Académique Documents
Professionnel Documents
Culture Documents
Meyer Et Al. 2004
Transféré par
Aline Freitas de MeloCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Meyer Et Al. 2004
Transféré par
Aline Freitas de MeloDroits d'auteur :
Formats disponibles
Steroids 69 (2004) 145159
Review
Sex steroids and growth factors in the regulation of mammary
gland proliferation, differentiation, and involution
I. Lamote, E. Meyer , A.M. Massart-Len, C. Burvenich
Department of Physiology, Biochemistry, and Biometrics, Faculty of Veterinary Medicine, Ghent University,
Salisburylaan 133, B-9820 Merelbeke, Belgium
Received 16 July 2003; received in revised form 10 December 2003; accepted 16 December 2003
Abstract
The mammary gland is subjected to major morphological and biochemical changes during the lactation cycle. It is therefore not surprising
that this dynamic process is strictly controlled. The importance of the sex steroid hormones 17-estradiol and progesterone for normal
development of the mammary gland was recognized several decades ago and has been unequivocally confirmed since. Furthermore, it is
now also established that the influence of sex steroids is not restricted to mammogenesis, but that these hormones also control involution.
Another important regulatory role is played by growth factors that have been shown to modulate survival (epidermal growth factor,
amphiregulin, transforming growth factor , insulin like growth factor, and tumor necrosis factor ) or apoptosis (tumor necrosis factor
, transforming growth factor ) of mammary cells. However, the molecular mechanism underlying the influence of sex steroid hormones
and/or growth factors on the development and function of the mammary gland remains largely unknown to date. Also scarce is information
on the interaction between both groups of modulators. Nevertheless, based on the current indications compiled in this review, an important
functional role for sex steroid hormones in the lactation cycle in co-operation with growth factors can be suggested.
2004 Elsevier Inc. All rights reserved.
Keywords: Lactation cycle; Growth factors; Steroids; Mammary gland
1. Introduction
Over the past several decades efforts have been made to
determine the relationship between hormones and the mammary gland. Steroid hormones of the ovary and placenta
were implicated very early as important stimulators of mammary gland development [1]. Since changes in development
of the female mammary apparatus are particularly evident
during gestation and lactation, these conditions were studied
more intensively than others. All these efforts have led to an
impressive number of original papers and excellent reviews
on steroids and lactation biology. The aim of the current
review was therefore not to provide the reader with completeness with respect to the current knowledge of this topic.
Rather, we wanted to contribute from an original point of
view putting the emphasis on what is now emerging as the
missing link between systemic sex steroids and local factors
in the control of the mammary gland throughout the lactation cycle. For this purpose, a comparison is made between
different mammalian species to enlarge the scope from the
Corresponding author. Tel.: +32-9-2647321; fax: +32-9-2647499.
E-mail address: evelyne.meyer@ugent.be (E. Meyer).
0039-128X/$ see front matter 2004 Elsevier Inc. All rights reserved.
doi:10.1016/j.steroids.2003.12.008
traditionally favorite mammary gland animal model, the rodent (rat, mouse), to less intensively studied, but equally
interesting species, such as ruminants (cow, goat, sheep).
Some studies in human, pig, and dog are also included.
For all species, the changes in the mammary gland during
gestation, lactation, and involution involve complex interactions between many hormones and cell types. Since most of
these hormones induce the production of local factors, insight into the endocrine control of the mammary gland is
complicated. The resulting interplay from systemic and local signals needs to be carefully considered to determine the
balance between proliferation, differentiation, and apoptosis
of the different cell populations at all stages of the lactation
cycle in the mammary gland.
2. Sex steroid hormones and the lactation cycle
The lactation cycle can be divided into different consecutive stages, including, in chronological order, mammogenesis, lactogenesis, galactopoesis, and involution. Each of
these phases is characterized by strict hormonal control. The
traditional role of steroids and other hormones in different
146
I. Lamote et al. / Steroids 69 (2004) 145159
mammalian species was already obtained from pioneer studies in the 1950, but has been since refined using new technologies like knock-out (KO) and transgenic animal models.
In the following paragraphs, the current view on the hormonal control of the mammary gland throughout the lactation cycle is summarized starting from the classical concepts
to the most recent state-of-the-art reviews.
2.1. Mammogenesis
From birth to the onset of puberty mammary growth
(mammogenesis) is minimal and proportional to that of the
body (isometric growth). This period is regarded as a quiescent phase in the growth of the gland. A phase of more
active mammary growth occurs around the time of puberty
and is characterized by a rapid extension and branching of
the duct system (allometric growth). Thereafter, the degree
of mammary proliferation depends on the nature of the reproduction cycle. The initial growth changes in the mammary gland in early gestation are related to the degree of development already attained during the reproduction cycles.
In many species, further duct extension and duct branching occur, followed by growth of lobules of alveoli. During
growth the proportion of parenchyma to stroma increases,
until eventually, the dense collection of lobes and lobules of
alveoli are separated only by septa of connective tissue. It
used to be thought that growth of the mammary parenchyma
brought about by cell division was completed in the first
two-thirds of gestation and that the subsequent increase in
size of the mammary glands was due to hypertrophy of the
existing alveolar cells and to the expansion of the alveoli
with secretion, but there is now clear evidence that cell division occurs throughout gestation and continues into the
early stages of lactation [2].
The importance of sex steroid hormones for normal mammogenesis has unequivocally been confirmed. Throughout
gestation, proliferation of mammary epithelium is induced
by the sex steroid hormones 17-estradiol (E2 ) and progesterone (P) [3]. E2 and P generally act as survival factors [4]
in hormone sensitive tissues, such as the ovaries, uterus, and
mammary gland [5]. Clinical studies on estrogen deficiency
syndromes in humans [6] have implicated estrogen in the
normal development of the breast. Definitive evidence for
the importance of estrogen signalling in normal mammary
gland development has been obtained from experiments in
mice. Firstly, castrated immature mice do not show ductal
growth through the fat pad of the mammary gland, signifying that mammary ductal development is hormone dependent [7]. Secondly, the mammary glands of ovariectomized
mice are stimulated to grow by implanted estrogen pellets
[8], and implants of pure anti-estrogens inhibit mammary
growth in intact mice [9]. Finally, female estrogen receptor
KO mice develop mammary glands with only vestigial ducts
present at the nipples [10]. Nevertheless, extensive proliferation of the mammary gland in response to the ovarian sex
steroid hormones occurs only if the pituitary gland is intact. Ovarian hormones in the absence of pituitary hormones
have little or no mammogenic activity. In both the rat and
mouse, detailed studies have been carried out on the hormonal requirements for mammary proliferation in the absence of endogenous mammogenic hormones, i.e. after removal of the pituitary and ovaries, or pituitary, ovaries, and
adrenals. These pioneer studies on the rat by Lyons and his
colleagues and on the mouse by Nandi are well-known, and
although some minor differences exist in the responses of
the two rodent species, they both show that the hormones
required for duct growth are estrogen, somatotropin (STH),
and adrenal corticoid. If P and prolactin (PRL) are added to
this combination, lobulo-alveolar growth is stimulated [2].
More recent studies with PRLR knock-out mice, i.e. without functional allele, and PRL hemizygous mice, i.e. with
one functional allele, showed that mammary development
is essentially blocked at the stage of extended ductal outgrowths during pregnancy but that a normal ductal network
is formed during puberty [11,12].
