marketing Area:
For materials that are produced in bulk quantities where the cost of the product per tonne
is relatively low and the cost of transport a significant fraction of the sales price, the plant
should be located close to the primary market. This consideration will be less important
for low volume production, high-priced products like Pharmaceuticals.
Raw Materials:
The availability and price of suitable raw materials will often determine the site location.
Plants producing bulk chemicals are best located close to the source of the major raw material
where this is also close to the marketing area.
Transport:
The transport of materials and products to and from the plant will be an overriding
consideration in site selection. If practicable, a site should be selected such that is close to at
least two major forms of transport: road, rail, waterway (canal or river), or a sea port. Road
transport is being increasingly used, and is suitable for long- distance transport of bulk
chemicals. Air transport is convenient and efficient for the movement personnel and essential
equipment and supplies, and the proximity of the site to a major airport should be considered.
Availability of Labour:
Labour will be needed for construction of the plant and its operation. Skilled construction
workers will usually be brought in from outside the site area, but there should be an adequate
() www.final-yearproject.com | www.finalyearthesis.com
pool of unskilled labour availability locally and labour suitable for training to operate the
plant. Skilled tradesmen will be needed for plant maintenance. Local trade union customs and
restrictive practices will have to be considered when assessing the availability and suitability
of the local labour for recruitment and training.
Availability of Utilities (Services):
Chemical processes invariably require large quantities of water for cooling and general
process use, and the plant must be located near a source of water of suitable quality. Process
water may be drawn from a river, from wells, or purchased from a local authority. At some
sites, the cooling water required can be taken from a river or lake, or from the sea; at other
locations cooling towers will be needed.
Electrical power will be needed at all sites. Electrochemical processes that require large
quantities of power; need to be located close to a cheap source of power. A competitively
priced fuel must be available on site for steam and power generation.
Environmental Impact and Effluent Disposal:
All industrial processes produce waste products, and full consideration must be given to the
difficulties and cost of their disposal. The disposal of toxic and harmful effluents will be
covered by local regulations, and the appropriate authorities must be consulted during the
initial site survey to determine he standards that must be met. An environmental impact
assessment should be made for each new project or major modification or addition or an
existing process.
Local Community Considerations:
The proposed plant must be fit in with and be acceptable to the local community. Full
consideration must be given to the safe location of the plant so that it does not impose a
significant additional risk to the community. On a new site, the local community must be able
to provide adequate facilities for the plant personnel: schools, banks, housing, and
recreational and cultural facilities.
Availability of Land:
Sufficient suitable land must be available for the proposed plant and for future expansion.
The land should ideally be flat, well drained and have suitable load-bearing characteristics. A
full site evaluation would be made to determine the need for piling or other special
foundations.
Climate:
Adverse climatic conditions at a site will increase costs. Abnormally low temperatures will
require the provision of additional insulation and special heating for equipment and pipe runs.
Stronger structures will be needed at locations subject to high winds (cyclone/hurricane) or
earthquakes.
() www.final-yearproject.com | www.finalyearthesis.com
Political and Strategic Considerations:
Capital grants, tax concessions, and other inducements are often given by governments to
direct new investment to preferred locations such as areas of high unemployment. The
overriding of such grants can be the overriding considerations in site selection.
In addition to the main plant, we also have to consider the associated services which have to
be amalgamated within a particular plant site. Canteens, parks, general utilities, emergency
medical services and places for storage must also be taken into consideration while deciding
on a particular site.
Actual Site Selection:
The plant has to be located at a place where cost of raw materials is less. Another factor
which has to be considered is demand for product in vicinity of production area. Reliance in
partnership with Canadian firm Niko Resources has discovered significant gas reserves in
Krishna Godavari basin estimated at 7 trillion cubic feet. Also demand for fertilizers in this
region is also high. According to FAI (2000) data, consumption of N + P2O5 + K2O is 154.9
kg/ha. This reserve is one of the biggest Natural Gas reserve of India and will amount to
around 50% of Indias total Natural Gas consumption.
We hence propose the site for the new Ammonia plant to be Kakinada Industrial
Area,Andhra Pradesh. The major reasons in support of this selection are as follows:
Available Market:
According to FAI (Fertilizer Association of India) reports, the region wise consumption of
Nitrogen based Fertilizers in the Southern Region is around 26% of the total consumption of
India, while only around 10% of the total Ammonia Production Capacity lies in the Southern
Region. So Southern Region has to import it from other Regions. Hence a suitable market is
present.
