Académique Documents
Professionnel Documents
Culture Documents
00032106
Transféré par
TAOUACHICopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
00032106
Transféré par
TAOUACHIDroits d'auteur :
Formats disponibles
IEEE T R A N S A C T I O N S O N B I O M E D I C A L E N G I N E E R I N G . VOL. 36. N O . 7.
JULY 1989
73R
FES and Spasticity
ANETA STEFANOVSKA, LOJZE VODOVNIK, S E N I O R MEMBER, I E E E , NUSA GROS, STANISLAV
REBERSEK, A N D R U ~ AACIMOVIC-JANE~IC
Abstrucf-A model of hemiplegic spasticity based on electromyographical and biomechanical parameters measured during passive
muscle stretching is presented. Two components of spasticity can be
distinguished-phasic and tonic. This classification depends on the pattern of stretch reflex activity which can be either phasic or tonic as well
as on the muscle stretchltension characteristic. Stretch reflex, as a control loop, is in phasic spasticity characterized by increased sensitivity
to velocity of stretching. In tonic spasticity, sensitivity to length of
stretching is increased. After the injury, phasic spasticity appears first
and invokes monosynaptic reflex pathways. The intensity of tonic spasticity increases with the duration of disability and hence causes changes
in muscle fiber biomechanical properties.
The model mentioned above has been used to evaluate the effects of
FES on spasticity. Hemiplegic patients with implanted peroneal nerve
stimulator for gait correction were followed up for one year starting a
week before implantation. Long-term use of FES resulted in decrease
of tonic spasticity in both ankle joint antagonistic muscle groups. In
stimulated tibialis anterior muscle, the phasic type of spasticity increased. Tu obtain the correlation between changes in spasticity and
functional abilities of patients, the maximal voluntary isometric contraction of both muscle groups was also measured. An improvement in
voluntary strength was also observed. This can be taken as additional
evidence that tonic spasticity is of greater physiological and clinical
significance than phasic spasticity. It may he concluded that use of FES
can decrease tonic spasticity and, if applied early after the injury, can
prevent the appearance of tonic spasticity.
INTRODUCTION
HERAPEUTIC and carry over effects of functional electrical stimulation (FES) have often been
observed and are still under investigation [I 11, [25], [29],
[41]. The functional aspects of changes caused by longterm FES have been tr_eated in various studies. Merletti
with co-workers [30], StefanCiE, ReberSek, and Merletti
[44] and Vodovnik and ReberPek [46] have reported
changes in voluntary control of paretic muscles in hemiplegic patients following FES. Improvement of motor coordination and gait abilities evaluated by biomechanical
and electromyographical measurements during gait have
also been studied by Carnstam, Larsson, and Prevec [8]
and Maleii? et al. 1261. However, it is still questionable
M;inu\cript recci\cd May 70. 1988; revised Novcmber 12. 1988. This
u o r h \-.a\ ~ u p p o i t c din part by the Slovene Research Coinmunity, Ljuhll:ttici. Yugo\la\ i c i . the National lnatitute for Diwbility and Rehabilitation
K e ~ c a r c l i Warhiiigtoii.
.
I>C. and the Vivian Smith Foundation for R e \ t ~ -
iati\c Nctirolog!. Hou>t(rn. TX.
A Sti.tano\\ha. L. VodoLnik. and S . ReberSek are with the Faculty of
tlcctrical Engineering. Edvard Kardelj University. 61000 I,jubl,jana, Yugo\l.i\ i;i
N. Groa and R. Ai.imo\iC-Jane2i? arc with the University Rehabilitation
In\tlturc. t;d\ ard Kardelj Uni\cr\it) . L,jubl.jana. Yugoslavia.
I L E t 1 ~ ) Nutiihcr
s
8927436.
whether long-term use of FES results in direct improvement of motor control or also has a certain impact on spasticity.
At least two reasons can explain the lack of quantitative
results of the effects of either short or long term electrical
stimulation on spasticity. First, since a unique definition
of spasticity is still lacking [21], [22] there are numerous
studies concerned with the different parameters of spasticity [4], [8], [15], [28], [38], [47] which can not be easily compared. Second, there is a lack of a simple and effective method for quantitative measurement of the
different components which characterize spasticity. A
large number of studies reported only qualitatively the effects of electrical stimulation on muscle spasticity [3],
[ 171, [23], [24]. Consequently, different and frequently
contradictory opinions about the effects of electrical stimulation on spasticity appear. In clinical practice, there
even exists a widespread attitude that one should not stimulate an already overstimulated spastic muscle.
In this paper, a model of hemiplegic spasticity is presented which is based on electromyographical and biomechanical parameters measured during passive muscle
stretching. The model was used to analyze the results obtained from measurement of ankle joint spasticity in hemiplegic patients with implanted peroneal nerve stimulator
for gait correction. Data were collected starting one week
before implantation and continuing for a maximum of one
year of FES. To obtain the correlation between changes
in spasticity and functional abilities of the patients, the
maximal voluntary isometric contraction of both antagonistic muscle groups was also measured.
MODELOF SPASTICITY
The definition of spasticity is a subject of diverse opinions. A frequently used definition is that of Landau [22],
which includes: 1) decreased dexterity, 2) loss of strength,
3) increased tendon jerks, 4) increased resistance to slower
passive muscle stretch, and 5 ) hyperactive flexion reflexes
(flexor spasms). On the other hand, Knutsson [ 181 described almost unlimited interindividual variation in patients with spastic paresis. This is the basis for the opinion
that spasticity has to be substituted by a detailed description of each particular patients motor disfunction. In this
paper, we have adopted the definition of Pedersen 13 I ] in
which spasticity is described as a part of the upper motoneurone syndrome. The increased phasic and tonic
stretch reflexes are the central part of this definition. Ac-
00 18-9294/89/0700-0738$0 I .OO C<J 1989 lEEE
STEFANOVSKA
er
739
d.:FES A N D SPASTICITY
cordingly , by applying passive muscle stretching at different velocities one can measure neurophysiological and
biomechanical parameters as an indicator of the degree of
spasticity.
