Académique Documents
Professionnel Documents
Culture Documents
tmp4B79 TMP
Transféré par
FrontiersDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
tmp4B79 TMP
Transféré par
FrontiersDroits d'auteur :
Formats disponibles
ARTICLE
Received 23 Sep 2014 | Accepted 3 Mar 2015 | Published 10 Apr 2015
DOI: 10.1038/ncomms7824
OPEN
Enhancing grain boundary ionic conductivity in
mixed ionicelectronic conductors
Ye Lin1, Shumin Fang1, Dong Su2, Kyle S. Brinkman3 & Fanglin Chen1
Mixed ionicelectronic conductors are widely used in devices for energy conversion and
storage. Grain boundaries in these materials have nanoscale spatial dimensions, which can
generate substantial resistance to ionic transport due to dopant segregation. Here, we report
the concept of targeted phase formation in a Ce0.8Gd0.2O2 ! dCoFe2O4 composite that
serves to enhance the grain boundary ionic conductivity. Using transmission electron
microscopy and spectroscopy approaches, we probe the grain boundary charge distribution
and chemical environments altered by the phase reaction between the two constituents. The
formation of an emergent phase successfully avoids segregation of the Gd dopant and
depletion of oxygen vacancies at the Ce0.8Gd0.2O2 ! dCe0.8Gd0.2O2 ! d grain boundary. This
results in superior grain boundary ionic conductivity as demonstrated by the enhanced
oxygen permeation flux. This work illustrates the control of mesoscale level transport
properties in mixed ionicelectronic conductor composites through processing induced
modifications of the grain boundary defect distribution.
1 Department of Mechanical Engineering, University of South Carolina, Columbia, South Carolina 29208, USA. 2 Center for Functional Nanomaterials,
Brookhaven National Laboratory, Upton, New York 11973, USA. 3 Department of Materials Science and Engineering, Clemson University, Clemson,
South Carolina 29634, USA. Correspondence and requests for materials should be addressed to K.S.B. (email: ksbrink@clemson.edu) or to F.C.
(email: chenfa@cec.sc.edu).
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
ixed ionicelectronic conductors (MIECs) are widely
used in semiconductors, electrochemical energy storage
materials, electrodes of fuel cells and batteries, separation membranes and catalysts with various requirements for
chemical, electrical, thermal and mechanical properties111.
Single-phase MIECs seldom fulfill all of the requirements for
these diverse applications. For example, typical perovskite-type
MIECs exhibit structural instability in the extreme oxygen
chemical potential gradients encountered in specific
applications, including their use as anodes for solid oxide fuel
cells and membrane reactors for partial oxidation of natural gas12.
Composite MIEC materials consisting of separate ionic and
electronic conductive phases offer a promising and robust
material solution to this challenge. Property tuning appears
to be flexible and straightforward in dual-phase MIECs
(DP-MIECs), as both the ionic conductors and the electronic
conductors have been well developed for many decades1316.
However, the performance of DP-MIECs is not necessarily
controlled by the properties of individual constituents. Phase
interactions and altered interfaces such as grain boundaries may
play a key role in determining the overall device performance.
Grain boundaries are believed to dominate the overall ionic
conductivity through structural disorder, solute segregation,
oxygen vacancy depletion and the formation of precipitates
from extraneous phases17,18. In the vicinity of extended structural
defects such as grain boundaries or edge dislocations, the
concentration of each type of point defect is controlled by the
difference in the standard chemical potential between the grain
interior and the grain boundary/dislocation core1921. A wellstudied example is Ce0.8Gd0.2O2 ! d (CGO), which is an excellent
choice for an oxygen ionic conductor due to its high oxygen ionic
conductivity and structural stability. Doping with Gd3 results in
negatively charged gadolinium dopants on Ce4 lattice sites
0
), which are charge compensated by positively charged
(GdCe
oxygen vacancies (VO:: ). These defect pairs form defect associates
due to Coulombic attraction, leading to an inhomogeneous
distribution of charges and an inherent increase in the barrier for
oxygen ion transport2225. In particular, a positive space charge
potential is observed in the grain boundary core of CGO,
indicative of an accumulation of positively charged oxygen
vacancies26. The characteristics of a space charge potential barrier
extending from the grain interior to grain boundary core include
an increase in the Gd/Ce atomic ratio and the Ce M5/M4 ratio,
together with a decrease in the O/Ce atomic ratio. For example,
the grain boundary core stoichiometry of Ce0.8Gd0.2O2 ! d has
been reported to be Ce0.590.04Gd0.410.04O1.240.17 (ref. 26).
This results in the depletion of oxygen vacancies in the space
charge layer, and leads to a decrease in the grain boundary
conductivity by several orders of magnitude as compared with the
bulk conductivity in single-phase CGO27,28.
Compared with single-phase CGO, the ionicelectronic transport paths in CGO-based DP-MIECs should be more complex
due to the emergence of new phases with additional grain
boundary interfaces. In addition, the structure, composition and
charge distribution in the grain boundaries can also change
during fabrication of multiphase composites. Additional phases
may be formed due to cation migration between the starting
components at elevated temperature. New crystalline phases have
the potential to either promote or obstruct ionicelectronic
transport, depending on their structures and compositions29. The
emergence of additional crystalline phases, therefore, provides an
opportunity for modification of the inherent ionic transport
barriers in the starting phases, such as the space charge-induced
depletion of oxygen vacancies in the CGOCGO grain boundary.
However, the charge distribution at the CGOCGO grain
boundary has not yet been investigated in DP-MIECs.
2
DP-MIECs based on CGOAFe2O4 (A Ni, Co or Mn)
possess both high tolerance to CO2 and significant oxygen
permeation performance, making them very attractive for
post-combustion carbon capture and sequestration3034, as well
as anode for solid oxide fuel cells. Therefore, we have chosen
CGOCoFe2O4 (CFO) as a model system for general MIEC
composite studies. We have investigated the influence of
emergent crystalline phase formation on the defect structure
at the grain boundary of the ionic conductive component
CGO. Lessons learned from these investigations will result in
significant benefit to related fields on the basis of ionic
conduction processes for energy conversion and storage such as
fuel cells and batteries.
Although the space charge layer effects have been observed for
decades in single-phase CGO, it has never been completely
controlled or modified due to the experimental difficulties
associated with the small spatial size of the grain boundaries
and oxygen ions (B150 and 0.14 nm, respectively), together
with the light weight of oxygen ions20,27,35. Microstructure and
chemical composition analysis at the atomic scale relies on
techniques such as scanning transmission electron microscopy
(STEM) coupled with electron energy loss spectroscopy (EELS).
STEM-EELS is capable of simultaneously mapping the atomic/
electronic structure of light elements such as oxygen at adequate
spatial resolution. Although the polycrystalline nature of the
composites makes it difficult to obtain an atomic resolution image
of both the CGO and CFO across the grain boundary, the
electronic state of the elements across the boundary can be
identified by STEM-EELS line scan crossing the grain boundary
in steps of a few nanometers36.
In this study, we improve the oxygen ion transport by
minimizing the space charge effect in the CGOCGO grain
boundaries through a controlled phase reaction between the CGO
and CFO phases. A panorama of the atomic/electronic structure,
relative atomic ratios and local dopant profiles near all types of
grain boundaries in the MIEC composites is simultaneously
probed and resolved by STEM-EELS in high spatial resolution
(o1.3 ) and energy resolution (o0.35 eV). The distribution,
chemical composition and electronic fingerprint of an emergent
Gd, Fe-rich phase formed during processing of the CGOCFO
composites are investigated by (S)TEM-energy dispersive X-ray
spectrometry (EDX)/EELS and selected area electron diffraction
(SAED). Finally, the oxygen permeation flux of these composites
is measured to demonstrate their enhanced oxygen ionic
conductivities.
Results
Morphology and phase structure of the composite MIECs.
Three composite MIECs were prepared using CGO and CFO with
volume ratios of 50:50, 60:40 and 80:20 (denoted as CGOCFO5050, CGO-CFO6040 and CGO-CFO8020, respectively).
The compositions of the sintered composites were then examined
by EDX mapping of Ce, Gd, Co and Fe in STEM mode. In
addition to the two starting phases (CGO and CFO), the emergence of a new phase was observed. The three different phases are
coloured in light green, orange and blue in the compositional
images (Fig. 1f,l,r). The light green phase contains Gd, Fe, Co and
Ce (denoted as GFCCO); the orange phase contains Ce and Gd
(denoted as CGO); and the blue phase contains Co and Fe
(denoted as CFO). High-angle annular dark-field (HAADF)
images (Fig. 1a,g,m) in the STEM mode also revealed three phases
from their contrasts (bright, grey and dark), corresponding to
GFCCO, CGO and CFO phases with different atomic number (Z)
contrast, respectively. The newly formed GFCCO phase is the
product of a chemical reaction between CGO and CFO,
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
Ce
Gd
Co
Fe
Ce
Gd
Co
Fe
Ce
Gd
Co
Fe
Figure 1 | Phase identification by STEM-EDX. (a) High angle annular dark-field (HAADF) image showing the area of EDX mapping, (be) EDX element
mapping in STEM mode for Ce, Gd, Co, Fe elements and (f) phase distinguishable map after combining the EDX signals of be for the CGOCFO5050
sample; (gl) and (mr) are corresponding results for CGOCFO6040 and CGOCFO8020 samples, respectively. Scale bars, 1 mm.
as shown in equation (1) in which CGOCFO6040 is chosen as
an example:
CGO CFO ! CGO6040 CFO6040 GFCCO6040 1
where CGO6040, CFO6040 and GFCCO6040 correspond to new
CGO, CFO and GFCCO phases formed in the CGOCFO6040
sample after sintering. The GFCCO phase is predominantly
surrounded by the CGO phase rather than the CFO phase. In
CGOCFO8020 (Fig. 1r), many GFCCO particles are found
inside the CGO at a great distance away from CFO particles,
suggesting that the GFCCO phase is formed through the migration of Fe and Co, probably in the form of a liquid phase. CGO
has a much higher sintering temperature than CFO (41,400 !C
and o1,100 !C, respectively)37,38. However, highly dense samples
(relative density 495%) have been achieved for all the three
CGOCFO composites after sintering at only 1,300 !C,
presumably because cobalt oxide leads to a liquid phasemediated densification of ceria during the sintering process39.