Availability of Raw Materials:
The huge reserves of Natural Gas at the Krishna River Basin in Andhra Pradesh, provides a
suitable source of our major raw material.
Availability of Transport, Labor & Utilities:
As the area is itself an industrial area, so infrastructure for all these is sufficient. Cheap labour
is easily available. Power and other utilities are also available at a reduced rate.
Availability of Land:
() www.final-yearproject.com | www.finalyearthesis.com
The area being an industrial area, the procurement of land should not be a problem.
Political considerations:
As Andhra Pradesh is a Industrially Oriented state, so there are no problems. Infact the state
encourages setup of industries and gives some special exemptions like lower taxes, etc.
PLANT LAYOUT
The economic construction and operation of a process unit will depend on how well the plant
equipment specified on the process flow sheet and laid out. The principal factors to be
considered are:
1.
2.
3.
4.
5.
6.
Economic consideration: construction and operation cost.
The process requirement
Convenience of operation
convenience of maintenance
Safety
Future expansion
Costs:
The cost of construction can be minimized by adopting a layout that gives shortest run of
connecting pipes between equipment, and adopting the least amount of structural steel work.
However, this will not necessarily be the best arrangement for operation and maintenance.
Process Requirement:
All the required equipment have to be placed properly within process. Even the installation of
the auxiliaries should be done in such a way that it will occupy the least space.
Operation:
Equipment that needs to have frequent operation should be located convenient to the control
room. Valves, sample points, and instruments should be located at convenient position and
height. Sufficient working space and headroom must be provided to allow easy access to
equipment.
Maintenance:
Heat exchangers need to be sited so that the tube bundles can be easily withdrawn for
cleaning and tube replacement. Vessels that require frequent replacement of catalyst or
() www.final-yearproject.com | www.finalyearthesis.com
packing should be located on the outside of buildings. Equipment that requires dismantling
for maintenance, such as compressors and large pumps, should be placed under cover.
Safety:
Blast walls may be needed to isolate potentially hazardous equipment, and confine the effects
of an explosion. At least two escape routes for operator must be provided from each level in
the process building.
Plant Expansion:
Equipment should be located so that it can be conveniently tied in with any future expansion
of the process. Space should be left on pipe alleys for future needs, service pipes oversized to
allow for future requirements.
PLANT LAYOUT
Main Sections:
A complex chemical plant is divided into sections to limit the capital at risk, some of these
areas should be located in areas where there is no fire risk. Care should be taken to give
enough vacant space so as to avoid a chain reaction during the times of fire ao any other
emergencies.
Pipe Bridges:
Equipments can be under pipe bridges but avoid large inventories of flammable materials or
say pumps, moving liquids above atmospheric temperature. Enough bends should be given
for the lines carrying hot liquids, otherwise thermal expansion may damage the lines.
Loading and Off-loading:
Locate the loading and offloading facilities at the terminal parts of the plant so that regular
traffic through the plant is minimized. Road tanker loading or offloading should be classified
as danger areas and should be located at the periphery of the plant. These facilities should be
so located that they are close to the railway terminal.
Plant Roads:
Plant roads should give access to plants and should be arranged so that vehicles do not pass
through process areas. Dead end roads should be avoided while access from any point to any
part of the plant should be from two directions. Hazardous areas should not overlap the plant
limits.
Administrative Block:
() www.final-yearproject.com | www.finalyearthesis.com
This can be located at any place, but preferably at a place away from the plant. Normally it is
kept at an enclosure slightly away from the production units. It should house the offices and
rooms meant for conferences and emergency meetings. A refreshment room can also be
provided in the building.
() www.final-yearproject.com | www.finalyearthesis.com
8
12
5
4
PLANT LAYOUT
() www.final-yearproject.com | www.finalyearthesis.com
NOMENCLATURE OF VARIOUS PLACES SHOWN IN THE ABOVE PLANT
LAYOUT
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
Administrative Block
Fire & Safety Department
Plant Utilities
Emergency Water
Packaging Plant
Process Plant
Expansion
Laboratory
Store
Stray Yard
Canteen
Storage Vessels
Workshops
Connecting Roads
() www.final-yearproject.com | www.finalyearthesis.com
COST ESTIMATION
Capacity of plant = 200 tonnes per day
Cost (FCI) in 1990 for 100,000 tonnes per year for ammonia plant = $ 20 million.