Methods of measuring the ankle joint resistance have
already been described [ l ] , [14], [34], [35]. Agarwal and
Gottlieb applied controlled sinusoidal torques and measured the resulting movement in normal [ l ] and spastic
subjects [14]. Rack [34] and Rack et al. [35] imposed
sinusoidal movement and measured resulting torque in
normal subjects. Our approach differs from ones already
mentioned since we applied frequencies of significantly
lower values (0.1-2 Hz) and constant, high amplitude oscillations. There are both engineering and physiological
reasons for such a decision: a) Due to nonlinearities in a
spastic ankle joint the applied frequencies are enough informative in the sense that changes in frequency imply
changes in peak values of the resistive torque. b) While
testing spasticity, the muscle has to be stretched in all of
its length. The range of ankle joint motion in normal subject is 80-90 and is reduced to 40-50 in spastic patients. c) Triggering and measuring the tonic component
of spasticity depend on the duration of stretching. d) For
the phasic component of spasticity, the velocity of
stretching is of high significance. For example, when a
sinusoidal displacement of 1 Hz and amplitude of 16 is
applied, the maximal velocity of stretching is 100/s
which is enough to trigger phasic reflex in the spastic
muscle. Practically, such high amplitude/low frequency
oscillations mimic clinical testing of spasticity.
Measurement of Spasticity
The spasticity of ankle joint muscles in hemiplegic patients was examined. Passive muscle stretching was obtained by sinusoidal movements of the foot in the sagittal
plane. The joint movements were performed by an electrohydraulic position-controlled servosystem [37]. Fig. 1
shows the experimental setup. The center of rotation was
always aligned with the ankle joint axis. Constant resting
(bias) and displacement angles were used. Velocity
dependence on passive resistance was tested at four different velocities of movements, i.e., frequencies of sinusoidal oscillations. Ten successive periods of oscillations were measured at each frequency, with the start and
stop position always at the same bias angle. Four parameters were recorded:
EMG of the tibialis anterior (TA) muscle,
EMG of the triceps surae (TS) muscle,
angular position of the ankle joint, and
resistive torque at the ankle.
Electromyographic activity was detected by silver chloride disc electrodes of 1 cm in diameter.
In the relaxed and cooperative subject a joint resists
movement as a result of its biomechanical properties (joint
inertia and viscoelastic properties of the muscles and joint)
and the degree of triggered stretch reflex, i.e.,
= f(Mc(cp.
CP)? R,(cp, CP,)
signal
electro-
generator
servosystem
ankle
joint
I li
rorque
A b
computer
tibialis soleus
anterior
conversion
analog
recorder
Fig. 1. Block diagram of ankle joint muscles spasticity measurement
where
is the angular position of the ankle joint, defined
as cp = cpo A sin 27r ft
(po is the resting angle; 30 < (po < 40 in plantar
flexion,
A is the amplitude of oscillation; A = 16,
f is the frequency of movement; f = 0.1,0.5, 1, and
2 Hz,
(b is the velocity of movement; ,,b,(
= 10, 50, 100,
and 200/s,
Mc-ankle joint muscle biomechanical properties,
cp
and
R,-characteristics
of the stretch reflex loop.
The above parameter values are relevant for this paper.
Components of Spasticity
The classification of the components of spasticity is
based on the measurements of resistive torque in more
than 100 hemiplegic patients. We have also made the same
measurements in at least 20 normal subjects. In a normal
subject, there is no electromyographic activity in either
the TS or the TA muscles at peak velocities of 10/s200 / s . The peak to peak value of resistive torque does
not change, depending only on the passive biomechanical
properties of the ankle joint, i.e. M = Mc = const. (see
Fig. 3). In the case of spasticity, both electromyographic
activity and resistive torque are increased, resulting in a
characteristic pattern for each particular patient. However, two general types of spasticity can be distinguished
in the hemiplegic population: tonic and phasic spasticity.
Neurophysiological Differences: The two expressions
are in fact used according to the type of reflex activity
[ 121, [ 191, [20], [45]. The term tonic spasticity is used
when tonic component of the stretch reflex activity is predominant, while phasic spasticity is used when phasic
component is predominant. In phasic spasticity, passive
muscle stretching beyond a certain muscle length threshold triggers reflex activity of phasic type. The EMG re-
740
IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING. VOL. 36. NO. 7. JULY 1989
301
l/-j
25
tonic
..
-=E 2 0 W
15-
10-
ut
200%
[ L N rn
0.1
I
I
0.5
1.0
2.0
Frequency ( i : z )
(b)
Fig. 2. (a) Typical pattern of exclusively phasic stretch reflex activity in
triceps surae muscle during passive ankle joint movement. EMG activity
and resistive torque linearly increased with an increase in the velocity of
niovcment. (b) Tonic reflex activity caused strong resistance during passive muscle stretching [note the different sensitivity of resistive torque
in (a) and (b)]. The increased velocity of movement, with simultaneously
decreased duration of stretching resulted in constant EMG activity and
rcsi9tive torque.
cord consists of brief discharge bursts of high frequencies. The intensity increases linearly with increasing
velocity of stretching. Fig. 2(a) shows a typical pattern of
exclusively phasic reflex activity during passive ankle
joint movement, and Fig. 2(b) shows a typical tonic pattern of reflex activity. In the case of tonic activity, the
EMG exhibits a tonic-maintained, low-frequency discharge pattern. The intensity of tonic activity depends on
the velocity and duration of stretching. Increase in frequency of movement results in increased velocity and decreased duration of stretching. The duration of stretching
for each muscle group-agonistic and antagonistic-at 2
Hz is half that at 1 Hz. Consequently, the tonic reflex
activity does not increase proportionally to the velocity of
movement, as shown in Fig. 2(b).
Biomechanical Differences: Velocityltorque characteristics measured on a large population of patients and normal subjects are clustered into three main groups: phasic,
tonic, and normal (Fig. 3 ) . Each of the measured velocity/torque curves falls within one of the three regions
characterized by the upper and lower boundary limit
curve.