The molecular mechanism of the influence of sex steroids
and other hormones on mammogenesis is still far from completely understood. It is generally accepted that sex steroids
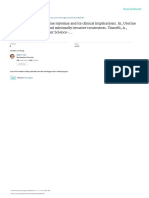
Fig. 1. Schematical representation of hormonal control of gestation, lactation and involution. During gestation, full lobulo-alveolar development takes
place under the continued stimulation of estrogen (E) and progesterone (P). In most of the positive mammary epithelial cells, ER and PR are colocalized,
while in stromal cells only ER is localized in some species (only changes in ER expression are represented). ER and PR expression decrease throughout
gestation compared to the non-gestating animals where ER and PR expression are relatively high. Activation of ER probably induces proliferation of
mammary epithelium through stimulation of the expression of growth factors, which may be locally secreted by stromal cells (arrow). Activation of the
PR-positive epithelial cells by P causes proliferation of the neighboring PR-negative cells (arrow). E can induce mammary PR expression via ER. Vice
versa PR probably also interacts with ER (see detail). Although not fully characterized, epithelial progenitors (in blue) have been described in a large
number of species. A dramatic decrease of P occurs around parturition and downregulation of PR expression is continued. ER is further downregulated
during the transition of gestation to lactation, while during full lactation expression is again upregulated. A fall in E is not uniform in all species and was
therefore not indicated in this figure. In addition, rising levels of prolactin (PRL) and/or somatotropin (STH) are necessary for successful lactogenesis.
PRL and STH induce functional differentiation through induction of transcription of milk protein genes. The importance of STH versus PRL is highly
species dependent (see detail). In early involution, ER expression decreases. Since an overlap between the periods of lactation and gestation exist in
some species, general changes in E and P could not be indicated. The absence of PRL and STH is critical for mammary gland involution. During
early involution, apoptotic epithelial cells and a decrease in the expression of milk protein genes have been detected (see detail). In late involution PR
expression increases. Proteolytic degradation of the basement membrane by plasmin and matrix metalloproteinases (MMP) is initiated (see detail) and
the apoptotic process is continued. As progenitor cells are limited in their proliferation capacity, they need to be renewed probably after some lactation
cycles. In the figure, extensive tissue degeneration is shown, although in a few species only isolated tissue degeneration is found.
I. Lamote et al. / Steroids 69 (2004) 145159
and growth hormones exert an influence on the mammary
gland and that the genomic biological responses in the mammary gland are predominantly mediated by receptors, but
it is surprising that most authors mention that specific receptors for these hormones are only expressed at very low
and even undetectable levels in the mammary gland [13,14].
However, recently Schams et al. [15] reported significant PR
147
and ER expression throughout gestation, lactation, and involution in the bovine. Furthermore, no non-genomic effects
of steroids have yet been described in the mammary gland.
For E2 , the genomic biological responses in the mammary
gland are predominantly mediated by the estrogen receptor
(ER) and not by ER [3]. ER is localized both in the
epithelial and stromal compartments of the mammary gland
148
I. Lamote et al. / Steroids 69 (2004) 145159
[16]. In contrast, human [17] and heifer [18] stromal cells
apparently do not express ER. During ductal development,
activation of ER induces proliferation of murine mammary
epithelium through stimulation of the expression of growth
factors (IGF-I), which are probably locally secreted by stromal cells (Fig. 1) (reviewed by Forsyth [14] and Hovey et al.
[16]). Stromal factors mediating development during gestation are postulated but not yet clearly demonstrated.
The biological responses of the mammary gland to P have
been intensively studied by many research groups [19,20].
Only genomic responses have been reported for P in the
mammary gland. In contrast with E2 , these genomic effects
are mediated by two isoforms of the progesterone receptor
(PR), PR-A and PR-B. The ratio in expression of both PR
isoforms in the mammary gland is critical for the normal response to P and is therefore strictly controlled. Recent studies (reviewed by Lydon et al. [20]) have demonstrated that
PR is selectively localized in the mammary epithelium and
not in the stroma. In addition, PR expression in the mammary
epithelium is strikingly heterogeneous; there are only few
PR-positive epithelial cells, which are apparently distributed
at random throughout the majority of PR-negative epithelial
cells (Fig. 1; gestation). This typical pattern of PR expression appears to be evolutionarily conserved as it is comparable in mouse and human. In both species, a paracrine
epithelialepithelial signalling via PR is present (Fig. 1; gestation); activation of the PR-positive cells causes proliferation of the neighboring PR-negative cells. Brisken et al. [21]
speculate that Wnt proteins might function as the paracrine
factors that operate downstream of PR. Shyamala [19] and
Lydon et al. [20] independently suggest that a possible explanation for this heterogeneous PR expression is the association of PR with a specific subtype of not fully differentiated, non-proliferating epithelial cells. These PR-positive
epithelial cells are presumed to remain in their progenitor
state during subsequent lactations. As for E2 , it has been
suggested that activation of PR sensitizes the epithelial cells
for proliferation following exposure to stromal growth factors [19,20]. Mammary PR expression gradually decreases
at the end of gestation, and in the final phase of differentiation to secretory epithelium, PR expression is completely
lost. Shyamala [19] suggests that this loss might even be
required to reach the stage of terminal differentiation.
Data obtained with ER-KO and PR-KO models confirm
that E mediated signalling via ER is essential for ductal
morphogenesis, while P signalling via PR is critical for
lobulo-alveolar development. P is required for the transition from ductal to lobulo-alveolar morphology. However,
it should be noticed that under normal physiological conditions, E2 indirectly stimulates lobulo-alveolar architecture
formation too because it can also induce mammary PR expression via ER [22]. Vice versa, it has been shown in vitro
that PR can also influence the biological responses to ER,
although further research is required to confirm this interaction on the steroid receptor level. It should be remarked
that ER and PR colocalize in 96% of PR-positive human lu-
minal mammary epithelial cells [23]. As in mice, cells that
express both ER and PR are nonproliferative [23,24], suggesting that E and/or P may stimulate adjacent ER/PR negative cells to divide by a paracrine mechanism [23] (Fig. 1;
gestation).
Shyamala et al. [25], Saji et al. [26] and Schams et al.
[15] refined these observations respectively for PR, ER and
the combination of both receptors and demonstrated differences in ER and PR mRNA and/or protein expression in
the mammary gland during gestation and lactation. These
authors found a relatively high mRNA expression of ER
and PR in the mammary tissue of non-gestating heifers. This
high level was down-regulated at the onset of lactation and
was due to constantly high levels of P and increasing levels
of E2 during the second-half of gestation. The mRNA data
were confirmed by demonstration of the protein for ER and
PR by immunohistochemistry with signals of staining of epithelial cell nuclei. Additionally, an increased cytoplasmic
PR staining of epithelial cells was obvious during lactogenesis (Fig. 1).
2.2. Lactogenesis and galactopoesis
Once substantial lobulo-alveolar growth has occurred, the
alveolar cells undergo organellar and biochemical differentiation and acquire the capacity to secrete milk. It is common
to differentiate between the initiation and the maintenance
of milk secretion. Upon parturition, withdrawal of P stimulates milk secretion. During the first few days after parturition (production of colostrum and first milk) this process is
called lactogenesis. Once milk secretion occurs, the suckling or milking stimulus promotes its maintenance, a process
called galactopoesis [27].
During lactogenesis and galactopoesis, milk production is
controlled by the lactogenic hormones PRL and STH. Both
PRL and STH are essential for the transition from a proliferative to a lactating mammary gland in all mammalian species
studied. Nevertheless, a quantitative distinction can be made
between ruminants (cow, goat, and sheep) where the influence of STH dominates over PRL during galactopoesis, and
other species like rodents and humans where the influence
of PRL dominates over STH during galactopoesis as well
as during lactogenesis. In fact, STH is dispensable for lactogenesis in mice and humans, as GHR knockout mice [28]
and human dwarfs with mutations in either GH or GHR can
lactate [29,30].
In general, basal levels of glucocorticosteroids are necessary to maintain metabolism and several specific hormone
actions and are also expected to play a permissive role during lactation. Nevertheless, there is no evidence that the
surge of glucocorticosteroids occuring around parturition is
involved in lactogenesis. PRL and STH induce functional
differentiation through milk protein and fatty acid synthesis.