=
$ 20(200/273.9)0.53 million
$ 16.9298 million
Cost index of 1990
356
Cost index for 2004
420
Cost (FCI) in 1990 for 200 tonnes per day
Cost of ammonia plant of capacity 200 TPD in 2004 = 19.973 million dollar
i.e., Fixed Capital Cost (FCI)
= Rs. 918775150
Estimation of total investment cost:
1) Direct cost:
a) Purchased equipment cost:
Range: (15 40% of FCI )
Assume 20% of FCI
PEC = Rs 183755030
b) Installation cost :
Range: (35 45% of PEC)
Assume 35% of PEC
Installation cost = Rs 64314260.5
c) Instrument and control installed:
Range: (6 30% of PEC)
Assume 25% of PEC
() www.final-yearproject.com | www.finalyearthesis.com
Instrumentation and control cost = Rs 45938757.5
d) Piping installation cost:
Range: (10 80% of PEC)
Assume 60% of PEC
= Rs. 110253018
e) Electrical installation cost:
Range: (10 40% of PEC)
Assume 35% of PEC
= Rs 64314260.5
f) Building process and auxilliary:
Range:(10-70% of PEC)
Assume 60%
=Rs 110253018
g) Service facilities and yard improvement:
Range: (30-80% 0f PEC)
Assume 60% of PEC
= Rs 110253018
i) Land:
Range: (4-8% of PEC)
Assume 6%
= Rs 11025301.8
Therefore direct cost = Rs 700106664.3
() www.final-yearproject.com | www.finalyearthesis.com
Indirect cost:
Expenses which are not directly involved with material and labour of actual installation or
complete facility
a) Engineering and supervision:
Range: (5-30% of DC)
Assume 25%
= Rs 175026666.1
b) Construction expenses and contractors fee:
Range: (6-22 % of DC)
Assume 16 %
= Rs 112017066.3
c) Contingency:
Range: (8-20% of DC)
Assume 12%
= Rs 84012799.72
Therefore total indirect cost = Rs 371056532.1
Fixed capital investment:
Fixed capital investment(FCI) = DC+IC
= Rs 1071163196
Working Capital = 10 20% of FCI
Assume 16%
() www.final-yearproject.com | www.finalyearthesis.com
WC = Rs 171386111.4
2) Total capital investment:
= FCI + WC
=Rs 124549308
Estimation of total product cost (TPC):
Fixed charges:
a) Depreciation: (depends on life period, salvage value and method of calculationabout 10% of FCI for machinery and equipment and 2-3% for Building Value for
Buildings)
(10% of FCI for machinery)
= Rs 107116319.6
b) Local taxes:
Range: (3-4% of TPC)
Assume 3%
= Rs 32134895.9
c) Insurances:
Range: (0.4-1% of FCI)
Assume 0.7%
= Rs 7498142.4
d)Rent:
Range: (8-12% of FCI)
Assume 10%
= Rs 107116319.6
() www.final-yearproject.com | www.finalyearthesis.com
Therefore total fixed charges = Rs 253865677.5
Direct Production Cost:
Fixed charges = (10-20% of TPC)
Assume 15%
Therefore Total product cost (TPC) = Rs. 1692437850
Direct production:
a) Raw material:
Range: (10-50% 0f TPC)
Assume 40%
= Rs 676975140.1
b) Operating labour (OL):
Range: (10-20% of TPC)
Assume 15%
= Rs 253865677.5
c) Direct supervisory and electric labour:
Range: (10-25% of OL)
Assume 20%
= Rs 50773135.5
d) Utilities:
Range: (10-20% of TPC)
Assume 15%
= Rs 253865677.5
() www.final-yearproject.com | www.finalyearthesis.com
e) Maintenance:
Range: (2-10% of FCI)
Assume 8%
= Rs 86693055.7
f) Operating supplies (OS):
Range: (10-20% of maintenance)
Assume 15%
= Rs 12853958.4
g) Laboratory charges:
Range: (10-20% of OL)
Assume 12%
= Rs 30463881.3
h) Patent and royalties:
Range: (2-6% of TPC)
Assume 4%
= Rs 67697514.0
Direct production cost = Rs 1432188040
Plant overhead cost:
50-70% of (OL+OS+M). It includes the following: general plant upkeep and
overhead, payroll overhead, packaging, medical services, safety and protection, restaurants,
recreation, salvage, laboratories, and storage facilities
Assume 65%
= Rs 229068249.5
() www.final-yearproject.com | www.finalyearthesis.com
Thus, Manufacture cost = Direct production cost + Fixed charges + Plant overhead costs.