At the frequency of 0.1 Hz, the maximal velocity ($,,,
= 10" / s ) is not high enough to trigger phasic reflex even
in the most hyperactive stretch reflex loop. Hence, in
Fig. 3 . Torque versus frequency (velocity) characteristics measured on a
large population of patients and normal subjects are clustered into three
main groups: phasic, tonic, and normal. Curves from each particular
group exhibit a similar pattern. For normal subjects peak to peak values
of resistive torque (RT) is constant at all measured frequencies. In the
curves related to the phasic group, RT increases roughly linearly or even
slightly faster at higher frequencies. In the tonic group there is a considerable increase in RT at lower frequencies while at higher frequencies it
remains practically saturated.
phasic spasticity, the resistive torque includes only biomechanical properties and takes on the values close to
normal subjects. In tonic spasticity, the resistive torque
at 0.1 Hz is higher and reflects either changed muscle fiber properties or a decreased threshold of tonic discharge.
For normal subjects peak-to-peak value of resistive torque
is constant at all measured frequencies. In the phasic
group, the resistive torque increases roughly linearly with
respect to the applied frequency (in some cases the increase can go slightly faster at higher frequencies). In the
tonic group, there is a considerable increase in resistive
torque at lower frequencies (velocities) while at higher
frequencies the resistive torque remains practically saturated. Furthermore, the absolute values of resistive torque
are also significantly higher in tonic than in phasic spasticity. An analytical expression of the above qualitative
descriptions is developed below. The linearity of velocity
dependence of resistive torque can be represented by the
difference in the linear regression characteristic and measured values (regression error)
C IM; I
where M is the measured value of resistive torque obtained by the averaging of peak-to-peak values at ten successive oscillations, A is the estimated value of resistive
torque by linear regression analysis, and for i = 1 ,
* * * , 4 the frequency of movement ( f ) is 0.1, 0.5, 1 ,
and 2 Hz.
The intensity of resistance can be represented by the
area constrained with the interval (0.1; 2 Hz) on the .x
axis and the measured values of resistive torque on the y
axis. In this way the quantitative measure of spasticity can
be defined bv
P = ep/k
where p is the area below the resistive torque curve and k
is a constant.
STEFANOVSKA
el
By choosing k
P
1
<P
I1
74 1
u l . : FES A N D SPASTICITY
TABLE I
PATIENTS
DATA
10 N2 m2 Hz:
would signify a pattern with no spasticity,
I 10 represents
dominant phasic spasticity,
Patient
Duration of
Disability
Before Impl.
Age at
Implant.
Cause of Spasticity
3m
25 Y
3m
11 m
6Y
4m
4m
1Y
50
42
37
54
41
48
50
41
CVI
Brain injury
CVI
CVI
Barotrauma
CVI
CVI
Tumor
and
10 < P
I20
represents dominant tonic spasticity.
LONG-TERM
EFFECTSOF FES
Methodology
The rationale for using implanted electrical stimulator
is that it enables simpler application and regular utilization for the patient, which is particularly important in
long-term study. Eight hemiplegic patients with implanted peroneal nerve stimulators for gait correction were
studied. Patient data are presented in Table I with respect
to the date of implantation. Additional clinical data are
reported elsewhere [4 11.
Before implantation each patient used surface electrical
stimulation for two to three weeks, 30 min per day. The
decision for implantation was made in cases when satisfactory effects of surface FES on gait pattern correction
during swing phase were obtained. All patients were able
to walk using a crutch, except for patient 6, who was able
to walk for 30 m only when assisted. After implantation
all stimulation was stopped for 11 f 1 days. Within the
following week, patients learned to use the peroneal implant and then continued to use chronic electrical stimulation for up to 2 h per day. Only patient 6 did not use
FES to such an extent, as a consequence of his initial inability to walk independently for long distances. Pulse
width of stimulation impulses was up to 0.5 ms using both
surface and implanted electrodes while the frequency was
30-33 Hz. Details on the implantable stimulator and the
technique of implantation are reported elsewhere [42],
WI.
The experimental schedule with particular phases is
given in Fig. 4. The measurements of spasticity and voluntary isometric contraction were made 8 & 4 days before, 21 & 5 days after, six months after and 12 months
after the implantation. Isometric contraction was measured in dorsal and plantar flexion in the same ankle angle
as the resting angle ( cpo) during measurement of spasticity. Maximal voluntary contraction in the TA and T 3
muscles was performed as selectively as possible and had
a duration of approximately 10 s for each muscle. During
the measurements, the patient was always given the same
instructions by the examiner to contract maximally only
one muscle group.
RESULTS
Spasticity: Measurements of resistive torque values
obtained before and six months after implantation are presented in Fig. 5 . Each point in the torque versus frequency plot is defined by coordinates ( f , % ) where f is
is the average of 10 succesthe applied frequency and
sive peak-to-peak values of resistive torque. Low and
constant resistance to passive movement was character-
1) M . A.
2) H. H.
3) R. H.
4) G. J .
5) P. M.
6) J. R.
7) S . A .
8) C . A .
implontation
regular
meosursment measurement
(71)
(T2)
1 week
u s e of FES
maosurament
(T3 )
6 months
mwsurament
(TI)
6 months
Fig. 4. Time track of the FES application and measurements of spasticity.
Fig. 5 . Resistive torque versus frequency (velocity) characteristic before
implantation (solid lines) and after six months of FES (dashed lines).