The transcription of several milk protein genes like -casein
and whey acidic protein (WAP) significantly increases upon
mid-lactation as compared to the onset of lactation. PRL
I. Lamote et al. / Steroids 69 (2004) 145159
acts directly through mammary epithelial receptors and activates various transcription factors. One of the key signalling
molecules activated by the PRL receptor is Signal transducer
and activator of transcription 5 (Stat5). The basic role of
Stat5 in the mammary gland is to mediate PRL signalling,
while the PRL receptor in turn relies heavily on Stat5 to
mediate its effects [31]. However, Miyoshi et al. [32] provide evidence that Stat5 has also other functions than mediation of the PRL effect alone and vice versa that PRL signalling is not strictly mediated by Stat5. In contrast to PRL,
demonstration of functional receptors for STH in the mammary gland is scarce [33]. Nevertheless, it is certain that the
mammary gland is a site of STH production as well as STH
action [34]. STH is found in canine mammary secretions
(particularly pre-partum and in colostrum) at concentrations
1001000 times those in plasma, although the role of milk
STH remains uncertain [35]. Because milk STH concentrations are not correlated with fetal plasma STH and STH is
not absorbed intactly through the canine gastrointestinal tract
[36,37]. The biological response to STH is thought to be indirectly mediated via the insulin-like growth factor I (IGF-I)
system. However, there are many contradictory results supporting or refreshing this role for IGF-I which seems to be
species dependent [35,38].
2.3. Involution
Upon fulfilment its functional purpose in the course of
normal lactation, the mammary gland regresses gradually
(gradual involution) and ultimately returns to a state of development only slightly in advance of that which existed at
the beginning of the first gestation. A much faster regression
of the mammary gland occurs following cessation of milking of animals in early lactation (initiated involution) [39].
The withdrawal of the suckling young (weaning) or the cessation of milking are both inducers of involution. One of the
first steps in the dry period following weaning or cessation
of milking is the interruption of the release of galactopoetic
hormones. As a result, milk stasis and a fast decrease in milk
secretion and in the expression of genes responsible for milk
synthesis, such as whey acidic protein, occur (Fig. 1). Next
to hormone withdrawal, another factor, feedback inhibitor
of lactation (FIL), has been proposed to be involved in the
reduction of milk synthesis and functional differentiation of
secretory cells at milk stasis. It has been shown that FIL has
an inhibitory effect on protein synthesis. FIL has an immediate and direct effect on casein and lactose synthesis and
long term, probably indirect effects on cell differentiation
by inhibiting synthesis of lactogenic hormone receptors on
secretory cells (reviewed by Knight et al. [40]).
Immediately after suckling was discontinued, apoptotic
mammary epithelial cells have been detected in rodents.
Nevertheless, during this initial phase, involution is still reversible (Fig. 1, early involution). In the second phase of involution in rodents, proteolysis of the extracellular matrix is
initiated and the apoptotic process is continued (Fig. 1, late
149
involution (remodeling)) with an almost complete loss of
epithelial cells [41]. The response to weaning in ruminants
appears to be slower and less expressed than in rodents. Although somewhat intermingled, both phases of involution
are also present in ruminants.
The later involution phase is characterized by a general
increase in expression of protease genes concomittant with
a decrease of their inhibitors. The expression of plasminogen activators, which induce the formation of active plasmin
from plasminogen and are inhibited by STH, increases after
drying off. This local increase of plasmin and plasminogen
activators is reflected in bovine milk as a result of the typical gradual involution in cows [42]. Although plasmin(ogen)
is important in the postlactational involution, Lund et al.
[43] demonstrated in plasminogen knock-out mice that involution can proceed with some retardation in the absence
of plasminogen. Following activation, the plasmin enzyme
will, in turn, activate members of the matrix metalloproteinase (MMP) family. MMP include some collagenases and
stromelysines and are essential catalysts in the proteolytic
degradation of the basement membrane and the extracellular matrix of the mammary gland. Other marked increases
in expression are observed for gelatinase and tissue transglutaminase (Fig. 1, late involution) [44]. Although tissue
transglutaminase is not a protease, it also plays an important
role in the apoptotic process. Its activation leads to the formation of a crosslinked protein scaffold in cells undergoing
apoptosis. This protein scaffold may stabilize the integrity of
the dying cells before their clearance by phagocytosis, thus
preventing the non-specific release of harmful intracellular
components and consequently inflammatory responses [45].
It should be emphasized that although MMP are undoubtedly
central to the process of mammary gland involution, apoptosis in rodents begins prior to degradation of extracellular
matrix. Apoptosis may be triggered by other mechanisms
which effect downstream MMP expression. One possibility
is that changes in cellmatrix interactions occur. Prince et al.
[46] suggest that the modulation of integrin ligand binding
activity might play a role.
Mammary gland involution has been extensively studied
in rodents, and this model is often considered to reflect the
process in other mammalian species as the specific knowledge on involution in other species is scarce. Nevertheless,
quantitative inter-species differences are considerable. Especially for ruminants, it seems not justified to assume that involution is similar to that in rodents because the sequence of
lactation and gestation differs fundamentally between both
groups of species. Indeed, in cows and goats, there is an
overlap between the periods of lactation and gestation, while
in rodents lactation is separated from gestation by a dry period. It should be remarked that although sheep are also ruminants, they are non-gestating at drying off. The speed of
involution is slowest in ruminants and fastest in rodents, although both in rodents and ruminants, a limited number of
epithelial cells are programmed to die starting from peak
lactation. The caprine mammary gland is exceptional as the
150
I. Lamote et al. / Steroids 69 (2004) 145159
speed of involution is intermediate. Mammary tissue regression in the bovine mammary gland remains limited even at
the end of the dry period prior to calving. In contrast to rodents where apoptosis and changes in gene expression are
observed to be major at 4 days after cessation of milking,
minor changes occur only at 7 days after cessation of milking in cows [47,48].
The quantitative apoptosis and gene expression data are
supported by morphological changes observed during involution. While the alveolar structure has completely degenerated after a dry period of 4 days in mice, it remains mostly intact at that time in the bovine mammary gland, and even after
a dry period of several weeks, only isolated tissue degeneration can be found. An important additional feature of rodent
involution is the proteolytical degradation of the basement
membrane between stroma and epithelium (Fig. 1) which
starts by an altered expression of MMP and their inhibitors.
Analogous to apoptosis, basement membrane degradation is
already maximal at 4 days. In sharp contrast, the basement
membrane in ruminants is still largely intact at 7 days of dry
period [47,48].
An explanation for this marked difference in cows is that
a similar number of epithelial cells as in rodents will undergo apoptosis between two lactations, but that secretory
epithelial cells are not readily eliminated at the start of the
dry period. This hypothesis is supported by data obtained in
the goat, the species characterized by an intermediate speed
of involution combined with a similar degree of cell death
as rodents. In the caprine model, there is a partial survival of
the secretory epithelium during the first period of mammary
involution.
A logical question is then which cells will survive and
which cells will be replaced during the dry period? It is evident from the subsequent reproductive and lactation cycles
that the mammary gland possesses a strong regeneration capacity. The presence of pluripotent stem cells and cell-line
committed progenitors in the normal mammary gland has
been described by several authors in different species such
as the mouse [49,50], rat [51,52], human [53,54] and cow
[55]. Nevertheless, the precise nature of these cells needs to
be further characterized using specific markers, especially
in humans. Kordon and Smith [56] first suggested the existence of a population of self-renewing and pluripotent stem
cells in the mammary gland of mice. In addition, indications
for the presence of precursor cells with a limited differentiation potential that can only generate ductular or alveolar
epithelial cells were also provided. As these precursor cells
are limited in their proliferation capacity, they need themselves to be renewed by cells originating from the pluripotent stem cell population. It can therefore be postulated that
candidate cells for renewal are the precursor cells that are
responsible for expanding and maintaining the number of
mammary epithelial cells in subsequent lactation. In this
way, involution prepares the mammary gland for an optimal
milk secretion capacity in the following lactation. Further
research from Chepko and Smith [57] confirmed the initial
indications on the replacement of a subpopulation of older
epithelial cells by new epithelial cells with a higher secretory capacity, generated from precursor cells. The presence
of morphologically distinct stem cells and different types of
precursor cells in the mammary gland was not only demonstrated in rodents (rat and mouse), but also in the human and
the cow. A similar hierarchy in progenitors was independently described by Stingl et al. [58] in human mammary
tissue. Subpopulations of cells with stem cell characteristics
were also found in the bovine mammary gland by Holland
et al. [55] on the basis of differences in ultrastructural features and gap junction intercellular communication.