Manufacturing cost = 1915121967
General expenses:
a) Administration cost:
Range: (40-60% of OL)
Assume 50%
= Rs 126932838.8
b) Distribution and selling price:
Range: (2-30% of TPC)
Assume 20%
= Rs 338487570.1
c) Research and development cost:
Range: (3% of TPC)
= Rs 50773135.51
d) Financing ( Interest):
Range: ( 0-10 % Of TCI)
Assuming 5 % of TCI
= Rs 62127465.4
Therefore general expenses (GE) = Rs 578321009.7
Total production cost:
Total production cost = MC + GE
= Rs 2493442977
Gross earnings and rate of return:
The plant is working for say 340 days a year
Selling price of ammonia =Rs. 40 /kg
() www.final-yearproject.com | www.finalyearthesis.com
Total income = 1500340100035 = Rs 2720000000
Gross income = Total income total product cost
= Rs 226557023.1
Let the Tax rate be 30% (common)
Tax = 30%
Tax = Rs 67967106.9
Net Profit = Gross income - Taxes = Gross income (1- Tax rate)
= Rs 158589916.1
Rate of return = net profit*100 / total capital investment
= 12.76 %
Break even Analysis:
Annual Direct Production Cost = Rs. 1432188040
Annual Fixed charges, overhead and general expenses = Rs. 1061254937
Total Annual sales = Rs. 2720000000
Selling Price of ammonia per tonne. = Rs. 40 000
Direct production cost per ton of ammonia = Rs. 21061.6 per ton
Let n TPA be the break even production rate.
Number of tons needed for a break-even point is given by
n = 56037.2 tons/year
n = 164.82 tons/day = 164.2 TPD
Hence, the break-even production rate is 164.2 TPD or 82.4 % of the considered plant
capacity.
() www.final-yearproject.com | www.finalyearthesis.com
CONCLUSION
The cost analysis shows that Ammonia is such a product whose costs are highly
variable depending on the costs of the raw materials, especially hydrogen.
The heat in the plant is integrated through a network of heat exchangers so that minimum
amount of steam is to be produced.
The product costing showed a rate of return of 12.75% which means that ammonia plant of
given capacity is profitable to operate.
The product quality depends on the maintenance of appropriate temperature & pressure
conditions in the reactors.
Various PID controllers in DDCS mode are used at different stages.
() www.final-yearproject.com | www.finalyearthesis.com
BIBLIOGRAPHY
1. Peters, Max S. and Timmerhaus, Klaus D., Plant Design & Economics, 4th edition,
McGraw Hill, Inc. (1980)
2. Wilbrant, Frank C. & Dryden, Charles E., Chemical Engineering Plant Design, 4th
edition, McGraw Hill, Inc. (1980)
3. Coulson, J. M. & Richardson, J. F., Chemical Engineering, Volume 6
4. Perry, J.H., Chemical Engineers Handbook, 7th edition, McGraw Hill, Inc. (1985)
5. Brownell, L.E. and Young, E.H., Process Equipment Design, 1st edition, Wiley, Prentice
Hall (1962)
6. McCabe, Warren L., Smith, Julian C., and Harriot, Peter, Unit Operations of
Chemical Engineering, 5th edition, Pergamon Press (1983)
7. Strelzoff Samuel L., Technology and manufacture of ammonia, Wiley
Interscience Publications.