The linearization which signifies the reduction of tonic spasticity is particularly noticeable in patients 1 and 4 .
istic of patient 8. Chronic FES did not cause any appearance of spasticity in this case. The other seven patients
studied had velocity-dependent resistance increased with
respect to normal. The compressed data for all patients
are presented in Fig. 6. The ordinate of the diagram represents the average of peak to peak values from all the
patients at four frequencies. Three curves are related to
the measurements before, after, and six months after implantation. The decrease in resistive torque after six
months of FES is evident. One can also recognize tile
tendency of the curves toward linearity. This corresponds
to a change in the pattern of reflex activity in the triceps
surae muscle to a phasic pattern. After six months of FES
the phasic reflex activity slightly increased in the stimulated tibialis anterior muscle. A typical increase can be
742
IEEE TRANSACTIONS ON BIOMEDICAL ENGINEERING, VOL. 36, NO. 7. JULY 1989
post
3 wk
- 10
6 mo post
gJ
0 01
05
0 10
50
I
Freourncvinzi
100
Md.,rndI
xi0
"eloClly I D I S )
Fig. 6. The average values of the resistive torque calculated from the re
sults obtained in all patients.
r -
tibialis anterior
*--*-*
-__-
,{~&"c.-c
.----
I
I
triceps surae
i
position of the ankle p n l
Fig 7 Record\ obtained during passive movement performed at the frequency of 2 Hz before and after six months of FES in patient 4 Increase
in phasic reflex activity in stimulated tibialis anterior muscle IS evident
QUANTITATIVE
TABLE I1
MEASURE
OF SPASTICITY
(P)
Patients
1 weekpreop
3 weekspostop
6 monthspostop
19.5
16.5
1.6
9.4
13.6
12.4
6.5
5.5
16.1
1.3
14.5
3.3
10.7
6.1
6.2
13.0
2.3
1.0
0.7
0.3
2.2
5.7
seen in Fig. 7 where records obtained from the same patient during passive movement with a frequency of 2 Hz
(patient 4-before and six months after the implantation)
are presented. To summarize, let us consider the values
of factor P representing the type of spasticity presented in
Table 11. The data clearly show that long-term use of FES
decreased the level of spasticity and influenced the
changes in the type of spasticity from tonic to phasic.
Voluntary Isometric Contraction: Results are presented in Fig. 8(a) for plantar flexion, and Fig. 8(b) for
dorsal flexion. Each point in the torque versus time plane
is the average value of isometric torque obtained during
10 s of voluntary contraction. Improvement of voluntary
strength in both plantar and dorsal flexors is evident. This
improvement is of higher extent in TA muscle where electrical stimulation was applied.
DISCUSSION
A model of spasticity is presented which includes two
types of spasticity influenced in different ways by FES. A
significant decrease in tonic spasticity in both TS and TA
(b)
Fig. 8. Values of maximal voluntary isometric contraction in plantar flexion (a) and dorsal flexion (b). TI-one week before implantation, 1mpI.time of implantation, T2-three weeks after, T3-six months after and
T4--12 months after implantation.
muscles and an increase in phasic spasticity in the stimulated TA muscle were observed. Those changes resulted
in improved voluntary strength in both ankle joint antagonistic muscle groups. Such results are in agreement with
the opinion that tonic reflex activity is of greater physiological and clinical significance than phasic stretch reflex
activity [ 191. The model is based on the measurements of
hemiplegic spasticity and does not include other manifestations of spasticity characteristics of additional pathologies. For example, flexor spasms are rarely present in
hemiplegic spasticity but are particularly dominant in paraplegic spasticity [40].
The method used in this study is based on the measurements of biomechanical and stretch reflex properties of
the spastic muscles. Such a technique has been used in
various studies of mechanisms of spasticity [5], [6], [ 141,
[16], [28], [37], while it has been used in few studies of
the influence of different therapeutic techniques on spasticity [27] and particularly the effects of electrical stimulation [4], [32], [38]. This measurement method enables
minimization of the influence of the measurement itself
on the variable state of spasticity [36]. However, the complexity of spasticity itself and the changes brought about
STEFANOVSKA
rl
U/.:
FES A N D SPASTICITY
by the different techniques are often difficult to interpret.
The understanding of the results obtained in this study
would be incomplete or even incorrect without distinguishing both components of hemiplegic spasticity, particularly due to their sometimes diametrically opposed effects.
During this study, the effects of FES on spasticity were
evaluated over a longer period of time, and spontaneous
changes were also possible. Measurements of hemiplegic
spasticity at different times after the injury [39] have
shown that the phasic type appears first, while the intensity of tonic spasticity increases with the duration of the
disability. An investigation of time changes in ATR
(Achilles tendon reflex) after a spinal cord injury has been
carried out by Ashby et al. [ 2 ] . From this paper, it is
evident that the ATRIM ratio (the M response is muscle
action potentials recorded from motor units which are activated by electrical stimulation of their motor axons),
which is a measure of monosynaptic reflex loop excitability, increases during the first year after injury, and decreases in the third year. However, the results obtained in
our study consistently show the positive effects of electrical stimulation, i.e., decrease of tonic spasticity and increase of voluntary strength. There was only one patient
with increased resistance during passive movement of the
ankle joint. Due to irregular use of FES a spontaneous
increase of reflex activity, which is possible in the early
stage after the injury, is more likely. The interruption for
two weeks due to implantation can be treated as a control
and demonstrated the increase in reflex activity when
stimulation had stopped for a certain time. Therefore, it
can be suggested that electrical stimulation should be continuously used in order to maintain its beneficial effects.
Long-term electrical stimulation, in fact, enhanced the results of its short term effects on tonic activity established
by the same measurement technique [38]. It is interesting
to notice that the influence on tonic activity has also been
reported using spinal cord stimulation in cats [9] and by
cerebellar stimulation in monkeys [ 131.
The improvement in voluntary strength confirms the results obtained by surface stimulation in numerous studies
[30], [44], [46]. Moreover, there are no evident differences regarding the effects of surface and direct nerve
stimulation.
To understand the effects of FES, one has to know the
mechanisms of tonic and phasic spasticity. Phasic spasticity first appears after the injury and phasic reflex activity almost linearly increases with the velocity of movement. Berger, Horstman, and Dietz [7] have stressed that
the exaggerated monosynaptic reflex obviously does not
tension' According to O u r re'Ontribute
much to
sults, phasic Spasticity generates resistive torque significantly lower than tonic spasticity (as shown in Fig. 3 ) .