The Fe-, Co-containing liquid phase can easily transport the Fe
and Co ions required for the reaction that forms the emergent
GFCCO phase. A similar compositional investigation of
single-phase CGO revealed that the Gd ion concentration at the
CGOCGO grain boundary core was much higher than the
average concentration in the grain interior of single-phase CGO
(B40% compared with B20%, ref. 26). Therefore, the reaction
that results in GFCCO phase formation is most likely initiated at
the CGOCGO grain boundary zone (reaction mechanism is
suggested in Supplementary Fig. 1), resulting in the reduction of
Gd ion concentration, which mitigates Gd accumulation in the
CGOCGO grain boundary. The origin of the space charge layer
and poor grain boundary ionic conductivity in CGO is attributed
to Gd accumulation, coupled with oxygen vacancy depletion in
the grain boundary. Therefore, a change in Gd ion concentration
in the CGOCGO grain boundary can dramatically impact the
oxygen ionic transport. To confirm this point, we carefully
investigated the energy-loss near-edge structure (ELNES) of the
element edges and the change of the extracted charge ratios near
the grain boundary regions in all the composites.
Grain boundary studies by STEM-EELS. Figure 2 shows the
compositional and charge distribution variations for the CGO
CGO grain boundary in the CGOCFO6040 composite acquired
by EELS line scan in the STEM mode. Figure 2g shows a significant difference in the Ce M5/M4, Gd/Ce and O/Ce ratios in
the CGOCGO grain boundary region in CGOCFO6040 as
compared with single-phase CGO26. The Gd/Ce ratio in the
CGOCGO grain boundary core of single-phase CGO is
significantly higher than that in the grain interior,
demonstrating the accumulation of Gd ions in the grain
boundary and concomitant formation of a space charge layer26.
The accumulation of Gd ions is closely correlated to the
concentration of oxygen vacancies in the space charge layer,
which is reflected by the ratio of Ce M5/M4. The Ce M4,5 and Gd
M4,5 edges of the CGOCGO grain boundary in single-phase
CGO shifted to higher energy, and the Ce M5/M4 ratio increased
26.3% (0.95-1.20) at the grain boundary core compared with
the grain interior40. In contrast, the ELNES spectra of Ce M4,5
edges, Gd M4,5 edges and O K edges at the CGOCGO grain
boundary core in the CGOCFO6040 composite are all very
similar to those from the CGO grain interior (B10 nm away from
core, Fig. 2df). There were no obvious edge position shifts or
shape changes observed, indicating similar local chemical states,
symmetry and atomic environment extending from the grain
boundary to the grain interior for the CGO phase inside the
CGOCFO6040 composite41,42. Furthermore, the Ce M5/M4 only
changed by 3.3% from the grain boundary core to the grain
interior (0.92-0.89). This provides additional confirmation that
the oxygen vacancy concentration at the CGOCGO grain
boundary core in CGOCFO6040 is similar to the concentration
in the CGO grain interior. Therefore, the depletion of oxygen
vacancies inside the space charge layer, observed in single-phase
CGO was significantly mitigated and a nearly constant
distribution of oxygen vacancy concentration is expected along
the CGOCGO grain boundary inside the CGOCFO6040
composite. A schematic is shown in Fig. 2h,i to conceptually
understand the difference of the space charge effect between
single-phase CGO and the modified CGO phase inside the
CGOCFO6040 composite. Figure 2h,i proposes that Gd ion
accumulation and the resulting oxygen vacancy depletion in the
CGOCGO grain boundary are avoided in the CGOCFO6040
composite through the in situ formation of a Gd- and Fe-rich
GFCCO phase. Although similar effects were observed inside the
CGOCFO8020 composite, the CGOCGO grain boundaries in
CGOCFO8020 were much thicker (B42 nm compared with
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
CGO
CGO
Gd M4
1,160 1,180 1,200 1,220 1,240 520
Energy loss (eV)
Space charge layer
900
920
940
Energy loss (eV)
[Concentration]
880
Intensity (a.u.)
Intensity (a.u.)
Ce(on)
Ce(off)
Space charge layer
Ce M5
Gd(on)
Gd(off)
O(on)
O(off)
Intensity (a.u.)
Gd M5
Ce M4
CGO
CGO
0.91
0.89
Gd/Ce
GI
GB
GI
O/Ce
8
530
540
550
Energy loss (eV)
0.96
CeM5/M4
0.92
4
0
8
4
Probe position (nm)
560
Space charge layer
CGO
0.95
Relative ratio intensity (a.u.)
Ce M4,5 edges
Gd M4,5 edges
Space charge layer
O K edges
Intensity (abr. units)
GB core
1.20
CGO
Figure 2 | CGOCGO grain boundaries in CGOCFO6040 revealed by STEM-EELS. (a) The survey image including the EELS line scan across the
CGOCGO grain boundary (GB) from the CGOCFO6040 composite; (b) and (c) are EELS line scan signal profiles presenting in two-dimensional and
three-dimensional mode, respectively; (d), (e) and (f) are ELNES spectra of Ce M4,5, Gd M4,5 and O K edges extracted at (marked by on) or B10 nm
away from (marked by off) the CGOCGO grain boundary core, respectively; (g) profile of the CeM5/M4, Gd/Ce and O/Ce ratio near the CGOCGO
boundary (The solid symbol results are from this work, while the hollow symbol results are from single-phase CGO26. The grain boundary thickness of
both samples is B4 nm); The proposed oxygen vacancy concentration profiles near the CGOCGO grain boundary zone of (h) single-phase CGO and
(i) CGOCFO6040 composite. The GB core was highlighted by red and green line in h and i, respectively. Scale bar, 100 nm (a).
4 nm in CGOCFO6040, shown in Supplementary Fig. 2). The
thicker grain boundary provides more resistance for the transport
of oxygen ions17.
In addition to the interface of the primary oxygen ion
conductor CGOCGO, there are five additional types of
grain boundaries inside these composites: CGOGFCCO,
CFOGFCCO, GFCCOGFCCO, CGOCFO and CFOCFO.
The CGOGFCCO grain boundary is especially important as it
will determine whether the GFCCO phase will serve as a blocker
or promoter for oxygen ion transport. Supplementary Figures 3
and 4 show the inter-diffusion of the elements and the change of
the oxygen stoichiometry along the CGOGFCCO boundaries
from all of the composites studied in this work. The
CGOGFCCO boundary inside the CGOCFO6040 composite
displayed the shortest transport path (B27 nm) and the least
oxygen vacancy concentration fluctuation among the three
composites. This resulted in the smallest resistance for oxygen
ions transport among the CGOGFCCO boundaries. On the
basis of recent results using X-ray nanotomography, the volume
4
ratio of GFCCO phases in both CGOCFO6040 and
CGOCFO5050 is B10%, and the continuity of the GFCCO
particles is zero43. Therefore, the GFCCOGFCCO gain
boundary is not a contiguous ion transport path. The
STEM-EELS line scan results on the remaining grain
boundaries are shown in Supplementary Figs 57. There were
no Co and Fe concentration variations observed near the
CFOCFO grain boundaries, and the CFOCFO boundaries are
expected to serve as excellent electronic conduction pathways.
Phase compositions and structures of CGOCFO composites.
The STEM-EDX quantification results for Gd, Fe, Co and Ce
contents of all the three CGOCFO composites are shown in
Supplementary Tables 13. In all the cases, the ratio of Gd/Ce and
Fe/Co in CGO and CFO is significantly lower than those in the
CGO and CFO starting powders (0.25 and 2.0, respectively).
Despite differences in composition, the CGO and CFO phases
retained their original structures inside the three CGOCFO
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
composites (fluorite structure with space group Fm-3m (225) and
spinel structure with space group Fd-3m (227), respectively), as
shown in the X-ray diffraction and SAED patterns in
Supplementary Figs 811. The STEM-EDX quantification results
also revealed that the GFCCO phases were rich in Gd and Fe.
Simultaneous growth of GdFeO3 (perovskite) and Gd3Fe5O12
(garnet) was observed in Gd2O3Fe2O3 diffusion couples at
1,2001,400 !C with activation energies of B400 and
B550 kJ mol ! 1, respectively44. Among the potential GFCCO
phases, GdFeO3 is thermodynamically more favoured to form
than Gd3Fe5O12. In all the samples, the peaks of the newly formed
GFCCO phase match well with that of GdFeO3 (Supplementary
Fig. 8). However, the peaks of Gd3Fe5O12 cannot be distinguished
due to significant overlap with the CGO, CFO and GdFeO3
diffraction peaks. Although X-ray diffraction patterns provide the
best macroscopic structural information for phase identification,
SAED patterns can provide more precise structural information
of selected GFCCO particles (Supplementary Fig. 12). The results
of SAED studies revealed that the GFCCO5050 and GFCCO8020
particles displayed a garnet A3B5O12-d-like structure, whereas the
GFCCO6040 particles possessed a perovskite-like structure.