At the end of gestation, the new population of epithelial
progenitors likely differentiates under sex steroid hormonal
influence as described above for juvenile secretory cells at
the onset of lactation (Fig. 1). In a recent publication, Wagner
et al. [59] additionally demonstrated that this newly matured
epithelial cell population is not replaced during the following
lactation cycle in humans and retains a limited proliferation
capacity.
The causes and the detailed mechanism of the remodeling process are not exactly defined, but one of the primary
stimuli identified in rodents and ruminants is the abrupt
withdrawal of lactogenic hormones. In vivo, systemic lactogenic hormone levels drop immediately after cessation of
milking. The importance of the absence of PRL and STH
for mammary gland involution was first demonstrated in rodents [60] and has recently been confirmed in vitro for the
cow [61]. The most pronounced effect on involution was
observed in the absence of PRL. As PRL represses the expression of the pro-apoptotic IGFBP-5 mRNA, PRL deletion leads to the halt of inhibition of IGFBP-5 expression in
epithelial cells. In consequence, more IGF-I is sequestered
by IGFBP-5 and thus prevented from binding to its receptor and from suppressing apoptosis and delaying involution
(Fig. 1) [61,62].
Data on the influence of sex steroids on mammary gland
involution are scarce. Athie et al. [63] studied the effect of
exogenous E2 on the involution in cows, by examining the
changes in milk composition. Using this criterium, an accelerated involution was found following administration of E2 .
However, the relevance of these observations for the physiological situation is not clear. In a second study, PR mRNA
becomes again detectable in glands from mice undergoing
lactational involution after a period of being undetectable
during lactation [25]. However, it has also been suggested
that the absence of PR expression is a potential key factor
in the remodeling of the basement membrane between the
stroma and the mammary epithelium during involution. In a
third study by Schams et al. [15], the mRNA expression and
protein data for ER and PR show clear regulatory changes
suggesting involvement of these receptors in bovine mammary gland involution. The increase of ER at 24 weeks of
involution and of ER 24 weeks after the end of lactation
can be interpreted as preparation of tissue for new mammogenic activity if specific signals are released. Although
I. Lamote et al. / Steroids 69 (2004) 145159
reported in a limited number of studies, these data obtained
in different animal models clearly indicate that sex steroid
hormones also modulate the mammary gland throughout the
involution stage (Fig. 1).
3. Growth factors and the lactation cycle
An increasing list of local growth factors has been
shown to modulate survival and apoptosis in the mammary
gland. Several of these proteins or polypeptides are also
cytokines. A stimulatory role in the proliferation and/or
differentiation of mammary epithelial cells is suggested for
most growth factors including epidermal growth factor, amphiregulin, transforming growth factor , and insulin like
growth factor. Tumor necrosis factor might play a dual
role, stimulating cell survival or death depending on the
presence or absence of other factors, whereas transforming
growth factor has been found to be growth inhibiting and
apoptosis inducing during several phases of the mammary
cycle.
3.1. EGF
One of the main groups of growth factors affecting the
mammary gland is the family of epidermal growth factors (EGF) including EGF, amphiregulin (AR), transforming
growth factor (TGF), heparin binding EGF, betacellulin,
and epiregulin. EGF family members exert direct mitogenic
effects and are therefore classified as typical survival factors
based on observations with transgenic mice and from murine
tumor cell line studies [6467]. EGFs all bind with varying
affinities the epidermal growth factor receptor (EGFR), the
first receptor of the ERBB-signalling network which comprises four homologous receptor tyrosine kinases (ERBB
14). In addition, heparin binding EGF, betacellulin, and
epiregulin also bind another receptor of the ERBB-signalling
network namely ERBB-4 (reviewed by Pinkas-Kramarski
et al. [68]).
Studies in mice on ERBB expression and activating
profiles revealed that signalling by EGFR (and possibly
ERBB-2) is critical for ductal outgrowth. It is less clear
whether this receptor also functions in alveolar morphogenesis and lactation. Since EGFR levels and phosphorylation
were shown to coordinately peak in late gestation and
lactation, such a role is suggestive although it remains controversial (Table 1). This is not the case for signalling by
ERBB-2, -3, and -4, which is clearly important for alveolar
morphogenesis and lactation (reviewed by Troyer and Lee
[69]).
Studies with AR- and/or EGF- and/or TGF-null mice
confirmed the fundamental role of EGFR in ductal morphogenesis and revealed a differential role for the EGFR
ligands. Mammary glands from adolescent AR null mice
displayed striking defects in ductal outgrowth. Additional
loss of EGF or TGF exacerbated the defect whereas mice
151
lacking only EGF and TGF had normal glandular arborization, underscoring the fundamental role of AR in ductal
elongation [70]. AR is expressed exclusively by the epithelium while EGFR would only be critical in the stroma.
Taken together, activation of stromal EGFR by epithelial derived AR provides the epithelialstromal signal previously
postulated to be necessary for ductal morphogenesis [69].
In order to understand the role of EGFR in ductal morphogenesis more in detail it will be necessary to identify
its critical downstream signalling partners. Obvious candidate effectors are molecules previously implicated in
ductal morphogenesis, cell adhesion/migration or remodeling of the extracellular matrix. These include integrin
subunits [70], the intracellular kinases, Src or FAK, and
MMP. MMP are particularly attractive since several of them
are expressed by stromal fibroblasts adjacent to advancing
ducts and are regulated by EGFR ligands in cultured cells
[69].
Next to studies on receptor expression and activating profiles, studies on the expression of EGFR ligands throughout
gestation, lactation, and involution in the mouse have also
been performed (Table 1) [6567]. The mRNA levels of all
subfamily members except for EGF decrease during gestation and disappear during lactation. In contrast, EGF expression increases dramatically at the end of gestation and
peaks during lactation, with high levels found in human and
murine milk [71]. Inversely, EGF decreases during involution when the expression of the other subfamily members
including TGF starts to increase again [72].
These observations also suggest a differential role for the
mammary EGF subfamily members in the lactation cycle. It
can be postulated that TGF together with other EGF subfamily members might contribute more specifically to epithelial proliferation during gestation as well as during the
dry period, while only EGF would also play a role in the
differentiation process during lactation. Data obtained from
studies in rodent mammary cell cultures [73] are in accordance with this differential role for EGF family members.
These data suggest that EGF is needed for proliferation and
for rendering cells responsive to lactogenic hormones, but
that following differentiation, EGFs role might be to prevent
apoptosis. It should be remarked that the survival growth
factor EGF has been associated with an increased expression
of the anti-apoptotic Bcl-2 family member Bcl-xL , which
could be involved in the regulatory mechanism [74]. Despite
these observations, it is also possible that EGF expression
during lactation relates specifically to its secretion in the
milk rather than having a role in the differentiation process
during lactation.
That the role of the EGF subfamily is likely evolutionarily
conserved is supported by the fact that EGFR have been
demonstrated in the bovine and ovine mammary gland in
addition to that of the rodent (reviewed by Forsyth [14]).
Moorby et al. [75] observed that a remarkable decrease in
TGF binding capacity occured at the end of gestation and
during lactation in sheep.