Therefore, the assumption that phasic spasticity reflects
enhanced monosynaptic reflex loop excitability appears
valid, Intensity of tonic spasticity increases with the duration of disability.In our previous paper, ,391 the correlation between the intensity of tonic activity and the
743
200ms
Fig. 9 . I)Twitch contraction of soleus muscle in normal subject. 2) spastic patient three years after the injury who has been using FES for two
years, and 3) spastic patient two years after the injury who has not been
using FES.
BioReduced
spasticity
Neurophysioloqical
Improved
voluntary control
i ...I
Fig. I O . Direct effects of FES (first level) resulted in long-term changes
of neurophysiological and biomechanical properties (second level).
prolonged twitch contraction time of spastic muscle has
been demonstrated. The structural changes of the muscles
due to spasticity have been shown by Mayer and Yung
[28] in a study of single motor unit activity, and by Dietz,
Ketelsen, Berger, and Quintern [ 101 in histochemical and
morphometrical analysis of spastic muscles. Further such
evidence can be found in the clinical study of Perry [33].
Dietz et al. [ 101 suggested that the muscle fiber transformation may be due to changes in discharge pattern, as
well as due to trophic substance which passes down the
axons. Chronic use of FES can preserve the changes in
muscle contractile properties as shown in Fig. 9 and can
decrease tonic spasticity, as shown in the present paper.
It is difficult to distinguish the origin of these influences
as neurophysiological or biomechanical. The most likely
is a combination of both mechanisms as suggested in Fig.
10. On the other hand, the increased phasic reflex activity
could be due to an increase of monosynaptic reflex loop
sensitivity in the stimulated muscle. The widespread attitude that electrical stimulation causes an increase in
spasticity therefore results from cases where most probably phasic spasticity was increased.
REFERENCES
[ 1 I G . C. Agarwal and G . L . Gottlieb, "Oscillation o f the human ankle
121
131
141
[Si
joint in response to applied sinusoidal torque on the foot." J . PhX.5i d . . vol. 268. pp. 151-176. 1977.
P . Ashby. M . Verrier, and E , Lighfoot, "Segnlental reflex pathwny
in spinal shock and spinal spasticity in man." J . ~ ( , ~ t r -,wrrro.\Itr~q.
o/.
P . \ y ~ h i c i r r ~vol.
.
37. pp. 1352-1360. 1974.
V. Alfieri. "Electrical treatment of spasticity-ReHex tonic activity
eICCtrOSti,nUI~,tiOn,~~
i n hcrniple:ric patients and
S(.cim/. J . Rrlicrh. M d . , vol. 14. pp. 177-182, 1982.
T . Bajd. M . Gregori?, L. Vodovnik. and H. Benko. "Electrical slimulation in treating spasticity due to spinal cord injury." Arc.11. P h ~ s .
T/7rr. R d i d 7 i / . , v o l . 66, pp. 515-517, 1985.
A . Berardelli. M . Hallett. C. Kaufman. E. Fine. W . Berenberg. and
74 4
IEEE TRANSACTIONS O N BIOMEDICAL ENGINEERING. VOL. 36. NO 7. JULY 1969
S . R. Simon, Stretch reflex of triceps surae in normal man, J .
Neurol. Neurosurg. Psychiatry, vol. 45, pp. 513-525, 1982.
[6] D. Burke, J . D . Gilles, and J. D. Lance, The reflex response to
sinusoidal stretching in spastic man, Brain, vol. 94, pp. 455-470,
1971.
[7] W . Berger, G. Horstmann, and V. Dietz, Tension development and
muscle activation in the leg during gait in spastic hemiparesis: Independence of muscle hypertonia and exaggerated stretch reflexes, J .
Neurol. Neurosurg. Psychiatry, vol. 47, pp. 1029-1033, 1984.
[8] B . Camstam, L. E. Larsson, and T. S . Prevec, Improvement of gait
following functional electrical stimulation, Scand. J . Rehabil. Med.,
vol. 9 , pp. 7-13, 1977.
[9] C. E. Chapman, D . G . Ruegg, and M. Wiesendanger, Effects of
dorsal cord stimulation on stretch reflexes, Brain Res., vol. 258,
pp. 211-215, 1983.
[ 101 V. Dietz, U.-P. Ketelsen, W. Berger, and J . Quintem, Motor unit
involvement in spastic paresis-Relationship between leg muscle activation and histochemistry, J . Neurol. Sei., vol. 75, pp. 89-103,
1986.
[ I I] M. R. Dimitrijevid, F . Graranin, T . Prevec, and J. Trontelj, Electronic control of paralyzed extremities, Bio. Med. E n g . , vol. 3 , pp.
8-14. 1968.
[I21 M. R. Dimitrijevid and J. Faganel, Motor control in the spinal
cord, in Recent Achievements in Restorative Neurology: I . Upper
Motor Neuron Functions and Disjunctions, J. Eccles and M. R. Dimitrijevic, Eds. Basel: Karger, 1985, pp. 150-163.
[I31 T . J . Ebner, J. R. Bloedel. J . L. Vitek, and A. B. Schwartz, The
effects of cerebellar stimulation on the stretch reflex in spastic monkey, Brain. vol. 105, pp. 425-442, 1982.
(141 G . L. Gottlieb, G. C . Agarwal, and R. Penn, Sinusoidal oscillation
of the ankle as a means of evaluating the spastic patient, J . Neurol.
Neurosurg. Psychiatry, vol. 41, pp. 32-39, 1978.
[ 151 F. Gratanin, Functional electrical stimulation in control of motor
output and movements, in Contemporary Clinical Neurophysiology,
EEG Suppl. No. 34, W. A. Cobb and H. Von Duijn, Eds. Amsterdam: Elsevier, 1978, pp. 355-368.
[I61 R. Herman, W. Freedman, and N. Mayer, Neurophysiologic mechanisms of hemiplegic and paraplegic spasticity: Implications for therapy, Arch. Phys. Med. Rehab., vol. 5 5 , pp. 338-343, 1974.
[ 171 H. J . Hufschmidt, Die Spastik-Theoretische Uberlegungen zu einer neuen Therapie. Der Nervenarzt, vol. 39, pp. 2-11, 1968.