Higher oxygen ionic conductivities have been observed in
perovskite-type phases as compared with garnet-type phases45.
Another pronounced feature of the GFCCO phase is its high
Z-contrast. HAADF images (Fig. 1a,g,m) indicated that the
average atomic number (Zavg) of the GCFFO phase was higher
than the Zavg for CGO. We have estimated that GFCCO phase
has a high level of oxygen vacancies on the basis of the results of
average atomic number (Supplementary Note 1). A high level of
oxygen vacancies, together with the unique perovskite structure
make the perovskite-type GFCCO a candidate for fast oxygen
ionic conduction, providing additional paths for oxygen ion
transport.
Proposed models for ionic and electronic transport paths.
Figure 3a shows the potential paths for oxygen ionic and electronic transport in DP-MIECs consisting solely of one ionic and
one electronic conductive phase. The effective oxygen ionic
conduction in DP-MIECs is limited by the space charge effect in
the grain boundaries of the oxygen ionic conductor (such as
CGO) and the increased tortuosity introduced by the electronic
conductive phase. In the case of CGOCFO composites, both the
grain boundary conductivity and tortuosity are changed by the
formation of an emergent GFCCO phase. The results of our
microscopy and EELS studies can be summarized as follows:
(a) the CGOCGO grain boundaries in the CGOCFO6040
composite conduct oxygen ions better than traditional CGO;
(b) the GFCCO phase in the CGOCFO6040 composite possesses
a perovskite-like structure and high level of oxygen vacancies,
providing additional pathways for oxygen ionic conduction; and
(c) the CGOGFCCO grain boundaries in the CGOCFO6040
composite conduct oxygen ions better than those in CGO
CFO5050 and CGOCFO8020 (Supplementary Fig. 3). On the
basis of the results above, the oxygen ionic/electronic transport
paths for all the composites are shown in Fig. 3bd. In general,
the CGOCGO and CGOGFCCO grain boundaries, along
with the GFCCO6040 and CGO grains in CGOCFO6040 are
fast oxygen-ionic transport paths. The CGOCFO5050 and
CGOCFO8020 possessed grain boundaries (CGOGFCCO) and
garnet-type GFCCO grains, which are expected to be slow
oxygen-ionic transport paths. The electronic transport in
CGOCFO8020 is also limited because the volume ratio of CFO
(20%) is below the percolation limit. Considering that the oxygen
permeation process involves both oxygen ionic and electronic
transportation, the oxygen permeation flux should increase
in the following order: CGOCFO60404CGOCFO50504
CGOCFO8020, which was verified by oxygen permeation
measurements.
Oxygen permeation performance of CGOCFO composites.
Figure 4a shows the oxygen permeation flux of different
CGOCFO composite membranes, along with literature data for
comparison. The flux of the membranes follows the predicted
order based on oxygen ion and electron transport pathways:
CGOCFO60404CGOCFO50504CGOCFO8020. It should
be noted that the CGOCFO6040 shows a significantly higher
flux than previously reported membranes based on the materials
system CGOAFe2O4. For example, a 1.0-mm-thick CGO
CFO6040 and 0.5-mm-thick Ce0.9Gd0.1O2 ! dNiFe2O4 (volume
ratio 60:40, CGONFO6040) membranes displayed oxygen
CGO bulk phase
CFO bulk phase
GFCCO5050
GFCCO6040
GFCCO8020
O2 slow transport path
O2 fast transport path
O2 transport blocking path
e transport path
e transport blocking path
e
O2
O2
e
O2
Figure 3 | Proposed oxygen ionic and electronic transport paths. (a) Traditional dual phase MIECs (DP-MIECs) without formation of the third phase.
(bd) Novel ternary phase MIECs (TP-MIECs) shown as (b) CGOCFO5050 (c) CGOCFO6040 and (d) CGOCFO8020 in this work.
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
15
2.5
CGO-CFO6040 (1 mm)
CGO-CFO5050 (1 mm)
CGO-CFO8020 (1 mm)
CGO-CFO6040 one pot (1 mm)
CGO-CFO6040 (1 mm, coated on feed side)
CGO-NFO6040 Luo (0.50 mm, coated on feed side)
CGO-NFO4060 balaguer (0.68 mm)
Log(T) (Scm1K1)
Flux (108 mol cm2 s1)
20
10
2.0
1.5
1.0
CGOEa=79.3 kJ mol1
0.5
CGOCFO6040 Ea=86.11.0 kJ mol1
CGOCFO5050 Ea=105.31.6 kJ mol1
CGOCFO8020 Ea=133.01.4 kJ mol1
850
875
900
925
Temperature (C)
950
0.0
CGOCFO6040 one pot Ea=145.91.0 kJ mol1
0.82
0.84
0.86
1,000/T (K1)
0.88
0.90
Figure 4 | Oxygen permeation performance of the CGOCFO composite MIECs. (a) Oxygen permeation flux of the CGOCFO composites with
different volume ratios measured between 850 and 950 !C. Feed gas: 150 ml min ! 1 air. Sweep gas: 30 ml min ! 1 helium. (b) The Arrhenius plot of
the calculated ambipolar conductivities of CGOCFO composites based on oxygen permeation flux. The ionic conductivity of single-phase CGO is
shown for comparison.
permeation flux values of 5.18 & 10 ! 8 and 6.47 & 10 ! 8
mol cm ! 2 s ! 1, respectively at 900 !C, with the presence of a
CGOSm0.5Sr0.5CoO3-d (SSC) catalyst coated on the feed side32.
The much thicker CGOCFO6040 membrane examined
in this work shows similar flux values compared with the
CGONFO6040 composite. A CGOCFO7525 sample prepared
by the one-pot method exhibited very low flux
(B1.0 & 10 ! 8 mol cm ! 2 s ! 1) at 900 !C (ref. 30), which may
have been due to differences in the volume ratio of ionic-toelectronic conductive phases and surface exchange limitations
due to the lack of a catalyst on the membrane surface. To clarify
this, we prepared a CGOCFO6040 sample by the one-pot
method and measured the oxygen flux under the same conditions
of a CGOCFO6040 sample prepared by the powder-mixing
method. Most CGO-based DP-composite membranes show
higher performance when the grain sizes are smaller. For
example, when CGOFe2O3 membranes prepared by the onepot method were sintered at different temperatures to produce
different grain sizes, the membrane with finer CGO and Fe2O3
grains showed higher oxygen flux46. The CGONiFe2O4
membrane prepared by the one-pot method also showed higher
performance than samples prepared by the powder-mixing
method. This is because the former contains finer CGO and
NiFe2O4 grains and less homoaggregation of grains of the same
phase than the latter32. One would expect CGOCFO6040
prepared by the one-pot method to show a higher flux than
samples prepared by the powder-mixing method. However, the
flux of the CGOCFO6040 samples prepared by the powdermixing method was approximately four times higher than those
prepared by the one-pot method at 900 !C (Fig. 4a). The total
electrical conductivity of the CGOCFO6040 samples was also
several times higher than those of various CGOCFO composites
prepared by the one-pot method measured at 300 !C (shown in
Supplementary Fig. 13, Supplementary Table 4). These major,
unexpected differences are apparently not due to the difference in
CGO, CFO grain sizes/distribution, but are closely related to the
difference in the CGOCGO grain boundaries and the formation
of GFCCO phases. In CGOFe2O3, CGONiFe2O4, and CGO
CFO6040 one-pot membranes, no apparent phase reactions or
new type of grains were found. Instead, a rim structure consisting
of CGO and CFO nanoparticles was found at the grain
boundaries in the CGOCFO composites made by the one-pot
method30. Therefore, the interfacial structure of CGOCGO grain
boundaries in these CGO-based composites will always hinder
the oxygen ion transport, leading to high activation energies for
oxygen permeation (135.3, 1284 and 123.40.6 kJ mol ! 1
for CGOFe2O3, CGONiFe2O4 and CGOCFO6040 one pot,
6
respectively) and relatively low flux32,46. In contrast, the space
charge effect near the grain boundary region was mitigated in the
CGOCFO composites prepared by the powder-mixing method
by the formation of oxygen ionic conducting GFCCO phases. The
activation energies calculated from the Arrhenius plot of flux and
temperature are 68.81.0, 86.41.5 and 113.00.9 kJ mol ! 1
for CGOCFO6040, CGOCFO5050, CGOCFO8020 prepared
by the powder-mixing method, respectively. This further
highlights the importance of the high CGOCGO grain
boundary conductivity in CGOCFO6040 samples prepared by
the powder-mixing method.
The flux ratio at 900 !C is 3.0:4.8:1.0 for the CGOCFO5050,
CGOCFO6040 and CGOCFO8020, respectively. The oxygen
permeation flux of the CGOCFO6040 membrane is B61%
higher than the flux of the CGOCFO5050 membrane at 900 !C.