152
I. Lamote et al. / Steroids 69 (2004) 145159
Table 1
Expression and/or blood concentration of growth factors in the mammary gland during the lactation cycle
Growth factor
EGF family
IGF family
Stage of lactation cycle
Reference
Gestation
Lactation
Involution
EGF(mRNA)
Other EGF family
members (mRNA)
EGFR
Increase
Decrease
Peak values
Disappearance
Decrease
Increase
Late gestation:
peak values
Peak values
IGF-I (mRNA)
IGF-I (blood concentrations)
Decrease
IGF-II (blood concentrations)
IGF-IR
IGF-IIR
IGFBP-1, -3, -6 (mRNA)
IGFBP-1, -3, -6 (blood
concentrations)
IGFBP-2, -4, -5 (mRNA)
Constant
Low levels
After parturition: decrease;
during lactation: increase
Constant
Parturition: decrease
Constant
Littleno expression
TNF
TNF
TNF
TNFR
TNFR
(mRNA)
(protein)
subtype p55 (mRNA)
subtype p75 (mRNA)
TGF
TGF1 (mRNA)
TGF3 (mRNA)
TGFR (mRNA)
Constant
High IGFBP-3
Increase
Increase
Decrease
High level
Early lactation: peak values
Increase
Decrease
Increase
Very low levels
Very low levels
Very low levels
3.2. IGF
The insulin-like growth factor (IGF) family of ligands
(IGF-I and IGF-II), binding proteins (IGFBP 16), and receptors (IGF-IR and IGF-IIR) play pivotal roles in growth
and development of the organism. The precise role of IGF
in the mammary gland is complex and not yet fully elucidated. There are indications that IGF-II would play a minor
role compared to IGF-I, as it is, for instance, not even expressed in the human breast [76]. Nevertheless, the synthesis
of IGF-I in the mammary gland has been described in many
species, and IGF-IR and IGF-IIR have been detected in the
mammary gland as well. Moreover, it has been shown that
IGF-I is a typical survival factor in the mammary gland. For
instance, Amundadottir et al. [67] demonstrated that mammary tumor cells overexpressing pro-apoptotic proteins survive in the presence of IGF-I. The activity of IGF-I is controlled by a family of specific binding proteins, the IGFBP.
Some IGFBP members induce, while others inhibit the stimulatory effect of IGF-I and are thus associated with cell survival or death, respectively.
The mammary expression and blood concentrations of
IGF, IGF-R, and IGFBP in the mammary gland have been
examined during the lactation cycle in several species
(Table 1). The highest expression levels of IGF-I are detected in nongestating heifers. There is a tendency for
IGF-I levels to decrease during gestation, relatively low
levels are found during lactogenesis and lactation, and an
up-regulation occurs during involution [77]. These expres-
[71,72]
[71,72]
[69]
Increase
[77]
[78]
Constant
Constant
Littleno expression
High IGFBP-3
[79]
[79]
[79]
[80,81]
[80,81]
Increase
[82]
[93]
[93]
[90]
[90]
High values
High values
[100,102,104]
[104]
[100,102]
sion levels are not fully paralleled by the IGF-I concentrations found in blood. Ronge et al. [78] showed that the
IGF-I concentration in cows decreases significantly after
parturition, followed by a gradual increase as lactation persists. Examination of the IGF-IR during the lactation cycle
shows that the number of IGF-IR declines at parturition, a
change that coincides with decreases in the blood level of
its ligand. In contrast, IGF-II and IGF-IIR remain largely
unchanged in cows (reviewed by Baumrucker and Erondu
[79]). During lactation and involution, there is little or no
expression of IGFBP-1, -3, or -6 mRNA in rat, while it
has been shown that IGFBP-3 blood concentrations are
higher during both the prepartum period and involution
in ruminants [80,81]. The latter pattern fits well with the
hypothesis that IGFBP-3 has a stimulatory effect on IGF-I
during involution. During postlactational involution, there
is also a four-fold increase in IGFBP-2 mRNA and sixand 10-fold increases in the expression of IGFBP-4 and -5
mRNA and protein within 24 h after weaning in rodents.
Increased expression of IGFBP-5 correlates with apoptotic
cell death in other tissues as well (reviewed by Rosfjord
and Dickson [82]). Baumrucker and Erondu [79] postulate
that there is an important species difference in IGFBP function. IGFBP-5 appears to be important in rodent mammary
gland involution, while IGFBP-3 exhibits the greatest concentration changes during involution in the bovine species.
However, LeRoith et al. [83] also demonstrated the importance of IGFBP-3 in rodents. These authors observed
that transgenic mice expressing either IGF-I or IGFBP-3 in
I. Lamote et al. / Steroids 69 (2004) 145159
mammary tissues produced milk, but had smaller alveoli
than non-transgenic mice. After weaning, the mice expressing either IGF-I or IGFBP-3 failed to fully undergo tissue
remodeling, had decreased postlactational apoptosis, and
their mammary glands retained enlarged lumens [83,84]. As
binding proteins are expected to inhibit IGF activity, these
observations may appear contradictory. However, IGFBP-3
has been shown to retain active IGF by prolonging its
half-life [8386]. It can therefore be suggested that the inhibition of postlactational involution observed in IGFBP-3
transgenic mice was due to a local accumulation of IGF-I
in the mammary gland. This was also indicated by studies
with transgenic mice overexpressing IGF-I des, a naturally occurring variant of IGF-I that lacks three N-terminal
amino acids with a much lower binding affinity for binding
proteins. Transgenic mice overexpressing IGF-I des have
altered ductular and lobular morphology, similar to mice
overexpressing IGF-I, suggesting again that IGF-I is the
active factor that inhibits postlactational involution and that
IGFBP-3 is prolonging the half-life of this survival factor.
IGF-I is also called somatomedin (A and C) to indicate
that it mediates the STH-action. Indeed, the initial focus of
animal scientists on the IGF system was brought about by an
effort to explain the galactopoetic effect of bovine STH [87].
Because STH receptors were not found on lactating bovine
mammary epithelial cells [38], the IGF were naively thought
to be the active endocrine compounds that directly stimulate milk production [79]. An increase of plasma IGF-I and
-II after STH administration in lactating cows has been observed. IGF-I, but not IGF-II levels, also increased in milk.
The question remains whether the ST-induced increase of
IGF-I could stimulate milk secretion (i.e. endocrine behavior) in lactating ruminants. Prosser et al. [88] infused IGF-I
into the pudic artery of lactating goats and demonstrated an
increase in mammary blood flow and milk secretion. The
most obvious effect occurred early in the homolateral gland
(direct action). In the heterolateral gland, the effect was
less pronounced and delayed by a few hours. It was further
demonstrated that intravascularly administered IGF-I can be
transported from blood to milk across the secretory epithelium, probably using receptor internalization. This is an indication that the increased levels of IGF-I in milk after STH
administration can originate from extramammary sources.
To understand the role of IGF in the proliferation and
differentiation of the mammary gland more clearly, downstream signalling molecules needs to be elucidated. In general, IGF-IR acts through two primary cascades, the mitogen
activated protein (MAP) kinase and phosphatidyl-3-kinase
(PI3-K) kinase pathways. The ultimate targets of the MAP
kinase and PI3-K kinase cascades include members of the
Ets and forkhead transcription factor families. Regulation of
transcription factors provides a mechanism by which IGF
mediates a proliferative and differentiative effect. However,
it should be remarked that this mechanism is not specific for
the mammary gland [89].
153
3.3. TNF
Another player in the mammary regulatory network is tumor necrosis factor (TNF). TNF is mostly known as an
inflammatory cytokine, but it has pleiotropic effects. TNF receptors (TNFR) have been demonstrated in most tissues including human and rat mammary cells [90,91]. Basolo et al.
[92] demonstrated the presence of TNF mRNA and protein
in human mammary epithelial cells. TNF was first reported
as a potential regulator in the context of mammary proliferation and differentiation in 1992 [93]. In an in vitro rat mammary gland model, TNF was found to stimulate epithelial
cell proliferation both in the presence or absence of EGF. It
should be remarked that in the paper from Dollbaum et al.
[91], no stimulatory effect of TNF was observed when using colony number as a parameter for the evaluation of proliferation. However, Ip et al. [93] compared colony number
with cell number and found that while there was no increase
in colony number, there was a systematic increase in cell
number. This suggests that TNF is stimulating proliferation of a selected colony population. TNF also stimulates
differentiation in vitro but only in the absence or upon deficiency of EGF. Remarkably, using a basement membrane
of inferior quality in the in vitro model, TNF induces the
formation of exquisite multi-lobularductal organoids very
reminiscent of the in vivo rat mammary gland during lactation.
These morphological observations were complemented
with data on the functional differentiation of the mammary
epithelium, which was evaluated by the casein production.
TNF inhibits casein production in the presence of EGF, but
stimulates it in the absence of EGF in a concentration dependent manner. Varela et al. [73] have shown that TNF does
not require the EGFR for its action on rat mammary epithelium and that the TNF and EGF mitogenic actions in the
mammary gland are mediated by independent pathways, although co-operativity may occur under some circumstances.