[I81 E. Knutsson, Studies of gait in patients with spastic paresis, in
Clinical Neurophysiology in Spasticity, P. J. Delwaide and R. R.
Young, Eds. Amsterdam: Elsevier, 1985, pp. 175-183.
[I91 J . W. Lance, P. de Gail, and P. D. Neilson, Tonic and phasic signal
cord mechanisms in man, J . Neurol. Neurosurg. Psychiatry, vol.
29, pp. 535-544, 1966.
[20] J . W. Lance and D. Burke, Mechanisms of Spasticity, Arch. Phys.
Med. Rehab., vol. 5 5 , pp. 332-337, 1974.
[21] J. W. Lance, Symposium synopsis, in Spasticity: Disordered Motor Control, R. G . Feldman, R. R . Young, and W. P. Koella, Eds.
Miami: Symposia Specialist, 1980, pp. 485-495.
[22] W. M. Landau, Spasticity: What is it?What is not?. in Spasticity:
Disordered Motor Control, R . G . Feldman, R. R . Young, and W . P.
Koella. Eds. Miami: Symposia Specialist. 1980, pp. 485-495.
[23] W . J . Lee, J. P. McGovem. and E. N. Duvall, Continuous tetanizing (low voltage) currents for relief of spasm. Arch. Phys. Med..
vol. 31, pp. 766-770, 1950.
[24] M. G . Levine, M. Knott, and H. Kabat, Relaxation of spasticity by
electrical stimulation of antagonist muscles, Arch. Phys. Med., vol.
33, pp. 668-673, 1952.
(251 W . T. Liberson, H. J . Holmquest, D. Scott, and M. Dow, Functional electrotherapy: Stimulation of the peroneal nerve synchronized
with the swing phase of the gait of hemiplegic patients, Arch. Phys.
Med. Rehab., vol. 42, pp. 101-105, 1961.
[26] M. Maleiit, U . Stanit, M. Kljajid, R. A?imoviC-Janeiit, J . Krajnik,
N. Gros, and M. Stopar, Multichannel electrical stimulation of gait
in motor disabled patients, Orthoped., vol. 7, pp. 1187-1 195, 1984.
[27] M. Mayer and C. Adorjani, Tonic stretch reflex for quantification
of pathological muscle tone, in Spasticiry: Disordered Motor Control. R. G . Feldman, R. R. Young, and W. P. Koella, Eds. Miami:
Symposia Specialists, 1980, pp. 315-330.
1281 R. F . Mayer and J. L. Young, The effects of hemiplegia with spasticity on single motor units in Spasticity: Disordered Motor Control,
R. G . Feldman, R. R . Young, and W . P. Koella, Eds. Miami: Symposia Specialists, 1980, pp. 133-146.
1291 D. R. McNeal and W. K. Wilemon, Treatment of spasticity through
functional electrical stimulation, in Neuroelectric Research: Electroneuroprosthesis and Nonconvulsive Electrotherupy, D. V, Rey-
nolds and A. E. Sjobert, Eds. Springfield, IL: Charles C Thomas,
1971, pp. 65-71.
[30] R . Merletti, F. Zeluschi, D. Lutella, M. Galli, S . Angeli, and M.
Bellucci Sessa. A control study of muscle force recovery in hemiparetic patients during treatment with functional electrical stimulation, Scand. J . Rehab. Med., vol. I O , pp. 147-154, 1978.
[3 I ] E. Pedersen, Evaluation of antispastic therapy, in Clinical Neurophysiology in Spasticity, P. J . Delwaide and R. R . Young, Eds.
Amsterdam: Elsevier, 1985, pp. 221-223.
[32] R. D. Penn, G . L. Gottlieb, and G . C. Aganval, Cerebellar stimulation in man-Quantitative changes in spasticity, J . Neurosurg.,
vol. 48, pp. 779-786, 1978.
1331 J . Perry, Rehabilitation of spasticity, in Spasticity: Disordered
Motor Control, R. G. Feldman, R . R . Young, and W . P. Koella, Eds.
Miami: Symposia Specialists, 1980, pp. 87-101.
[34] P. M . H. Rack, Limitations of somatosensory feedback in control
of posture and movement, in Handbook of Physiology: Motor control, Vol. I I , V. B. Brooks, Ed. Bethesda: American Physiological
Society, 1981, pp. 229-256.
[ 3 5 ] P. M. H . Rack, H . F. Ross, A. F. Thilmann, and D. K. W. Walters,
Reflex responses at the human ankle: The importance of tendon
compliance. J . Physiol., vol. 344, pp. 503-524, 1983.
1361 S . ReberSek, A. Stefanovska, and L. Vodovnik, Short term variations of ankle joint resistance during rest in spastic hemiplegic patients, in Proc. XIV. ICMBE and X I I ICMP, Espoo, Finland, 1985,
pp. 432-433.
[37] S . ReberSek, A. Stefanovska, L. Vodovnik, and N . Gros, Some
properties of spastic ankle joint muscles in hemiplegia, Med. Biol.
Eng. Conipur., vol. 24, pp. 19-26, 1986.
[38] S . ReberSek, L. Vodovnik, A. Stefanovska, T . Bajd, M. Gregori?.
and N . Gros, Modification of spasticity with electrical stimulation, in Progress Reports on Electronics in Medicine & Biology.
London: Inst. Electron. Radio Eng., 1986, pp. 159-166.
[39] A. Stefanovska, L. Vodovnik, S . ReberSek, and N . Gros, Contractile properties of spastic ankle joint muscles, in Final Report: Measurement and Quantification ofSpasticity, NIHR-441, Ljubljana, July
1985, pp. 45-59.
1401 A. Stefanovska, T. Bajd, L. Vodovnik, and N. Gros, Dermatome
stimulation: Differences in paraplegic and hemiplegic spasticity,
Electroencephalogr. clin. Neurophysiol., vol. 66, S 100, 1987.
[41] A. Stefanovska, N . Gros, L. Vodovnik, S . ReberSek, and R . A?imovid-Janeiit, Chronic electrical stimulation for the modification
of spasticity in hemiplegic patients, Scand. J . Rehab. Med. Suppl.,
1988, vol. 17, pp. 115-12!, 1988.