This is unexpected based solely on a 10% difference in the volume
ratio of the oxygen ion conducting CGO phase. It was
demonstrated that the CGO and CFO phases maintained the
fluorite and spinel structures and exhibited similar Gd/Ce and
Fe/Co ratios in these two composites (Supplementary Figs 9 and
10, Supplementary Tables 1 and 2). Therefore, the large difference
in oxygen permeation flux may be closely related to the different
structure of the GFCCO phases and CGOGFCCO grain
boundaries. The perovskite-type GFCCO6040 phase in CGO
CFO6040 has a structure permitting greater oxygen transport
compared with the garnet-type GFCCO5050 in the CGO
CFO5050 (ref. 45). Moreover, the change in the oxygen
vacancy concentration across the CGOGFCCO grain boundary
in the CGOCFO6040 is much smoother than variations in the
CGOCFO5050 (Supplementary Fig. 3), which facilitates the
transport of oxygen ions. The high level of oxygen flux in the
CGOCFO6040 sample is attributed to the cooperative effect of
these factors. The poor performance of the CGOCFO8020
composite is mainly attributed to the lack of electronic transport
paths on the basis of percolation theory (Fig. 3d). The formation
of nano-domains with oxygen vacancy associates (Supplementary
Fig. 11c,f) inside the CGO phase in CGOCFO8020 could also be
a barrier for the oxygen ionic transportation47.
The ambipolar conductivities of the CGOCFO composites
were calculated (details shown in Supplementary Note 2,
Supplementary Fig. 14) on the basis of the Wagner equation
and are shown in Fig. 4b (ref. 48). The ambipolar conductivities
of CGOCFO6040 are 0.057, 0.042 and 0.029 S cm ! 1 at 950, 900
and 850 !C. As the electronic conductivity of CFO is 41 S cm ! 1
in this temperature region, the ambipolar conductivity can be
estimated as the ionic conductivity12,43. After correcting for the
CGO volume fraction in CGOCFO6040 (57%, as determined by
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
X-ray nanotomography43), the ionic conductivities are 0.10, 0.073
and 0.051 S cm ! 1 at 950, 900 and 850 !C, respectively.
These values are very close to the ionic conductivity of CGO
(0.14, 0.11 and 0.078 S cm ! 1 at 950, 900 and 850 !C, respectively)
measured by the DC four-probe method and corrected by
the ionic transference number49. The ionic conductivities of
CGOLa0.8Sr0.2Fe0.8Co0.2O3 ! d and CGOLa0.7Sr0.3MnO3 ! d, in
contrast, are about one order of magnitude lower than that of
CGO29. Furthermore, the activation energies for ionic
conductivity are 79.30.0, 86.11.0, 105.31.6 and
133.01.4 kJ mol ! 1 for single-phase CGO, CGOCFO6040,
CGOCFO5050 and CGOCFO8020, respectively, as plotted
from Fig. 4b. The activation energy of CGOCFO6040 is very
close to that of single-phase CGO in the similar temperature
range. In comparison, the activation energies for ionic
conductivity of conventional DP-MIECs based on doped ceria
are much higher (10814, 1067 and 15410 kJ mol ! 1 for
CGOLa0.8Sr0.2Fe0.8Co0.2O3 ! d, CGOLa0.7Sr0.3MnO3 ! d, and
Ce0.8Sm0.2O2 ! dLa0.8Sr0.2CrO3 ! d, respectively)29,50. The high
ionic conductivity and the low activation energy of the CGO
CFO6040 composite suggest the effectiveness of fast oxygen-ionic
transport paths through the GFCCO6040 phase and the
CGOCGO, plus the CGOGFCCO grain boundaries.
Discussion
We correlated the performance of CGOCFO composite MIECs
with the charge distribution near the CGOCGO grain
boundaries and the structure of the newly formed GFCCO phase
using spatially resolved EDX/EELS, HADDF-STEM imaging and
SAED. Negligible differences in Gd/Ce, Ce M5/M4 and O/Ce
ratios were found near the CGOCGO grain boundary region in
the CGOCFO composites. The homogeneous charge distribution and uniform chemical environment along the grain
boundary enable fast oxygen ionic transport. In comparison,
the accumulation of Gd ions and depletion of oxygen vacancies at
the CGOCGO grain boundary in single-phase CGO deteriorate
its grain boundary conductivity. Our findings show that this
difference originates from cation migration driven by the
formation of Gd- and Fe-rich GFCCO phases. The reaction most
likely initiates at the CGOCGO grain boundaries where Gd ions
are known to accumulate. The reaction forming the GFCCO
phase reduces the Gd content in the grain boundaries. The
GFCCO6040 phase possesses a perovskite structure and high level
of oxygen vacancies, which are characteristic of fast oxygen ion
conductors. Furthermore, the CGOGFCCO grain boundaries
inside CGOCFO6040 show nearly constant local oxygen
stoichiometry, which also facilitates oxygen transport. The
synergistic effect of fast oxygen transport in CGOCGO, CGO
GFCCO grain boundaries, and the GFCCO6040 phase in CGO
CFO6040 results in the highest oxygen flux observed in the
CGOAFe2O4 materials system. These findings point out a novel
method to optimize the performance of composite MIECs: an
in situ phase reaction, which simultaneously reduces the space
charge effect at the grain boundaries and creates new oxygen
ionic conducting pathways. Engineering interfaces such as grain
boundaries through an in situ phase reaction provides a
promising path toward the design of high-performance materials
for energy conversion and storage. This concept of intentionally
targeting emergent phase formation, involving elements known to
segregate at grain boundaries provides a new tool for materials
system design. This concept was demonstrated for composite
MIEC systems, but the approach can also be applicable to studies
involving sintering aids in ionic conductors used as solid oxide
electrolytes for a variety of energy conversion and storage
systems. One example may be BaZr0.8Y0.2O3 ! d, which possesses
very high bulk proton conductivity but poor grain boundary
proton conductivity18,51,52.
Methods
Sample preparation. CGO and CFO powders (1250 nm and 2050 nm respectively, based on TEM characterization, InfraMat Advanced Materials LLC, USA)
with volume ratios of 50:50, 60:40 and 80:20 were ball-milled in ethanol for 20 h
and then dried to obtain CGOCFO composite powders. These powders were
pressed into pellets in a 20-mm stainless steel die. The pellets were sintered in air at
1,300 !C for 2 h. CGOCFO6040 was also prepared by a one-pot combustion
method using metal nitrates and citric acid. The relative densities of the sintered
composites measured by the Archimedes method were all 495%.
Electron microscopic and spectroscopic characterizations. Due to the polycrystalline nature of both CGO and CFO in the composites, a large thin area of the
samples was needed for the TEM and STEM-EELS studies to obtain averaged
information. Thin areas with a thickness B50 nm were obtained for all the three
composites from the classic dimpling and ion milling method. The sintered CGO
CFO composites were first cut by a diamond saw and then by an ultrasonic disk
cutter (Model 170) to produce disks of 3 mm in diameter. The thickness of the
samples was then reduced to 150 mm by polishing using sandpaper, and then
reduced to 30 mm by dimpling using a dimpler (Gatan 626). The final sample
thickness of B50 nm was achieved by ion milling using an ion milling system
(Fischione Model 1010). The same voltage, current and milling angel programs
were applied for all the samples to keep the sample preparation consistent. No
surface damage and obvious amorphous phases were observed with the final low
energy ion milling processes for all of the three CGOCFO samples. The powder
samples for the TEM test were first prepared by supersonic dispersion of the
powders in ethanol, dipping the powder suspension onto a 400 mesh Cu grid (Ted
Pella, Inc.), followed by drying in air. HRTEM images and SAED patterns were
acquired by using the JEOL 2100F TEM equipped with a Schottky field-emission
gun (FEG) with Cs 1.0 mm operated at 200 kV. EDX mapping tests in the TEM
mode were performed before taking HRTEM images and SAED to distinguish the
different types of grains/grain boundaries from the composites. For each phase
from different composites, local atomic composition and electron-ELNES for different edges both for the grain boundary and grain interior were characterized
using a Cs-corrected Hitachi HD-2700C STEM equipped with a Cold-FEG gun,
operated at 200 kV coupled with EELS. Images were acquired using a probe convergence semi-angle of 27 mrad, and the inner collection angle of the ADF detector
was 53 mrad. The EELS data were collected using an Engina ER (Gatan) detector,
with a collection half-angle of 26 mrad. STEM-EDX mapping was also taken by a
5030 EDX detector from Bruker, attached with the Hitachi STEM. The energy
resolution is B1.3 eV at the dispersion of 1.25 eV per channel. The probe size was
1.3 , the beam current was 30B40 pA, and the dwelling time was 3 s. Steps
of 3B5 were carefully selected to avoid the possible effects induced by the
electron-beam damage. The signals of O K-edge, Gd M4,5-edges, Ce M4,5-edges,
Fe L2,3-edges and Co L2,3-edges were obtained simultaneously and performed using
a non-linear least squares fitting integrated with Digital Micrograph software.
Oxygen permeation flux measurement. The sintered CGOCFO membranes
were polished to a thickness of 1.0 mm using sandpapers with different grits from
P120 to P600. CGOSm0.5Sr0.5CoO3-d (SSC) catalyst layers with thickness of
B20 mm were painted on both surfaces of the membranes to accelerate the oxygen
exchange process on the surfaces. After drying, the membranes were directly sealed
to an alumina tube using a glass ring (Schott 8252) at 1,000 !C for 2 h. The catalyst
layers were in situ calcined in the sealing process. The oxygen permeation flux was
measured between 850 and 950 !C. The feed gas was 150 ml min ! 1 air, while the
sweep gas was 30 ml min ! 1 He. The sweep gas from the outlet was analysed by gas
chromatography (Agilent 7890A). The leakage due to incomplete sealing was o1%
and corrected before the calculation of oxygen flux.