It is not yet clear whether the mammary effects of TNF
are mediated through a direct or an indirect mechanism of
action. Ip et al. [93] speculate that one indirect action of
TNF could be to induce the expression of TGF.
How all these in vitro observations can be related to the
physiological situation was further studied by Varela and Ip
[90]. Changes in the TNF and TNFR mRNA and protein
levels were followed throughout the lactation cycle in the rat
(Table 1). The obtained results are in accordance with the
data from the initial study from Ip et al. [93] and strongly
suggest a potential role for TNF in mammary epithelium
proliferation and morphogenesis during the lactation cycle
of rats. During gestation there is a pronounced increase of
TNF at mRNA and protein expression levels, which probably inhibits casein production until the onset of lactation.
Throughout lactation, TNF mRNA levels decline, but a
high expression of the TNF protein is observed. A possible
explanation for this difference could be that the protein has
154
I. Lamote et al. / Steroids 69 (2004) 145159
a longer half-life and persists even when the corresponding
mRNA has already disappeared. A marked differential expression was also observed for the two TNFR subtypes p55
and p75. As for the TNFR, the p55 subtype mRNA peaks
in early lactation and act as the sole mediator of proliferation. In contrast, mRNA of the p75 TNFR subtype increases
steadily throughout lactation and is responsible for functional stimulation as reflected by casein production. These
findings in rat are not always in accordance with human
studies, which are moreover seemingly contradictory. Basolo et al. [92] observed TNF in human mammary epithelial cells, while Miles et al. [94] localized TNF and TNFR
in the mammary stroma, and Pusztai et al. [95] failed to detect both proteins in normal human breast tissue although
the p55 TNFR subtype was occasionally expressed in the
stroma.
Is TNF also involved in mammary gland involution? An
active role for TNF in this mechanism seems contradictory with its proposed function as a growth and differentiation factor. However, Varela and Ip [90] hypothesize that
the well-known apoptosis-inducing action of TNF as observed in many other cell types might be inhibited in the
mammary gland by a yet unidentified protein in the other
stages of the lactation cycle and is no longer present at the
involution stage. A link between TNF and MMP production was furthermore suggested [73] and has recently been
confirmed in detail. The latter authors show that the secretion of MMP-9 is stimulated by TNF in vitro and postulate
that it may play a role in extracellular matrix degradation
and subsequent controlled invasion of the stroma that occurs
upon mammary remodeling.
It should be remarked that the trigger for the release of
TNF and its exact source in the mammary gland remain
largely unknown to date. One possibility is that interstitial
cells, such as leukocytes, found in the mammary gland serve
as a source for TNF. Indeed, lymphocytes have been reported to aggregate in the mammary gland during gestation
and lactation [96], and the proportion of macrophages was
reported to be greatest in the periparturient period in the
cow [97,98]. Interestingly, not only the number of mammary
macrophages, but also their TNF production capacity was
significantly increased with respect to their monocyte counterparts in blood.
3.4. TGF
A last group of cytokines that have been demonstrated in
the mammary gland of different species, such as the mouse
[99], the sow [100], ruminants, i.e. the cow [101], and the
goat [102] are the members of the transforming growth factor (TGF1, 2, and 3) family. In contrast to the other
growth factors, these cytokines have been classified as local
apoptosis inducing or growth inhibiting factors (reviewed
by Rosfjord and Dickson [82]). The detailed mechanism of
TGF action is not yet known. While TGF regulation of
gene expression patterns of cell cycle elements such as cy-
clins and cell adhesion elements such as integrins have been
reported in mice, further research on the exact function of
the TGF targeted genes in growth inhibition will increase
knowledge of the mechanisms by which TGF mediates its
cellular effect [103].
In the mammary gland, a considerable amount of evidence
has accumulated indicating that TGF plays a critical role
during several phases of the mammary cycle. TGF regulates growth and patterning of the mammary ductal tree in
the virgin mouse. During gestation, TGF is required for
alveolar development and functional differentiation, while at
the same time inhibiting secretion of milk proteins. At parturition this inhibition is lifted, permitting initiation of lactation. During the dry period, TGF was found to support
remodeling of the mammary gland [104].
Patterns of expression of different TGF isoforms during
the mammary cycle have been examined and provide interesting insights into the action of TGF in mice (Table 1).
Substantial expression of TGF1 and TGF3 was found in
all stages of mammary development with the exception of
lactation. TGF3 was substantially increased during gestation, falling to negligible levels immediately following parturition. TGF1 expression was strong in the virgin animal
during ductal development, declined during gestation, disappeared during lactation, and increased during involution
[104]. In two comparable studies on the mammary gland
of goats and sows, respectively, the expression of TGF1
and its receptor (TGFR type III or -glycan) was monitored throughout the lactation cycle [100,102] (Table 1). The
mammary expression of TGF1 and TGFR increases significantly from early lactation (very low levels) to the dry
period (peak values) in the lobulo-alveolar tissue of both
species. In accordance, TGF family members are not found
in porcine milk [105].
During ductal development, accumulations of TGF
around mammary tissues are probably associated with the
avoidance behavior of growing end buds, whose turning determines the pattern of interductal spacing. TGF1 induces
the synthesis of stromal/extracellular matrix and basement
membrane proteins, which trap TGF, creating a locally
nonpermissive environment for growth, resulting in cessation of DNA synthesis and regression of the end bud [104].
High TGF3 expression has been reported in the murine
mammary gland during gestation where it was stated to
inhibit the translation and probably also the secretion of
casein [106] as has been postulated above for TNF. Alveolar cells expressing TGF3 are thus fully prepared for
lactation, but are inhibited from synthesizing and secreting
abundant milk proteins. At parturition the level of TGF3
drops abruptly, presumably permitting full expression of
the lactational phenotype [104].
Wareski and Motyl [100,102] postulate that the TGF
proteins are involved in the induction of programmed
cell death during mammary involution in the caprine and
porcine species. Although the specific mechanism of this
process was not investigated, an increase after TGF
I. Lamote et al. / Steroids 69 (2004) 145159
administration in the epithelial Bax (a pro-apoptotic Bcl-2
family member)/Bcl-2 (anti-apoptotic) ratio and in caspase-3
activity was found. The ratio of pro- versus anti-apoptotic
Bcl-2 family members is essential in regulating the balance
between survival and death in the mammary gland [107].
A previous study in mice mammary epithelial cells showed
that PRL inhibits TGF1 transcription, which may explain
the low TGF1 mRNA and protein synthesis during lactogenesis and galactopoesis as well as the TGF1 increase in
late lactation and the dry period that was also measured in
the sow.
4. Interaction between sex steroid hormones and
growth factors
Cross-talk between receptors and their signalling pathways has been shown to play a critical role in various
cellular responses to ligands. Such cross-talk may occur
between receptors within the same family, such as the
EGFR and IGF-IR, which are both tyrosine kinase receptors, or between different families, such as nuclear steroid
receptors ER and PR and IGF-IR. This last interaction has
been frequently described in mammary cells, but as most
of these experiments were exerted with breast cancer cells,
they need to be confirmed in the normal mammary gland
(reviewed by Hamelers and Steenbergh [108]).
Estrogens and IGFs act as mitogens promoting cell proliferation in the mammary gland. Originally, it was considered that these agents manifest their mitogenic actions
through separate pathways, but a growing body of evidence
gathered over the last decade suggests that the IGF- and
estrogen-mediated signalling pathways are intertwined.