[42] P. Strojnik. E. Vavken, S . Zerovnik, V. Sirnit, M. Stopar, U. Stanit,
and R . AfimoviC-Janezit, Implantable peroneal underknee stimulator. in Proc. 8rh Int. Symp. ECNE. Dubrovnik. 1984. pp. 479485.
[43] P. Strojnik, R. Acimovid, E. Vavken, V. Simi?. and U . Stani:,
Treatment of drop foot using an implantable peroneal underknee
stimulator, Scand. J . Rehab. Med., vol. 19. pp. 37-43. 1987.
(441 M. Stefantit. S. Reberiek, and R. Merletti, The therapeutic effect
of the Ljubljana functional electronic peroneal brace, Europa Medicophy., vol. 12. pp. 1-9, 1979.
[45] R. R. Tasker, F. Gentili, P. Hwang, and K. Sogabe, Animal models
of spasticity and treatment with dentatectomy, in Spasticity: Disordered Motor Control. R . c . Feldman. R. R . Young, and P. Koella,
Eds. Miami: Symposia Specialist, 1980, pp. 155-177.
[46] L. Vodovnik and S . ReberSek. Improvement in voluntary control of
paretic muscles due to electrical stimulation, in Neural Organization, W. Fields and L. A. Leavitt, Eds. New York: Inter. Cont.
Med. Book. Corp.. 1973. pp. 101-116.
[47] L. Vodovnik, B. R. Bowman, and P. Winchester, Effect of electrical stimulation on spasticity in hemiparetic patients, Int. Rehubil.
Med.. vol. 6 , pp. 153-156, 1984.
Aneta Stefanovska received the Dipl. Eng. and
M.Sc. degrees from the Faculty of Electrical Engineering, Edvard Kardelj University, Ljubljana,
Yugoslavia, and is now working towards the
Ph.D. degree.
She is a Research Associate at the Faculty and
teaching assistant of Biocybemetics and Digital
Electronics. Her research interest includes modeling of normal and pathological neuromuscular
systems and the study of the effects of electrical
stimulation
STEFANOVSKA er al.: FES AND SPASTICITY
Lojze Vodovnik (SM73) received the Dipl.Eng.
and the D.Sc. degrees from the Faculty of Electrical Engineering, Edvard Kardelj University,
Ljubljana, Yugoslavia, where he is Professor of
Biocybemetics.
He was Research Associate and Visiting Professor from 1964 to 1969 at Case Western Reserve
University, Cleveland, OH, and World Rehabilitation Fund Fellow in 1981 at Rancho Los Amigos Hospital, Los Angeles, CA. His research interest is electrical stimulation of neuromuscular
systems.
Dr. Vodovnik is a senior member of the Bioengineering Society, a member of the Slovene Academy of Sciences and Arts, an honorary member of
the Yugoslave Engineering Society, and a member of the New York Academy,of Science
NuSa Gros is a Senior Physical Therapist at the
University Rehabilitation Institute, Ljubljana,
Yugoslavia. She has almost 25 years of experience as a specialist in development and application of new physical therapy methods and teaching, especially functional electrical stimulation
methods and devices. She is mentor for students
of the Department of Physical Therapy, Edvard
Kardelj University, Ljubljana.
145
Stanislav ReberSek, received the Dipl.Eng. and
D.Sc. degrees from the Faculty of Electrical Engineering, Edvard Kardelj University, Ljubljana.
Yugoslavia, where he is Associate Professor of
Biomedical Signal Processing, Analog, and Digital Electronics, and Nonelectrical Control Systems. He has been working on functional electrical stimulation for over ten years and is
experienced in hardware, measuring techniques,
and various stimulation methods including work
on spasticity and therapeutic effects of ES.
Ruia AEimoviC-Jane%, received the M.D. from
the Medical School in Ljubljana, Yugoslavia, in
1953.
She practiced medicine in various hospitals in
Yugoslavia and received a specialization degree
in physical medicine and rehabilitation in 1962.
also from the Medical School in Ljubljana. She
received the specialization degree after the study
of rehabilitation in the years 1963-1964 in Kopenhagen, Denmark. During the years 1964-1969
she was with the Rehabilitation Hospital in LaSko,
Yugoslavia, as a specialist and the head of the rehabilitation program. After
1969 she joined the Ljubljana Rehabilitation Institute where she promoted
the department for rehabilitation of hemiplegic patients. She also takes part
in research in the field of abnormal motor functions. She is presently the
head of the professional activities in the Institute, and vice-director of the
Institute. She is also principal investigator and co-investigator of several
research projects and a senior lecturer on the E. Kardelj University of Ljubljana, Department of Physical Therapy, covering area of the rehabilitation
of neurological diseases.
Dr. AEimoviC-JaneiiE is a member of the Slovene Research Council.