8. Treybal R .E. Mass Transfer Operations, III Edition, McGraw Hill, Inc (1981)
8. IFFCO KALOL Ammonia plant manual.
9. Rase Howard F., Chemical Reactor Design for Process Plant, Wiley Interscience
Publications. (1977)
9. McKetta, John J. and Cunninghum, William A., Encyclopedia of Chemical
Processing & Design, Revised edition, Marcel Deker Inc.(1984)
10. www.chemical-engineering-design.com
Vous aimerez peut-être aussi
- Ammonia PlantDocument16 pagesAmmonia Plantganeshan67% (6)
- Chemical Ammonia Report PDFDocument72 pagesChemical Ammonia Report PDFAli J. Hojeij100% (1)
- Production of AmmoniaDocument29 pagesProduction of AmmoniaBhavna Bajpai83% (6)
- Chemical Ammonia ReportDocument75 pagesChemical Ammonia Reportibiceng100% (1)
- Ammonia-Urea Industry in BangladeshDocument28 pagesAmmonia-Urea Industry in BangladeshHumayun Rashid Khan100% (1)
- 2500 MTPD of Ammonia From Naphtha: Nfc-Iet MultanDocument120 pages2500 MTPD of Ammonia From Naphtha: Nfc-Iet MultanKashan Aslam100% (2)
- Ammonia PlantDocument10 pagesAmmonia PlantHemal Patel Sam100% (3)
- Ammonia Plant Design For 1 MtpaDocument43 pagesAmmonia Plant Design For 1 MtpaPrateek Mall67% (3)
- KRIBHCO SHYAM FERTILISERS LTD AMMONIA PROCESS OVERVIEWDocument51 pagesKRIBHCO SHYAM FERTILISERS LTD AMMONIA PROCESS OVERVIEWSaad Khan89% (9)
- ENGINEERING DESIGN GUIDELINES Ammonia Plant Rev2.2web PDFDocument23 pagesENGINEERING DESIGN GUIDELINES Ammonia Plant Rev2.2web PDFazzatul amira0% (1)
- Ammonia Plant Material Balance PresentationDocument66 pagesAmmonia Plant Material Balance Presentationsagar dasgupta100% (1)
- Introduction to India's Fertilizer IndustryDocument11 pagesIntroduction to India's Fertilizer IndustryrvnesariPas encore d'évaluation
- Ammonia Plant Fundamentals PDFDocument27 pagesAmmonia Plant Fundamentals PDFMubarik Ali100% (1)
- Process Flow Diagram of A HALDOR TOPSOE Process Ammonia PlantDocument34 pagesProcess Flow Diagram of A HALDOR TOPSOE Process Ammonia PlantJatinder Saini81% (32)
- Ammonia ManualDocument389 pagesAmmonia Manualahmed100% (3)
- Ammonia TechnologyDocument13 pagesAmmonia TechnologyBai Singh100% (4)
- Ammonia Urea Mass BalanceDocument44 pagesAmmonia Urea Mass BalanceSenthil NathanaPas encore d'évaluation
- Ammonia Plant DescriptionDocument41 pagesAmmonia Plant Descriptionhussainbeds75% (4)
- Ammonia Technology TodayDocument22 pagesAmmonia Technology Todayrvnesari100% (3)
- Tkis AmmoniaDocument28 pagesTkis AmmoniaAhmed Nagy100% (1)
- Ammonia Energy 2520 BalanceDocument7 pagesAmmonia Energy 2520 Balanceapi-3714811Pas encore d'évaluation
- AmmoniaDocument69 pagesAmmoniaGanesh Kumar100% (1)
- Electric Heaters For Safe Startup ofDocument9 pagesElectric Heaters For Safe Startup ofSteve WanPas encore d'évaluation
- KBR - Ammonia Specific ExamplesDocument16 pagesKBR - Ammonia Specific Examplesqwerty9123460% (5)
- Packaged Air Separation PlantsDocument12 pagesPackaged Air Separation PlantsSrinivas VemulapalliPas encore d'évaluation
- Urea PlantDocument58 pagesUrea PlantMarcusWerteck100% (3)
- Ammonia Storage Tank Group No 18Document17 pagesAmmonia Storage Tank Group No 18Anonymous Xf4w0D2cPas encore d'évaluation
- Final ReportDocument46 pagesFinal ReportVarun Gupta100% (1)
- Ammonia AspenDocument24 pagesAmmonia AspenMohammadAlAmeenPas encore d'évaluation
- 4000 MTPD Ammonia Plant PaperDocument8 pages4000 MTPD Ammonia Plant PaperJose DenizPas encore d'évaluation
- KFCL Ammonia Production Training ReportDocument32 pagesKFCL Ammonia Production Training Reportrohit100% (1)