E2 has been shown to enhance IGF signalling at multiple levels (long-term and rapid effects). The expression of
IGF-IR and IGFBPs was found to be up-regulated by E2
[109,110]. Huff et al. [111] and Cohen et al. [112] report
IGF-I mRNA and protein expression in MCF-7 cells, which
are up-regulated by E2 , TGF, and EGF. However, it remains unclear whether breast cancer cells secrete IGF-I, as
other groups [113115] have reported that MCF-7 cells do
not express IGF-I. Next to these long-term effects, rapid effects of the liganded ER on the IGF-IR could also be observed in transformed cell lines, but not in the cancer cell
lines studied by Hamelers et al. [116]. In the presence of
ER, but not ER, E2 rapidly induced phosphorylation of
the IGF-IR [117]. Vice versa, several studies have demonstrated that IGF signalling as well as signalling of other survival factors, like EGF, result in the transcriptional activation of ER [118122]. Co-administration of E2 and growth
factors to cells has been shown to result in an additive effect
on the expression of endogenous estrogen-regulated genes
[122]. Finally, IGF-I and E2 have been shown to synergistically stimulate proliferation of various cancer cell lines
[114]. In analogy with the influence on IGF, estrogen was
reported to synergize with EGF in stimulating cell prolifer-
155
ation by upregulating EGFR [123]. A role for EGF in mediating estrogen induction of end bud formation and expression of PR has been proposed [124]. EGFR is also believed
to be a prominent downstream effector of estrogen action in
several tissues.
In comparison to E2 , few studies describe the interaction
between P and growth factors. Moreover, in analogy with
E2 , the studies on P-associated growth factors were carried
out in mammary tumor cells and await confirmation in the
normal mammary gland. P induces the expression of several
growth factors via its PR. Since studies suggest a role for PR
as well as for EGFR in ductal morphogenesis, these various
observations support the view that EGFR is an essential mediator of hormone action in the adolescent mammary gland
[69].
Complementary to these data, which were mostly obtained from experiments with cancer cell lines, are the recent
results from Schams et al. [15] providing strong evidence
that interactions between ER and PR and TGF and IGF-I
also occur in normal mammary gland tissue. These authors
demonstrate that the mRNA expression pattern of some proliferative growth factors, such as TGF and IGF-I, in the
bovine mammary gland during development and function
is comparable for ER and PR expression. The presence
of high ER, ER, and PR levels in the bovine mammary
gland before the onset of lobulo-alveolar development and
significantly lower levels during gestation and lactogenesis,
suggests an important functional role for the initiation of
lobulo-alveolar development, possibly in co-operation with
the proliferative actions of growth factors [15].
It should be remarked that additional evidence on the
cross-talk between sex steroid hormones and other local factors than IGF and EGF in the mammary gland is not only
limited but also highly suggestive.
Sordillo et al. [98] postulate that local TNF production
might be under steroid hormonal regulation, and Ip et al.
[93] have suggested that the inhibitory effect of TNF on
casein production might be in concert with P. The means
by which TGF3 levels are regulated are not known, but
regulation by changes in reproductive hormones such as P,
ST, and PRL are possibilities [104]. Finally, it is not yet
clear whether there is a link between the mammary effects of
TGF and TNF during involution, but it has nevertheless
been suggested that hormonal influences could modulate the
expression of both cytokines [90,125]. Further research on
this topic is therefore clearly warranted.
5. Conclusion
The well known importance of the sex steroid hormones
E2 and P for normal mammogenesis has unequivocally been
confirmed and refined by data obtained with KO and transgenic animal models. These latter studies have established
that ER mediated signalling is essential for ductal morphogenesis, while PR signalling is critical for lobulo-alveolar
156
I. Lamote et al. / Steroids 69 (2004) 145159
development. ER and PR expression has also been determined in the mammary gland during the lactation cycle and
the regulatory role of these steroid receptors and their ligands can now be expanded from mammogenesis to all other
phases including lactogenesis, galactopoesis, and especially
involution. These data also suggest that mammary proliferation and differentiation are perfectly counterbalanced by
cell death throughout the lactation cycle.
In addition to the regulatory role of sex steroid hormones,
an increasing list of local growth factors has also been shown
to modulate survival and apoptosis in the mammary gland.
A stimulating role in the proliferation and/or differentiation
of mammary epithelial cells is suggested for most growth
factors including EGF, TGF, AR and IGF. The cytokine
TNF might play a dual role, stimulating survival or cell
death depending on the presence or absence of other factors,
whereas TGF has been found only to be growth inhibiting
and apoptosis inducing. For most of these growth factors,
the expression patterns of their receptors were also monitored during the lactation cycle. These data reveal cross-talk
between the ER and PR and the receptors for some growth
factors, suggesting an important functional role for estrogens
and P in the lactation cycle in co-operation with these growth
factors. Nevertheless, the cross-talk between sex steroid and
growth factor receptors has not extensively been reported.
Moreover, the interaction with the IGF-IR and EGFR was
described in breast cancer cell lines and still needs to be
confirmed in the normal mammary gland. In conclusion, it
remains a challenge for future research to further unravel the
interaction between sex steroid and growth factor signalling
pathways in the mammary gland.
References
[1] Anderson R. Endocrinological control. In: Larson B, Smith V,
editors. Lactation I: a comprehensive treatise. New York, London:
Academic Press; 1974. p. 97140.
[2] Cowie A. Influence of hormones on mammary growth and milk
secretion. In: Falconer I, editor. Lactation. London: Butterworths;
1971. p. 12340.
[3] Clarke R. Introduction and overview: sex steroids in the mammary
gland. J Mammary Gland Biol Neoplasia 2000;5:24550.
[4] Kucharova S, Farkas R. Hormone nuclear receptors and their
ligands: role in programmed cell death. Endocr Regul 2002;36:37
60.
[5] Kiess W, Gallaher B. Hormonal control of programmed cell
death/apoptosis. Eur J Endocrinol 1998;138:48291.
[6] Pertzelan A, Yalon L, Kauli R, Laron Z. A comparative study of
the effect of oestrogen substitution therapy on breast development
in girls with hypo- and hypergonadotrophic hypogonadism. Clin
Endocrinol 1982;16:368.
[7] Borellini F, Oka T. Growth control and differentiation in mammary
epithelial cells. Environ Health Perspect 1989;80:8599.
[8] Daniel C, Silberstein G, Strickland P. Direct action of 17-estradiol
on mouse mammary ducts analyzed by sustained release implants
and steroid autoradiography. Cancer Res 1987;47:60527.
[9] Silberstein G, Van Horn K, Shyamala G, Daniel C. Essential
role of endogenous estrogen in directly stimulating mammary
growth demonstrated by implants containing pure antiestrogens.
Endocrinology 1994;134:8490.
[10] Korach K. Insights from the study of animals lacking functional
estrogen receptor. Science 1994;266:15247.
[11] Kelly P, Bachelot A, Kedzia C, Hennighausen L, Ormandy C,
Kopchick J, et al. The role of prolactin and growth hormone in
mammary gland development. Mol Cell Endocrinol 2002;197:127
31.
[12] Ormandy C, Sutherland R. Mechanisms of prolactin receptor
regulation in mammary gland. Mol Cell Endocrinol 1993;91:C16.
[13] Horwitz K, Clarke C. Estrogens and progestins in mammary
development and neoplasia. J Mammary Gland Biol Neoplasia
1998;3:12.
[14] Forsyth I. The insulin-like growth factor and epidermal growth
factor families in mammary cell growth in ruminants: action and
interaction with hormones. J Dairy Sci 1996;79:108596.
[15] Schams D, Kohlenberg S, Amselgruber W, Berisha B, Pfaffl
M, Sinowatz F. Expression and localisation of oestrogen and
progesterone receptors in the bovine mammary gland during
development, function and involution. J Endocrinol 2003;177:305
17.
[16] Hovey R, Trott J, Vonderhaar B. Establishing a framework for the
functional mammary gland: from endocrinology to morphology. J
Mammary Gland Biol Neoplasia 2002;7:1738.
[17] Bartow S. Use of the autopsy to study ontogeny and expression
of the estrogen receptor gene in human breast. J Mammary Gland
Biol Neoplasia 1998;3:3748.
[18] Capuco A, Akers R, Ellis S, Wood D. Mammary growth in
Holstein calves: bromodeoxyuridine incorporation and steroid
receptor localization. J Dairy Sci 2000;83:17.
[19] Shyamala G. Progesterone signaling and mammary gland
morphogenesis. J Mammary Gland Biol Neoplasia 1999;4:89104.
[20] Lydon J, Sivaraman L, Conneely O. A reappraisal of progesterone
action in the mammary gland. J Mammary Gland Biol Neoplasia
2000;5:32538.