Other characterization. For the impedance tests, both surfaces of the sintered
CGOCFO pellets were polished using sandpaper with P2500 grit and painted with
Pt paste. To ensure good contact between Pt electrodes and the samples, the pellets
were sintered in air at 1,000 !C for 5 h with a heating and cooling speed of
5 !C min ! 1. The impedance tests were performed in air between 150 and 800 !C,
using an IM6 electrochemical workstation (Zahner) in the frequency range of
10 ! 2106 Hz. The fittings of the obtained impedance spectra with the corresponding equivalent circuit model were done using the software Z-View.
References
1. Mihailetchi, V. D., Wildeman, J. & Blom, P. W. M. Space-charge limited
photocurrent. Phys. Rev. Lett. 94, 126602 (2005).
2. Whitesides, G. M. & Crabtree, G. W. Dont forget long-term fundamental
research in energy. Science 315, 796798 (2007).
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
3. Xiao, Y., Shenoy, V. B. & Bhattacharya, K. Depletion layers and domain
walls in semiconducting ferroelectric thin films. Phys. Rev. Lett. 95, 247603
(2005).
4. Maier, J. Nanoionics: ion transport and electrochemical storage in confined
systems. Nat. Mater. 4, 805815 (2005).
5. Shao, Z. P. & Haile, S. M. A high-performance cathode for the next generation
of solid-oxide fuel cells. Nature 431, 170173 (2004).
6. Baker, R. W. Future directions of membrane gas separation technology. Ind.
Eng. Chem. Res. 41, 13931411 (2002).
7. Wang, H., Werth, S., Schiestel, T. & Caro, J. Perovskite hollow-fiber
membranes for the production of oxygen-enriched air. Angew. Chem. Int. Ed.
44, 69066909 (2005).
8. Aoki, Y. et al. Bulk mixed ion electron conduction in amorphous gallium oxide
causes memristive behaviour. Nat. Commun. 5, 3473 (2014).
9. Ding, D., Li, X., Lai, S. Y., Gerdes, K. & Liu, M. Enhancing SOFC cathode
performance by surface modification through infiltration. Energ. Environ. Sci.
7, 552575 (2014).
10. Lee, J. G., Park, J. H. & Shul, Y. G. Tailoring gadolinium-doped ceria-based
solid oxide fuel cells to achieve 2 W cm ! 2 at 550!C. Nat. Commun. 5, 4045
(2014).
11. Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel
cells. Science 334, 935939 (2011).
12. Bouwmeester, H. J. M. Dense ceramic membranes for methane conversion.
Catal. Today 82, 141150 (2003).
13. Verwey, E. J., Haayman, P. W. & Romeijn, F. C. Physical properties and cation
arrangement of oxides with spinel structures II. Electronic conductivity.
J. Chem. Phys. 15, 181187 (1947).
14. Steele, B. C. H. Ceramic ion conducting membranes. Curr. Opin. Solid State
Mater. Sci. 1, 684691 (1996).
15. Ponpandian, N., Balaya, P. & Narayanasamy, A. Electrical conductivity and
dielectric behaviour of nanocrystalline NiFe2O4 spinel. J. Phys. Condens. Matter
14, 32213237 (2002).
16. Kharton, V. V. et al. Oxygen transport in Ce0.8Gd0.2O2 ! d-based composite
membranes. Solid State Ionics 160, 247258 (2003).
17. Guo, X., Sigle, W. & Maier, J. Blocking grain boundaries in yttria-doped
and undoped ceria ceramics of high purity. J. Am. Ceram. Soc. 86, 7787
(2003).
18. Pergolesi, D. et al. High proton conduction in grain-boundary-free yttriumdoped barium zirconate films grown by pulsed laser deposition. Nat. Mater. 9,
846852 (2010).
19. Tschope, A. Grain size-dependent electrical conductivity of polycrystalline
cerium oxide II: space charge model. Solid State Ionics 139, 267280 (2001).
20. Maier, J. Ionic conduction in space charge regions. Prog. Solid State Chem. 23,
171263 (1995).
21. An, J. et al. Atomic scale verification of oxide-ion vacancy distribution near a
single grain boundary in YSZ. Sci. Rep. 3, 2680 (2013).
22. Delmo, M. P., Yamamoto, S., Kasai, S., Ono, T. & Kobayashi, K. Large positive
magnetoresistive effect in silicon induced by the space-charge effect. Nature
457, 11121115 (2009).
23. Ruhle, M. Microscopy of structural ceramics. Adv. Mater. 9, 195217 (1997).
24. Dholabhai, P. P., Adams, J. B., Crozier, P. & Sharma, R. A density functional
study of defect migration in gadolinium doped ceria. Phys. Chem. Chem. Phys.
12, 79047910 (2010).
25. Ou, D. R. et al. Oxygen vacancy ordering in heavily rare-earth-doped ceria.
Appl. Phys. Lett. 89, 171911 (2006).
26. Lei, Y. Y., Ito, Y., Browning, N. D. & Mazanec, T. J. Segregation effects at grain
boundaries in fluorite-structured ceramics. J. Am. Ceram. Soc. 85, 23592363
(2002).
27. Guo, X. & Waser, R. Electrical properties of the grain boundaries of oxygen ion
conductors: acceptor-doped zirconia and ceria. Prog. Mater. Sci. 51, 151210
(2006).
28. Avila-Paredes, H. J., Choi, K., Chen, C.-T. & Kim, S. Dopant-concentration
dependence of grain-boundary conductivity in ceria: a space-charge analysis.
J. Mater. Chem. 19, 48374842 (2009).
29. Shaula, A. L. et al. Oxygen permeability of mixed-conducting composite
membranes: effects of phase interaction. J. Solid State Electrochem. 10, 2840
(2006).
30. Takamura, H., Kawai, M., Okumura, K., Kamegawa, A. & Okada, M.
Preparation and oxygen permeability of Gd-doped ceria and spinel-type ferrite
composites. MRS Proceedings 756, EE8.1116 (2002).
31. Takamura, H. et al. Oxygen permeation properties and surface modification of
acceptor-doped CeO2/MnFe2O4 composites. J. Electroceram. 17, 741748
(2006).
32. Luo, H. et al. CO2-stable and cobalt-free dual-phase membrane for oxygen
separation. Angew. Chem. Int. Ed. 50, 759763 (2011).
33. Balaguer, M., Garca-Fayos, J., Sols, C. & Serra, J. M. Fast oxygen separation
through SO2- and CO2-stable dual-phase membrane based on NiFe2O4
Ce0.8Tb0.2O2-d. Chem. Mater. 25, 49864993 (2013).
8
34. Luo, H. et al. Novel cobalt-free, noble metal-free oxygen-permeable
40Pr0.6Sr0.4FeO3-d60Ce0.9Pr0.1O2 ! d dual-phase membrane. Chem. Mater. 24,
21482154 (2012).
35. Tuller, H. L., Litzelman, S. J. & Jung, W. Micro-ionics: next generation power
sources. Phys. Chem. Chem. Phys. 11, 30233034 (2009).
36. Kim, Y. M. et al. Probing oxygen vacancy concentration and homogeneity in
solid-oxide fuel-cell cathode materials on the subunit-cell level. Nat. Mater. 11,
888894 (2012).
37. Hutlova, A., Niznansky, D., Rehspringer, J. L., Estourne`s, C. & Kurmoo, M.
High coercive field for nanoparticles of CoFe2O4 in amorphous silica solgel.
Adv. Mater. 15, 16221625 (2003).
38. Zhu, T. et al. Evaluation of Li2O as an efficient sintering aid for gadolinia-doped
ceria electrolyte for solid oxide fuel cells. J. Power Sources 261, 255263 (2014).
39. Balaguer, M., Sols, C. & Serra, J. M. Study of the transport properties of the
mixed ionic electronic conductor Ce1 ! xTbxO2 ! d Co (x 0.1, 0.2) and
evaluation as oxygen-transport membrane. Chem. Mater. 23, 23332343
(2011).
40. Pena, M. A. & Fierro, J. L. G. Chemical structures and performance of
perovskite Oxides. Chem. Rev. 101, 19812018 (2001).
41. Muller, D. A., Singh, D. J. & Silcox, J. Connections between the electronenergy-loss spectra, the local electronic structure, and the physical properties of
a material: a study of nickel aluminum alloys. Phys. Rev. B 57, 81818202
(1998).
42. Jang, H. W. et al. Metallic and insulating oxide interfaces controlled by
electronic correlations. Science 331, 886889 (2011).
43. Harris, W. M. et al. Characterization of 3D interconnected microstructural
network in mixed ionic and electronic conducting ceramic composites.
Nanoscale 6, 44804485 (2014).
44. Buscaglia, V. et al. Growth of ternary oxides in the Gd2O3Fe2O3 system.
A diffusion couple study. Solid State Ionics 146, 257271 (2002).
45. Kharton, V. V. et al. Ionic transport in Gd3Fe5O12- and Y3Fe5O12-based
garnets. J. Electrochem. Soc. 150, J33J42 (2003).