Vous aimerez peut-être aussi
- Post-Stroke Lower Limb Spasticity Alters The Interlimb Temporal Synchronisation of Centre of Pressure Displacements Across Multiple TimescalesDocument10 pagesPost-Stroke Lower Limb Spasticity Alters The Interlimb Temporal Synchronisation of Centre of Pressure Displacements Across Multiple TimescalesTAOUACHIPas encore d'évaluation
- Improved Biomedical Device For Spasticity QuantificationDocument4 pagesImproved Biomedical Device For Spasticity QuantificationTAOUACHIPas encore d'évaluation
- Adjacent Channel Interference Impact On The Capacity of Wcdma FDDDocument5 pagesAdjacent Channel Interference Impact On The Capacity of Wcdma FDDTAOUACHIPas encore d'évaluation
- Haptic Simulation of Elbow Joint SpasticityDocument2 pagesHaptic Simulation of Elbow Joint SpasticityTAOUACHIPas encore d'évaluation
- Sample HF ArchitectureDocument1 pageSample HF ArchitectureTAOUACHIPas encore d'évaluation
- RANAP Location Reporting Control MessageDocument1 pageRANAP Location Reporting Control MessageTAOUACHIPas encore d'évaluation
- RANAP RAB Assignment Request For A CS Video CallDocument1 pageRANAP RAB Assignment Request For A CS Video CallTAOUACHIPas encore d'évaluation
- RANAP RAB Assignment Request For A CS Speech CallDocument1 pageRANAP RAB Assignment Request For A CS Speech CallTAOUACHIPas encore d'évaluation
- Compressed Mode Using SFdiv2 - HLSDocument1 pageCompressed Mode Using SFdiv2 - HLSTAOUACHIPas encore d'évaluation
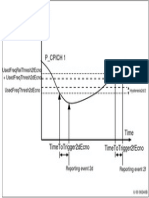
- Measurement Quantity P - Cpich 1: Usedfreqthresh2Decno + Utranrelthresh3AecnoDocument1 pageMeasurement Quantity P - Cpich 1: Usedfreqthresh2Decno + Utranrelthresh3AecnoTAOUACHIPas encore d'évaluation
- Measurement Quantity: Usedfreqrelthresh2Fecno + Usedfreqthresh2Decno Usedfreqthresh2DecnoDocument1 pageMeasurement Quantity: Usedfreqrelthresh2Fecno + Usedfreqthresh2Decno Usedfreqthresh2DecnoTAOUACHIPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Cva Power PointDocument167 pagesCva Power PointJen Passilan100% (14)
- Presbycusis JournalDocument11 pagesPresbycusis JournalJairah CandaoPas encore d'évaluation
- Local Anaesthesia TechniquesDocument21 pagesLocal Anaesthesia TechniquesZaki MubaraqPas encore d'évaluation
- Jurnal Keracunan Merkuri - Case ReportDocument6 pagesJurnal Keracunan Merkuri - Case ReportStanley LesmanaPas encore d'évaluation
- Clinical Science of Guilen Barren SyndromeDocument2 pagesClinical Science of Guilen Barren SyndromemanakimanakuPas encore d'évaluation
- Exercise 7 Embryology 1Document10 pagesExercise 7 Embryology 1Jerjer KingPas encore d'évaluation
- Chapter 13 - The Peripheral Nervous System and Reflex ActivityDocument53 pagesChapter 13 - The Peripheral Nervous System and Reflex Activityramadan100% (1)
- Forever AcupunctureDocument35 pagesForever AcupunctureAbud Arizpe Jorge Hafid100% (4)
- Writers Cramp - A Major Conundrum: Review Article44Document7 pagesWriters Cramp - A Major Conundrum: Review Article44IJAR JOURNALPas encore d'évaluation
- Tuberculoma OF THE Brain (Revisited)Document7 pagesTuberculoma OF THE Brain (Revisited)Nyoman SuryaPas encore d'évaluation
- CNS - Important QuestionsDocument2 pagesCNS - Important Questionsavantika rajeev100% (3)
- M-Caps-03: Physics: (Medical-Classroom Assessment Practice Sheet)Document16 pagesM-Caps-03: Physics: (Medical-Classroom Assessment Practice Sheet)duraibiotechPas encore d'évaluation
- Inside The ADD Mind, by Dr. Thomas BrownDocument4 pagesInside The ADD Mind, by Dr. Thomas BrownDerekPas encore d'évaluation
- Visual Field InterpretationDocument11 pagesVisual Field Interpretationhameed28885Pas encore d'évaluation
- Lecture Neural-Networks ..Document114 pagesLecture Neural-Networks ..Jaya ShuklaPas encore d'évaluation
- Kleptomania: Recent Advances in Symptoms, Etiology and TreatmentDocument4 pagesKleptomania: Recent Advances in Symptoms, Etiology and TreatmentHelwa SalsabelaPas encore d'évaluation
- What Me Worry - 04 - Attention Training PDFDocument9 pagesWhat Me Worry - 04 - Attention Training PDFEudasio CalvacantePas encore d'évaluation
- Vestibular Dysfunction and Its TherapyDocument253 pagesVestibular Dysfunction and Its TherapyalvinoPas encore d'évaluation
- La Teoría de La Compuerta - AlDocument12 pagesLa Teoría de La Compuerta - AlAlterOsPegiamPas encore d'évaluation
- 3CNCPDocument7 pages3CNCPHelen MontesPas encore d'évaluation
- The Kiki-Bouba Paradigm: Where Senses Meet and Greet (Aditya Shukla)Document13 pagesThe Kiki-Bouba Paradigm: Where Senses Meet and Greet (Aditya Shukla)Aditya ShuklaPas encore d'évaluation
- Paper D (KMU) 2nd Year - 0Document2 pagesPaper D (KMU) 2nd Year - 0G.R. JamesPas encore d'évaluation
- Case Report On Bipolar Affective Disorder: Mania With Psychotic SymptomsDocument3 pagesCase Report On Bipolar Affective Disorder: Mania With Psychotic SymptomsmusdalifahPas encore d'évaluation
- Three Theories Commonly Cited To Explain The Origin of Anxiety DisordersDocument13 pagesThree Theories Commonly Cited To Explain The Origin of Anxiety DisordersNor-aina Edding AbubakarPas encore d'évaluation
- Classification of StrokeDocument3 pagesClassification of StrokeafhamazmanPas encore d'évaluation
- Dysarthria - StatPearls - NCBI BookshelfDocument10 pagesDysarthria - StatPearls - NCBI BookshelfdaohailongPas encore d'évaluation
- LESSON PLAN Sensation and PerceptionDocument13 pagesLESSON PLAN Sensation and PerceptionBrylle DeeiahPas encore d'évaluation
- The Principles of Brain Based Learning and Constructivist Models in EducationDocument9 pagesThe Principles of Brain Based Learning and Constructivist Models in EducationRobert WilliamsPas encore d'évaluation
- 10.3 The Nerve ImpulseDocument20 pages10.3 The Nerve ImpulseazwelljohnsonPas encore d'évaluation
- Physiology of VisionDocument49 pagesPhysiology of VisionPrakash PanthiPas encore d'évaluation