- Ammonia Plant Basic EquationsDocument87 pagesAmmonia Plant Basic Equationschichosango100% (2)
- Ammonia SOPDocument51 pagesAmmonia SOPShamsul Azhar SulaimanPas encore d'évaluation
- Introduction to Urea: Properties, Production, and ApplicationsDocument70 pagesIntroduction to Urea: Properties, Production, and Applicationsravichem823Pas encore d'évaluation
- Ammonia & Ammonia Solution STORAGE AND HANDLINGDocument28 pagesAmmonia & Ammonia Solution STORAGE AND HANDLINGmah_abdelaalPas encore d'évaluation
- Co2 StripperDocument5 pagesCo2 StripperOkta Ochan Chandra100% (1)
- AmmoniaDocument9 pagesAmmoniaAditya Anugerah Putra100% (1)
- Ammonia ProductionDocument44 pagesAmmonia ProductionBalamurali BalamPas encore d'évaluation
- Methanol Plant Experience in RussiaDocument14 pagesMethanol Plant Experience in RussiaFer MugrabiPas encore d'évaluation
- Design For A High Temperature Shift ConverterDocument43 pagesDesign For A High Temperature Shift ConverterAaron GyamfiPas encore d'évaluation
- K-Green™: KBR'S Green Ammonia TechnologyDocument23 pagesK-Green™: KBR'S Green Ammonia Technologynixon manik100% (1)
- Cryogenic Separation Plants PDFDocument20 pagesCryogenic Separation Plants PDFHemanth Kumar Sarosh KiranPas encore d'évaluation
- Material balance for nitric acid production processDocument4 pagesMaterial balance for nitric acid production processyogeshdama100% (1)
- Ammonia Plant 2520Location&LayoutDocument4 pagesAmmonia Plant 2520Location&Layoutapi-3714811100% (1)
- Industrial Refrigeration Ammonia & CO2 Applications - Handbook Part IIDocument70 pagesIndustrial Refrigeration Ammonia & CO2 Applications - Handbook Part IIBaher SalehPas encore d'évaluation
- Conversion of SulfinolSM To BASF's aMDEADocument12 pagesConversion of SulfinolSM To BASF's aMDEAAna Carolina AbrantesPas encore d'évaluation
- Urea SynthesisDocument10 pagesUrea SynthesisCHEMICALPas encore d'évaluation
- Casale Advanced Ammonia TechnologiesDocument35 pagesCasale Advanced Ammonia TechnologiesMaribel ParragaPas encore d'évaluation
- Ammonia Safety ReviewDocument57 pagesAmmonia Safety ReviewFABIO MOACIR KORNDOERFERPas encore d'évaluation
- PurifierDocument29 pagesPurifierRaghvendra Pratap Singh100% (1)
- SULPHURIC ACID PLANT PROCESSoDocument33 pagesSULPHURIC ACID PLANT PROCESSojagadeesh100% (2)
- Olmi Urea Stripper & Carbamate Condenser LeafletDocument6 pagesOlmi Urea Stripper & Carbamate Condenser LeafletHamzaNoumanPas encore d'évaluation
- Sulfuric Acid Manufacture: Analysis, Control and OptimizationD'EverandSulfuric Acid Manufacture: Analysis, Control and OptimizationÉvaluation : 3.5 sur 5 étoiles3.5/5 (3)
- Fuel Property Estimation and Combustion Process Characterization: Conventional Fuels, Biomass, Biocarbon, Waste Fuels, Refuse Derived Fuel, and Other Alternative FuelsD'EverandFuel Property Estimation and Combustion Process Characterization: Conventional Fuels, Biomass, Biocarbon, Waste Fuels, Refuse Derived Fuel, and Other Alternative FuelsPas encore d'évaluation
- Ammonia Plant DesignDocument75 pagesAmmonia Plant Designrkm_rkmPas encore d'évaluation
- 7212 0Document11 pages7212 0nguyenPas encore d'évaluation
- Ammonia Properties and HandlingDocument25 pagesAmmonia Properties and HandlingJohn Harken100% (2)
- Ammonia ScrubbersDocument7 pagesAmmonia Scrubbersangelo pascuaPas encore d'évaluation
- Chemical Reaction Engineering: INTRODUCTION TO COMPANY (Pak American Fertilizers LTD.)Document24 pagesChemical Reaction Engineering: INTRODUCTION TO COMPANY (Pak American Fertilizers LTD.)Badar RasheedPas encore d'évaluation