46. Luo, H. et al. CO2-tolerant oxygen-permeable Fe2O3-Ce0.9Gd0.1O2-d dual phase
membranes. Ind. Eng. Chem. Res. 50, 1350813517 (2011).
47. Li, Z. P., Mori, T., Auchterlonie, G. J., Zou, J. & Drennan, J. Direct evidence of
dopant segregation in Gd-doped ceria. Appl. Phys. Lett. 98, 093104 (2011).
48. Wagner, C. Equations for transport in solid oxides and sulfides of transition
metals. Prog. Solid State Chem. 10, 316 (1975).
49. Eguchi, K., Setoguchi, T., Inoue, T. & Arai, H. Electrical properties of ceriabased oxides and their application to solid oxide fuel cells. Solid State Ionics 52,
165172 (1992).
50. Yi, J. X., Zuo, Y. B., Liu, W., Winnubst, L. & Chen, C. S. Oxygen permeation
through a Ce0.8Sm0.2O2-d-La0.8Sr0.2CrO3-d dual-phase composite membrane.
J. Membr. Sci 280, 849855 (2006).
51. Helgee, E. E., Lindman, A. & Wahnstrom, G. Origin of space charge in grain
boundaries of proton-conducting BaZrO3. Fuel Cells 13, 1928 (2013).
52. Shirpour, M., Gregori, G., Houben, L., Merkle, R. & Maier, J. High spatially
resolved cation concentration profile at the grain boundaries of Sc-doped
BaZrO3. Solid State Ionics 262, 860864 (2014).
Acknowledgements
This work was supported, in part, by the National Science Foundation (DMR-1210792)
and the HeteroFoaM Center, an Energy Frontier Research Center (EFRC) funded by the
U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (Award
no. DE-SC0001061). Funding to Clemson University from the EFRC is provided by
SCUREF/SRNS/DOE award #B139010. Electron microscopy research is carried out at
the Center for Functional Nanomaterials, Brookhaven National Laboratory, which is
supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under
contract no. DE-AC02-98CH10886 and DE-SC-0011270. We thank Ms Hsin-Hui Huang
and Dr Huolin Xin for the discussion and their help on analysing our TEM and
STEM-EELS data.
Author contributions
Y.L. conceived and designed the experiments with assistance from S.F. and D.S. K.S.B.
prepared the MIECs powder and composites. Y.L. and D.S. prepared the TEM samples
and performed the microscopy tests and analysis. S.F. and Y.L. carried out the oxygen
permeation experiments and X-ray diffraction test. Y.L. made the conductivity test. F.C.
and K.S.B. conceived and supervised the project. Y.L. and S.F. composed the manuscript
and prepared the figures. All the authors discussed the results, commented and revised
the manuscript.
Additional information
Supplementary Information accompanies this paper at http://www.nature.com/
naturecommunications
Competing financial interests: The authors declare no competing financial interests.
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
ARTICLE
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms7824
Reprints and permission information is available online at http://npg.nature.com/
reprintsandpermissions/
How to cite this article: Lin, Y. et al. Enhancing grain boundary ionic conductivity in
mixed ionicelectronic conductors. Nat. Commun. 6:6824 doi: 10.1038/ncomms7824
(2015).
This work is licensed under a Creative Commons Attribution 4.0
International License. The images or other third party material in this
article are included in the articles Creative Commons license, unless indicated otherwise
in the credit line; if the material is not included under the Creative Commons license,
users will need to obtain permission from the license holder to reproduce the material.
To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
NATURE COMMUNICATIONS | 6:6824 | DOI: 10.1038/ncomms7824 | www.nature.com/naturecommunications
& 2015 Macmillan Publishers Limited. All rights reserved.
Vous aimerez peut-être aussi
- Simulation of Transport in NanodevicesD'EverandSimulation of Transport in NanodevicesFrançois TriozonPas encore d'évaluation
- 10 1016@j Apsusc 2020 147047Document23 pages10 1016@j Apsusc 2020 147047Daniel MontalvoPas encore d'évaluation
- Applied Surface Science: Chen Hao, Xiaokun Wang, Xingli Wu, Yaning Guo, Linli Zhu, Xiaohong WangDocument11 pagesApplied Surface Science: Chen Hao, Xiaokun Wang, Xingli Wu, Yaning Guo, Linli Zhu, Xiaohong Wangreca adiyanti rahmanPas encore d'évaluation
- Applied Surface Science: Chen Hao, Xiaokun Wang, Xingli Wu, Yaning Guo, Linli Zhu, Xiaohong WangDocument3 pagesApplied Surface Science: Chen Hao, Xiaokun Wang, Xingli Wu, Yaning Guo, Linli Zhu, Xiaohong Wangreca adiyanti rahmanPas encore d'évaluation
- 1 s2.0 S2352152X22001086 MainDocument10 pages1 s2.0 S2352152X22001086 MainAKASHPas encore d'évaluation
- Achievements and Trends in PhotoelectrocatalysisDocument27 pagesAchievements and Trends in Photoelectrocatalysiscarlos1a1ramosPas encore d'évaluation
- Accepted Manuscript: Chemical Engineering JournalDocument24 pagesAccepted Manuscript: Chemical Engineering JournalQuân ĐặngPas encore d'évaluation
- Convergent and Divergent Paired Electrodeposition of Metal-Organic Framework Thin FilmsDocument13 pagesConvergent and Divergent Paired Electrodeposition of Metal-Organic Framework Thin Filmsyookarina8Pas encore d'évaluation
- Thermally Oxied Mno2 Based SupercapacitorsDocument25 pagesThermally Oxied Mno2 Based Supercapacitorsdush35Pas encore d'évaluation
- Wang Et Al 2018 Defect and Interface Engineering For Aqueous Electrocatalytic CO 2 Reduction2Document32 pagesWang Et Al 2018 Defect and Interface Engineering For Aqueous Electrocatalytic CO 2 Reduction2MinhPas encore d'évaluation
- Oriented Electron Transmission in Polyoxometalate-Metalloporphyrin Organic Framework For Highly Selective Electroreduction of CO2Document8 pagesOriented Electron Transmission in Polyoxometalate-Metalloporphyrin Organic Framework For Highly Selective Electroreduction of CO2Jam imtiazPas encore d'évaluation
- 2 Sun 2010 CathodeMaterialsForSolidOxideFDocument20 pages2 Sun 2010 CathodeMaterialsForSolidOxideFIsmail ShhaPas encore d'évaluation
- Reducing Energy Barriers by Multi-Interface Design On MXene With Confined Fe-Doped CoSe2 For Ultra-Efficient OER ElectrocatalysisDocument8 pagesReducing Energy Barriers by Multi-Interface Design On MXene With Confined Fe-Doped CoSe2 For Ultra-Efficient OER ElectrocatalysisDuc-Viet NguyenPas encore d'évaluation
- Top 1Document19 pagesTop 1toufik bendibPas encore d'évaluation
- Effect of Buffer Layers On Performance of Organic Photovoltaic Devices Based On Copper Phthalocyanine-Perylene Dye HeterojunctionDocument6 pagesEffect of Buffer Layers On Performance of Organic Photovoltaic Devices Based On Copper Phthalocyanine-Perylene Dye HeterojunctionAnonymous cYpEVvoPas encore d'évaluation
- 2022 MOGENS Current Understanding of Ceria Surfaces For CO2 Reduction in SOECs and Future Prospects - A ReviewDocument19 pages2022 MOGENS Current Understanding of Ceria Surfaces For CO2 Reduction in SOECs and Future Prospects - A Reviewantonello tebanoPas encore d'évaluation
- Book Chapter Page 485-494Document10 pagesBook Chapter Page 485-494rajeshayrPas encore d'évaluation
- Electrochimica Acta: N.M. Farandos, L. Kleiminger, T. Li, A. Hankin, G.H. KelsallDocument8 pagesElectrochimica Acta: N.M. Farandos, L. Kleiminger, T. Li, A. Hankin, G.H. Kelsallgogubila2501Pas encore d'évaluation
- 3 - The Role of Oxygen-Permeable Ionomer For Polymer Electrolyte Fuel CellsDocument9 pages3 - The Role of Oxygen-Permeable Ionomer For Polymer Electrolyte Fuel CellsFaseeh KKPas encore d'évaluation
- Modelagem em CFDDocument7 pagesModelagem em CFDclaralisiePas encore d'évaluation
- CV MoS2 Beilstein J. Nanotechnol. 2022, 13, 528-537Document10 pagesCV MoS2 Beilstein J. Nanotechnol. 2022, 13, 528-537gengan saravananPas encore d'évaluation
- Effects of KOH Etching On The Properties of Ga-PolarDocument14 pagesEffects of KOH Etching On The Properties of Ga-PolarAmor BchetniaPas encore d'évaluation
- Co Doped With Ni-MOFDocument8 pagesCo Doped With Ni-MOFBilal KhalidPas encore d'évaluation
- Band GapDocument10 pagesBand Gappetru apopeiPas encore d'évaluation