- Ammonia and Urea Production PDFDocument10 pagesAmmonia and Urea Production PDFئارام ناصح محمد حسێنPas encore d'évaluation
- Historic DeLand Building Earns LEED CertificationDocument30 pagesHistoric DeLand Building Earns LEED Certificationlockas222Pas encore d'évaluation
- 04 - AbsorbersDocument11 pages04 - AbsorbersRafael ReyesPas encore d'évaluation
- Germany Guideline SBDocument123 pagesGermany Guideline SBlockas222Pas encore d'évaluation
- Supply Chain QuestionDocument22 pagesSupply Chain Questionlockas22280% (5)
- PDC Chapter 10.1Document16 pagesPDC Chapter 10.1lockas222Pas encore d'évaluation
- Green Building RevolutionDocument229 pagesGreen Building Revolutionlockas222Pas encore d'évaluation
- Design of Acetone HYSYSDocument6 pagesDesign of Acetone HYSYSlockas222100% (1)
- Manufacturing Process FertilizerDocument14 pagesManufacturing Process FertilizermoentaseerPas encore d'évaluation
- 04 - AbsorbersDocument11 pages04 - AbsorbersRafael ReyesPas encore d'évaluation
- MVC Desalination Process Modeling and AnalysisDocument44 pagesMVC Desalination Process Modeling and Analysislockas222Pas encore d'évaluation
- Fluid Mechanics IntroductionDocument49 pagesFluid Mechanics IntroductionIastraPas encore d'évaluation
- Chapter 4 Shell and Tube Heat ExchangersDocument45 pagesChapter 4 Shell and Tube Heat Exchangerslockas222Pas encore d'évaluation
- Manufacturing Process FertilizerDocument14 pagesManufacturing Process FertilizermoentaseerPas encore d'évaluation
- Chapter 4Document106 pagesChapter 4lockas222Pas encore d'évaluation
- PDC CH 10.3 (Problems)Document6 pagesPDC CH 10.3 (Problems)lockas222Pas encore d'évaluation
- Chapter 7Document28 pagesChapter 7lockas222Pas encore d'évaluation
- Tech - 16a2d0707412ca70pct 100 071Document2 pagesTech - 16a2d0707412ca70pct 100 071lockas222Pas encore d'évaluation
- PCT100Document2 pagesPCT100lockas222Pas encore d'évaluation
- Start AgainDocument1 pageStart Againlockas222Pas encore d'évaluation
- Start AgainDocument1 pageStart Againlockas222Pas encore d'évaluation
- BackgroundDocument9 pagesBackgroundlockas222Pas encore d'évaluation
- Acetone BDocument9 pagesAcetone BIrdani IdrisPas encore d'évaluation
- A Summary of Thermodynamic FundamentalsDocument11 pagesA Summary of Thermodynamic Fundamentalslockas222Pas encore d'évaluation
- Neraca Massa ATKDocument49 pagesNeraca Massa ATKMuhammad KholidinPas encore d'évaluation
- Neraca Massa ATKDocument49 pagesNeraca Massa ATKMuhammad KholidinPas encore d'évaluation
- BackgroundDocument9 pagesBackgroundlockas222Pas encore d'évaluation
- Material BalanceDocument42 pagesMaterial Balancealireza_e_20% (1)
- Design and Construction of A Water Scrubber For The Upgrading of BiogasDocument6 pagesDesign and Construction of A Water Scrubber For The Upgrading of BiogasPreetham BharadwajPas encore d'évaluation
- Energies: Combustion and Heat Release Characteristics of Biogas Under Hydrogen-And Oxygen-Enriched ConditionDocument11 pagesEnergies: Combustion and Heat Release Characteristics of Biogas Under Hydrogen-And Oxygen-Enriched Conditionjuan_891211Pas encore d'évaluation
- Optimization of LNG RegasificationDocument30 pagesOptimization of LNG RegasificationShashwat OmarPas encore d'évaluation
- 1100-0111 enDocument14 pages1100-0111 enMohamed HamdallahPas encore d'évaluation
- (HIL-12) Integrated Procs SysDocument11 pages(HIL-12) Integrated Procs SysNicolas Esguerra HernandezPas encore d'évaluation
- 7 Urea Synthesis Using Chemical Looping ProcessDocument10 pages7 Urea Synthesis Using Chemical Looping ProcessandiesPas encore d'évaluation
- Molecular Models ActivityDocument28 pagesMolecular Models ActivityMa. Celeste LipPas encore d'évaluation
- Chemistry Test 1 July 2019 BatchDocument3 pagesChemistry Test 1 July 2019 BatchDaPas encore d'évaluation
- Hydrogen Production Steam Methane ReformingDocument4 pagesHydrogen Production Steam Methane ReformingrhyantoPas encore d'évaluation
- Dissolved Gas Analysis in Tap-Changer Oil: Product InformationDocument4 pagesDissolved Gas Analysis in Tap-Changer Oil: Product InformationHiro ItoPas encore d'évaluation
- GCSE Style Questions Crude OilDocument19 pagesGCSE Style Questions Crude OilSteve Bishop100% (4)
- Module 1.2 Chem Eng-Converted (1Document9 pagesModule 1.2 Chem Eng-Converted (1Nuclear PotatoesPas encore d'évaluation
- Biogas Technology: Construction, Utilization and Operation ManualDocument52 pagesBiogas Technology: Construction, Utilization and Operation ManualDwi Cahyo NugrohoPas encore d'évaluation
- Utilization of Aspen Hysys SimulationDocument6 pagesUtilization of Aspen Hysys SimulationKhuram MaqsoodPas encore d'évaluation
- 1 Hour Bullish Divergence ChartinkDocument35 pages1 Hour Bullish Divergence ChartinksaiPas encore d'évaluation
- Methane Hydrate - The World's Largest Natural Gas ResourceDocument5 pagesMethane Hydrate - The World's Largest Natural Gas ResourceThePomboPas encore d'évaluation
- 5-E-Mass Transfer I PDFDocument2 pages5-E-Mass Transfer I PDFMani SinghPas encore d'évaluation
- Direct observation of rapid permafrost soil carbon lossDocument1 pageDirect observation of rapid permafrost soil carbon lossAngela M.Pas encore d'évaluation
- Separación de Metano y NitrógenoDocument6 pagesSeparación de Metano y NitrógenoAnonymous jqevOeP7Pas encore d'évaluation
- Introductiontogastransportation 161222145926 PDFDocument26 pagesIntroductiontogastransportation 161222145926 PDFAl Jawad100% (1)
- Guide to Calculating Greenhouse Gas EmissionsDocument61 pagesGuide to Calculating Greenhouse Gas EmissionsAnthony McdonaldPas encore d'évaluation
- Waras Budi Santosa, Head of Monetization Division Oil & Gas, SKK MigasDocument16 pagesWaras Budi Santosa, Head of Monetization Division Oil & Gas, SKK MigasShah Reza DwiputraPas encore d'évaluation
- Tutorial Questions On Free Radical Reactions PDFDocument3 pagesTutorial Questions On Free Radical Reactions PDFHarry ZgamboPas encore d'évaluation
- Heat EngineDocument27 pagesHeat Engineali105Pas encore d'évaluation
- Catalysts 09 00722Document21 pagesCatalysts 09 00722daraj darajPas encore d'évaluation
- IBERDROLA GÜIRIA - SGT6-5000F(4) GENERIC CORRECTION CURVESDocument12 pagesIBERDROLA GÜIRIA - SGT6-5000F(4) GENERIC CORRECTION CURVESENMANUELPas encore d'évaluation
- Report - CBMDocument57 pagesReport - CBMYash GurjarPas encore d'évaluation
- Effect of climate change on human health and adaptive strategiesDocument6 pagesEffect of climate change on human health and adaptive strategiesDivina TamayoPas encore d'évaluation
- ResearchWhitepaper Green Energy Report - pdfv1Document42 pagesResearchWhitepaper Green Energy Report - pdfv1Haitham AskarPas encore d'évaluation
- Simulation and Experimental Results of PSA Process For Production of Hydrogen Used in Fuel CellsDocument18 pagesSimulation and Experimental Results of PSA Process For Production of Hydrogen Used in Fuel Cellswww.beatricechongPas encore d'évaluation