- 1 s2.0 S0360319916002184 AmDocument19 pages1 s2.0 S0360319916002184 Amdogars123Pas encore d'évaluation
- ZIF-derived holey electrode with enhanced mass transfer and N-rich catalytic sitesDocument10 pagesZIF-derived holey electrode with enhanced mass transfer and N-rich catalytic sitesNoah CHENPas encore d'évaluation
- Surfaces and InterfacesDocument9 pagesSurfaces and InterfacesBaurzhan IlyassovPas encore d'évaluation
- CO Poisoning - 1Document17 pagesCO Poisoning - 1Faseeh KKPas encore d'évaluation
- Nio-Cgo in Situ Nanocomposite Attainment: One Step SynthesisDocument6 pagesNio-Cgo in Situ Nanocomposite Attainment: One Step SynthesisgrazielelopessPas encore d'évaluation
- Strain Effects On The Ionic Conductivity of Y-Doped Ceria: A Simulation StudyDocument17 pagesStrain Effects On The Ionic Conductivity of Y-Doped Ceria: A Simulation StudyABHISHEK SINGH MSMEPas encore d'évaluation
- Karbon - Meso - Supercapacitor - 2011 PDFDocument5 pagesKarbon - Meso - Supercapacitor - 2011 PDFAndiQonitaPas encore d'évaluation
- Pa Jpet Vol 01 Issue 01 - 09Document5 pagesPa Jpet Vol 01 Issue 01 - 09Isaac Hyuk DanielPas encore d'évaluation
- Shinde 2019Document8 pagesShinde 2019vijayamathubalan pandyPas encore d'évaluation
- 1 s2.0 S1385894718324276 MainDocument9 pages1 s2.0 S1385894718324276 MainharrisonPas encore d'évaluation
- A 0230106Document6 pagesA 0230106AJER JOURNALPas encore d'évaluation
- Intro Me So Porous ElectrodeDocument14 pagesIntro Me So Porous ElectrodePallab BhattacharyaPas encore d'évaluation
- 1 s2.0 S221128552301056X MainDocument11 pages1 s2.0 S221128552301056X MainABDELKHALK ABOULOUARDPas encore d'évaluation
- Ordered Mesoporous Carbon SphereDocument11 pagesOrdered Mesoporous Carbon SphereŞebnem Gül İlarslanPas encore d'évaluation
- Ion ExchangeDocument13 pagesIon Exchangebreadfalling752Pas encore d'évaluation
- (Done) Hollow Porous Co3O4 - NC@rGO Derived From Reuleaux Tetrahedral ZIF-67 As A Promising Anode Material For LDocument10 pages(Done) Hollow Porous Co3O4 - NC@rGO Derived From Reuleaux Tetrahedral ZIF-67 As A Promising Anode Material For Luser728384940Pas encore d'évaluation
- Modified Electrode With ZeoliteDocument20 pagesModified Electrode With ZeoliteAseet AnsumanPas encore d'évaluation
- Fundamentals and Challenges of Electrochemical CO Reduction Using Two-Dimensional MaterialsDocument28 pagesFundamentals and Challenges of Electrochemical CO Reduction Using Two-Dimensional MaterialsIman QurbanovPas encore d'évaluation
- Achieving Superior Energy Storage and Microwave Absorption byDocument12 pagesAchieving Superior Energy Storage and Microwave Absorption bynoumanPas encore d'évaluation
- Int J Applied Ceramic Tech - 2023 - Moghadam - The Effect of Foamed Cement Nanocomposite As Counter Electrode On TheDocument26 pagesInt J Applied Ceramic Tech - 2023 - Moghadam - The Effect of Foamed Cement Nanocomposite As Counter Electrode On Theali abdolahzadeh ziabariPas encore d'évaluation
- Comparative Study of The Conductivity of Synthesized Bivalent Vanadates CaV2O6 and MnV2O6Document5 pagesComparative Study of The Conductivity of Synthesized Bivalent Vanadates CaV2O6 and MnV2O6Noremozachhom JahidPas encore d'évaluation
- Improvement in VRFB by modified graphite felt_Mahanta_2020Document13 pagesImprovement in VRFB by modified graphite felt_Mahanta_2020SureshBharadwajPas encore d'évaluation
- A Carbon Electrode Fabricated Using A Poly (Vinylidene Fluoride) Binder Controlled The Faradaic Reaction of Carbon PowderDocument5 pagesA Carbon Electrode Fabricated Using A Poly (Vinylidene Fluoride) Binder Controlled The Faradaic Reaction of Carbon PowderAlice1923Pas encore d'évaluation
- 10 31127-Tuje 1290655-3114991Document9 pages10 31127-Tuje 1290655-3114991amir ijazPas encore d'évaluation
- Molecular modeling of carbon dioxide transport and storage in porous carbon 5สค 56Document9 pagesMolecular modeling of carbon dioxide transport and storage in porous carbon 5สค 56Buarin Meen BoorinPas encore d'évaluation
- 1 s2.0 S0048969722062040 MainDocument12 pages1 s2.0 S0048969722062040 MainAlbanny PerezPas encore d'évaluation
- Eur J Inorg Chem - 2010 - Duan - Synthesis and Characterization of Metallic Co With Different Hierarchical StructuresDocument6 pagesEur J Inorg Chem - 2010 - Duan - Synthesis and Characterization of Metallic Co With Different Hierarchical Structuresmahdi ghamsariPas encore d'évaluation
- Single Wall Carbon Nanotubes/Polypyrrole Composite Thin Film Electrodes: Investigation of Interfacial Ion Exchange BehaviorDocument14 pagesSingle Wall Carbon Nanotubes/Polypyrrole Composite Thin Film Electrodes: Investigation of Interfacial Ion Exchange BehaviormiguelPas encore d'évaluation
- Optical Photocatalytic Electrochemical Magnetic DiDocument19 pagesOptical Photocatalytic Electrochemical Magnetic DiTasawer Shahzad AhmadPas encore d'évaluation
- 1-s2.0-S001623612102425X-mainDocument13 pages1-s2.0-S001623612102425X-mainfoyahov478Pas encore d'évaluation
- High-Loading Single Cobalt Atoms On Ultrathin MOF Nanosheets For Efficient Photocatalytic CO ReductionDocument8 pagesHigh-Loading Single Cobalt Atoms On Ultrathin MOF Nanosheets For Efficient Photocatalytic CO Reductionbin caiPas encore d'évaluation
- ReviewDocument17 pagesReviewfedemarcianoPas encore d'évaluation
- DSC Articles and ThesisDocument12 pagesDSC Articles and ThesisMohamedPas encore d'évaluation
- 2017 ZhiguoYeDocument7 pages2017 ZhiguoYethong.dataphdPas encore d'évaluation
- Ce Mo 3Document12 pagesCe Mo 3Tanvir HossainPas encore d'évaluation
- Non-Covalent Modification of Graphene Sheets in PEDOT Composite Materials by Ionic LiquidsDocument11 pagesNon-Covalent Modification of Graphene Sheets in PEDOT Composite Materials by Ionic LiquidsCedric Omar Hdz RiescoPas encore d'évaluation
- tmp3CAB TMPDocument16 pagestmp3CAB TMPFrontiersPas encore d'évaluation
- tmpCE8C TMPDocument19 pagestmpCE8C TMPFrontiersPas encore d'évaluation
- tmpFFE0 TMPDocument6 pagestmpFFE0 TMPFrontiersPas encore d'évaluation
- tmpE7E9 TMPDocument14 pagestmpE7E9 TMPFrontiersPas encore d'évaluation
- tmp6F0E TMPDocument12 pagestmp6F0E TMPFrontiersPas encore d'évaluation
- tmpE3C0 TMPDocument17 pagestmpE3C0 TMPFrontiersPas encore d'évaluation
- tmpF178 TMPDocument15 pagestmpF178 TMPFrontiersPas encore d'évaluation
- tmp80F6 TMPDocument24 pagestmp80F6 TMPFrontiersPas encore d'évaluation
- tmpEFCC TMPDocument6 pagestmpEFCC TMPFrontiersPas encore d'évaluation
- Tmp1a96 TMPDocument80 pagesTmp1a96 TMPFrontiersPas encore d'évaluation
- tmpF3B5 TMPDocument15 pagestmpF3B5 TMPFrontiersPas encore d'évaluation
- Tmpa077 TMPDocument15 pagesTmpa077 TMPFrontiersPas encore d'évaluation
- tmp72FE TMPDocument8 pagestmp72FE TMPFrontiersPas encore d'évaluation
- tmpF407 TMPDocument17 pagestmpF407 TMPFrontiersPas encore d'évaluation
- tmpC0A TMPDocument9 pagestmpC0A TMPFrontiersPas encore d'évaluation
- tmp60EF TMPDocument20 pagestmp60EF TMPFrontiersPas encore d'évaluation
- tmp8B94 TMPDocument9 pagestmp8B94 TMPFrontiersPas encore d'évaluation
- tmp6382 TMPDocument8 pagestmp6382 TMPFrontiersPas encore d'évaluation
- tmp998 TMPDocument9 pagestmp998 TMPFrontiersPas encore d'évaluation
- tmp4B57 TMPDocument9 pagestmp4B57 TMPFrontiersPas encore d'évaluation
- tmp9D75 TMPDocument9 pagestmp9D75 TMPFrontiersPas encore d'évaluation
- tmp37B8 TMPDocument9 pagestmp37B8 TMPFrontiersPas encore d'évaluation
- tmpC30A TMPDocument10 pagestmpC30A TMPFrontiersPas encore d'évaluation
- tmpD1FE TMPDocument6 pagestmpD1FE TMPFrontiersPas encore d'évaluation
- tmpB1BE TMPDocument9 pagestmpB1BE TMPFrontiersPas encore d'évaluation
- tmp3656 TMPDocument14 pagestmp3656 TMPFrontiersPas encore d'évaluation
- tmpA0D TMPDocument9 pagestmpA0D TMPFrontiersPas encore d'évaluation
- Tmp75a7 TMPDocument8 pagesTmp75a7 TMPFrontiersPas encore d'évaluation
- tmp27C1 TMPDocument5 pagestmp27C1 TMPFrontiersPas encore d'évaluation
- tmp2F3F TMPDocument10 pagestmp2F3F TMPFrontiersPas encore d'évaluation
- MT R 108 000 0 000000-0 DHHS B eDocument68 pagesMT R 108 000 0 000000-0 DHHS B eRafal WojciechowskiPas encore d'évaluation
- The Essence of Success - Earl NightingaleDocument2 pagesThe Essence of Success - Earl NightingaleDegrace Ns40% (15)
- Itec 3100 Student Response Lesson PlanDocument3 pagesItec 3100 Student Response Lesson Planapi-346174835Pas encore d'évaluation
- Sampling Fundamentals ModifiedDocument45 pagesSampling Fundamentals ModifiedArjun KhoslaPas encore d'évaluation
- 2010 Yoshimura Book of SpeedDocument83 pages2010 Yoshimura Book of Speedacornwoods_brentPas encore d'évaluation
- Duty Entitlement Pass BookDocument3 pagesDuty Entitlement Pass BookSunail HussainPas encore d'évaluation
- Localization Strategy in Vietnamese Market: The Cases ofDocument25 pagesLocalization Strategy in Vietnamese Market: The Cases ofHồng Thy NguyễnPas encore d'évaluation
- Management Reporter Integration Guide For Microsoft Dynamics® SLDocument22 pagesManagement Reporter Integration Guide For Microsoft Dynamics® SLobad2011Pas encore d'évaluation
- GSM Multi-Mode Feature DescriptionDocument39 pagesGSM Multi-Mode Feature DescriptionDiyas KazhiyevPas encore d'évaluation
- Rust Experimental v2017 DevBlog 179 x64 #KnightsTableDocument2 pagesRust Experimental v2017 DevBlog 179 x64 #KnightsTableIngrutinPas encore d'évaluation
- Perbandingan Sistem Pemerintahan Dalam Hal Pemilihan Kepala Negara Di Indonesia Dan SingapuraDocument9 pagesPerbandingan Sistem Pemerintahan Dalam Hal Pemilihan Kepala Negara Di Indonesia Dan SingapuraRendy SuryaPas encore d'évaluation
- Job Hazard Analysis TemplateDocument2 pagesJob Hazard Analysis TemplateIzmeer JaslanPas encore d'évaluation
- STS Chapter 5Document2 pagesSTS Chapter 5Cristine Laluna92% (38)
- Contemporary World Prelim Exam Test DraftDocument5 pagesContemporary World Prelim Exam Test DraftGian Quiñones93% (45)
- Harry Styles: The Rise of a Pop StarDocument9 pagesHarry Styles: The Rise of a Pop StarBilqis LaudyaPas encore d'évaluation
- 13 Daftar PustakaDocument2 pages13 Daftar PustakaDjauhari NoorPas encore d'évaluation
- HetNet Solution Helps Telcos Improve User Experience & RevenueDocument60 pagesHetNet Solution Helps Telcos Improve User Experience & RevenuefarrukhmohammedPas encore d'évaluation
- DS 20230629 SG3300UD-MV SG4400UD-MV Datasheet V16 ENDocument2 pagesDS 20230629 SG3300UD-MV SG4400UD-MV Datasheet V16 ENDragana SkipinaPas encore d'évaluation
- Lead Magnet 43 Foolproof Strategies To Get More Leads, Win A Ton of New Customers and Double Your Profits in Record Time... (RDocument189 pagesLead Magnet 43 Foolproof Strategies To Get More Leads, Win A Ton of New Customers and Double Your Profits in Record Time... (RluizdasilvaazevedoPas encore d'évaluation
- Amended ComplaintDocument38 pagesAmended ComplaintDeadspinPas encore d'évaluation
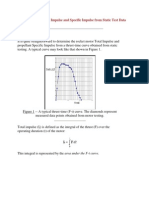
- Determining Total Impulse and Specific Impulse From Static Test DataDocument4 pagesDetermining Total Impulse and Specific Impulse From Static Test Datajai_selvaPas encore d'évaluation
- CaseHistoriesOnTheApplication of Vacuum PreloadingDocument25 pagesCaseHistoriesOnTheApplication of Vacuum PreloadingvaishnaviPas encore d'évaluation
- Youtube AlgorithmDocument27 pagesYoutube AlgorithmShubham FarakatePas encore d'évaluation
- Activate Adobe Photoshop CS5 Free Using Serial KeyDocument3 pagesActivate Adobe Photoshop CS5 Free Using Serial KeyLukmanto68% (28)
- 2014 March CaravanDocument48 pages2014 March CaravanbahiashrinePas encore d'évaluation
- Marketing Management NotesDocument115 pagesMarketing Management NotesKajwangs DanPas encore d'évaluation
- Joint Memorandum Circular (JMC) No. 2021Document49 pagesJoint Memorandum Circular (JMC) No. 2021Nicey RubioPas encore d'évaluation
- Software Engineering Modern ApproachesDocument775 pagesSoftware Engineering Modern ApproachesErico Antonio TeixeiraPas encore d'évaluation
- NDU Final Project SP23Document2 pagesNDU Final Project SP23Jeanne DaherPas encore d'évaluation
- BCM Risk Management and Compliance Training in JakartaDocument2 pagesBCM Risk Management and Compliance Training in Jakartaindra gPas encore d'évaluation
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsD'EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsPas encore d'évaluation
- Guidelines for Chemical Process Quantitative Risk AnalysisD'EverandGuidelines for Chemical Process Quantitative Risk AnalysisÉvaluation : 5 sur 5 étoiles5/5 (1)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksD'EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksÉvaluation : 5 sur 5 étoiles5/5 (1)
- Guidelines for the Management of Change for Process SafetyD'EverandGuidelines for the Management of Change for Process SafetyPas encore d'évaluation
- Piping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationD'EverandPiping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationÉvaluation : 4 sur 5 étoiles4/5 (18)
- Nuclear Energy in the 21st Century: World Nuclear University PressD'EverandNuclear Energy in the 21st Century: World Nuclear University PressÉvaluation : 4.5 sur 5 étoiles4.5/5 (3)
- Trevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationD'EverandTrevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationPas encore d'évaluation
- Produced Water Treatment Field ManualD'EverandProduced Water Treatment Field ManualÉvaluation : 4.5 sur 5 étoiles4.5/5 (5)
- Guidelines for Siting and Layout of FacilitiesD'EverandGuidelines for Siting and Layout of FacilitiesPas encore d'évaluation
- Process Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentD'EverandProcess Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentPas encore d'évaluation
- Well Control for Completions and InterventionsD'EverandWell Control for Completions and InterventionsÉvaluation : 4 sur 5 étoiles4/5 (10)
- Guidelines for Vapor Cloud Explosion, Pressure Vessel Burst, BLEVE, and Flash Fire HazardsD'EverandGuidelines for Vapor Cloud Explosion, Pressure Vessel Burst, BLEVE, and Flash Fire HazardsPas encore d'évaluation
- Guidelines for Enabling Conditions and Conditional Modifiers in Layer of Protection AnalysisD'EverandGuidelines for Enabling Conditions and Conditional Modifiers in Layer of Protection AnalysisPas encore d'évaluation
- An Applied Guide to Water and Effluent Treatment Plant DesignD'EverandAn Applied Guide to Water and Effluent Treatment Plant DesignÉvaluation : 5 sur 5 étoiles5/5 (4)
- Robotics: Designing the Mechanisms for Automated MachineryD'EverandRobotics: Designing the Mechanisms for Automated MachineryÉvaluation : 4.5 sur 5 étoiles4.5/5 (8)
- Guidelines for Developing Quantitative Safety Risk CriteriaD'EverandGuidelines for Developing Quantitative Safety Risk CriteriaPas encore d'évaluation
- Chemical Process Safety: Learning from Case HistoriesD'EverandChemical Process Safety: Learning from Case HistoriesÉvaluation : 4 sur 5 étoiles4/5 (14)
- Guidelines for Engineering Design for Process SafetyD'EverandGuidelines for Engineering Design for Process SafetyPas encore d'évaluation
- Oxygen to the Rescue: Oxygen Therapies, and How They Help Overcome Disease and Restore Overall HealthD'EverandOxygen to the Rescue: Oxygen Therapies, and How They Help Overcome Disease and Restore Overall HealthÉvaluation : 4.5 sur 5 étoiles4.5/5 (3)
- Major Accidents to the Environment: A Practical Guide to the Seveso II-Directive and COMAH RegulationsD'EverandMajor Accidents to the Environment: A Practical Guide to the Seveso II-Directive and COMAH RegulationsPas encore d'évaluation
- The HAZOP Leader's Handbook: How to Plan and Conduct Successful HAZOP StudiesD'EverandThe HAZOP Leader's Handbook: How to Plan and Conduct Successful HAZOP StudiesPas encore d'évaluation
- Bow Ties in Risk Management: A Concept Book for Process SafetyD'EverandBow Ties in Risk Management: A Concept Book for Process SafetyPas encore d'évaluation
- Guidelines for Hazard Evaluation ProceduresD'EverandGuidelines for Hazard Evaluation ProceduresÉvaluation : 5 sur 5 étoiles5/5 (4)