[21] Brisken C, Heineman A, Chaviarra T, Elenbaas B, Tan J, Dey S,
et al. Essential function of Wnt-4 in mammary gland development
downstream of progesterone signaling. Genes Dev 2000;14:6504.
[22] Atwood G, Hovey R, Glover J, Chepko G, Ginsburg E, Robinson W,
et al. Progesterone induces side-branching of the ductal epithelium
in the mammary glands of peripubertal mice. J Endocrinol
2000;167:3952.
[23] Anderson E, Clarke R, Howell A. Estrogen responsiveness and
control of normal human breast proliferation. J Mammary Gland
Biol Neoplasia 1998;3:2335.
[24] Russo J, Ao X, Grill C, Russo I. Pattern of distribution of cells
positive for estrogen receptor and progesterone receptor in relation
to proliferating cells in the mammary gland. Breast Cancer Res
Treat 1999;53:21727.
[25] Shyamala G, Schneider W, Schott D. Developmental regulation
of murine mammary progesterone receptor gene expression.
Endocrinology 1990;126:28829.
[26] Saji S, Jensen E, Nilsson S, Rylander T, Warner M, Gustafsson J.
Estrogen receptors and in the rodent mammary gland. Proc
Natl Acad Sci USA 2000;97:33742.
[27] Burvenich C, Vandeputte-Van Messom G, Roets E, Massart-Leen
A, Peeters G. Physiological factors affecting milk production in
lactating ruminants. In: Proceedings of Atti IX Congresso Nazionale
ASPA, Tavola Rotonda; 1991. p. 1191203.
[28] Zhou Y, Xu B, Maheshwari H, He L, Reed M, Lozykowski M, et
al. A Mammalian model for Laron syndrome produced by targeted
disruption of the mouse growth hormone receptor/binding protein
gene (the Laron mouse). Proc Natl Acad Sci USA 1997;94:13215
20.
[29] Rimoin D, Holzman G, Merimee T, Rabinowitz D, Barnes A, Tyson
J, et al. Lactation in the absence of human growth hormone. J Clin
Endocrinol Metab 1968;28:11838.
[30] Rosenbloom A, Guevara-Aguirre J, Rosenfield R, Francke U.
Growth hormone receptor deficiency in Ecuador. J Clin Endocrinol
Metab 1999;84:443643.
I. Lamote et al. / Steroids 69 (2004) 145159
[31] Brisken C, Ayyanan A, Doppler W. Prolactin signaling and Stat5:
going there own separate ways? Breast Cancer Res 2002;4:20912.
[32] Miyoshi K, Shillingford J, Smith G, Grimm S, Wagner K, Oka
T, et al. Signal transducer and activator of transcription (Stat) 5
controls the proliferation and differentiation of mammary alveolar
epithelium. J Cell Biol 2001;155:53142.
[33] van Garderen E, van der Poel H, Swennenhuis J, Wissink
E, Rutteman G, Hellmen E, et al. Expression and molecular
characterization of the growth hormone receptor in canine mammary
tissue and mammary tumors. Endocrinology 1999;140:590714.
[34] Mol J, van Garderen E, Rutteman G, Rijnberk A. New insights
in the molecular mechanism of progestin-induced proliferation of
mammary epithelium: induction of the local biosynthesis of growth
hormone (GH) in the mammary gland of dogs, cats and humans. J
Steroid Biochem Mol Biol 1996;517:6771.
[35] Hull K, Harvey S. Growth hormone: roles in female reproduction.
J Endocrinol 2001;168:123.
[36] Selman P, Mol J, Rutteman G, Rijnberk A. Progestin treatment in
dog. I. Effects on growth hormone, insulin-like growth factor I and
glucose homeostasis. Eur J Endocrinol 1994;131:41321.
[37] Schoenmakers I, Kooistra H, Okkens A, Hazewinkel H, Bevers M,
Mol J. Growth hormone concentrations in mammary secretions and
plasma of the periparturient bitch and in plasma of the neonate. J
Reprod Fertil 1997;51:3637.
[38] Flint D, Knight C. Interactions of prolactin and growth hormone
(GH) in the regulation of mammary gland function and epithelial
cell survival. J Mammary Gland Biol Neoplasia 1997;2:418.
[39] Lascelles A, Lee C. Involution of the mammary gland. In: Larson B,
editor. Lactation IV: a comprehensive treatise. New York, London:
Academic Press; 1978. p. 11577.
[40] Knight C, Peaker M, Wilde C. Local control of mammary
development and function. Rev Reprod 1998;3:10412.
[41] Streuli C, Gilmore A. Adhesion-mediated signaling in the regulation
of mammary epithelial cell survival. J Mammary Gland Biol
Neoplasia 1999;4:18391.
[42] Politis I. Plasminogen activator system: implications for mammary
cell growth and involution. J Dairy Sci 1996;79:1097107.
[43] Lund L, Bjorn S, Sternlicht M, Nielsen B, Solberg H, Usher P, et
al. Lactational competence and involution of the mouse mammary
gland require plasminogen. Development 2000;127:448192.
[44] Tenniswood M, Guenette R, Lakins J, Mooibroek M, Wong P,
Welsh J. Active cell death in hormone-dependent tissues. Cancer
Metastasis Rev 1992;11:197220.
[45] Szondy Z, Sarang Z, Molnar P, Nemeth T, Piacentini M,
Mastroberardino P, et al. Transglutaminase 2/ mice reveal
a phagocytosis-associated crosstalk between macrophages and
apoptotic cells. Proc Natl Acad Sci USA 2003;100:78127.
[46] Prince J, Klinowska C, Marshman E, Lowe E, Mayer U, Miner J,
et al. Cellmatrix interactions during development and apoptosis of
the mouse mammary gland in vivo. Dev Dyn 2002;223:497516.
[47] Capuco A, Akers R. Mammary involution in dairy animals. J
Mammary Gland Biol Neoplasia 1999;4:13744.
[48] Wilde C, Knight C, Flint D. Control of milk secretion and apoptosis
during mammary involution. J Mammary Gland Biol Neoplasia
1999;4:12936.
[49] Smith G, Boulanger C. Mammary stem cell repertoire: new insights
in aging epithelial populations. Mech Ageing Dev 2002;123:1505
19.
[50] Deugnier M, Faraldo M, Janji B, Rousselle P, Thiery J, Glukhova
M. EGF controls the in vivo developmental potential of a mammary
epithelial cell line possessing progenitor properties. J Cell Biol
2002;159:45363.
[51] Kim N, Oberley T, Yasukawa-Barnes J, Clifton K. Stem cell
characteristics of transplanted rat mammary clonogens. Exp Cell
Res 2000;260:14659.
[52] Kamiya K, Gould M, Clifton K. Quantitative studies of ductal
versus alveolar differentiation from rat mammary cologens. Proc
Soc Exp Biol Med 1998;219:21725.
157
[53] Bocker W, Moll R, Poremba C, Holland R, van Diest P, Dervan
P, et al. Common adult stem cells in the human breast give rise
to glandular and myoepithelial cell lineages: a new cell biological
concept. Lab Invest 2002;82:73745.
[54] Dontu G, Abdallah W, Foley J, Jackson K, Clarke M, Kawamura
M, et al. In vitro propagation and transcriptional profiling of human
mammary stem/progenitor cells. Genes Dev 2003;17:125370.
[55] Holland M, Tai M, Trosko J, Griffin L, Stasko J, Cheville N, et al.
Isolation and differentiation of bovine mammary gland progenitor
cell populations. Am J Vet Res 2003;64:396403.
[56] Kordon E, Smith G. An entire functional mammary gland
may comprise the progeny from a single cell. Development
1998;125:192030.
[57] Chepko G, Smith G. Mammary epithelial stem cells: our current
understanding. J Mammary Gland Biol Neoplasia 1999;4:3552.
[58] Stingl J, Eaves C, Zandieh I, Emerman J. Characterization of
bipotent mammary epithelial progenitor cells in normal adult human
breast tissue. Breast Cancer Res Treat 2001;67:93109.
[59] Wagner K, Boulanger C, Henry M, Sgagias M, Hennighausen
L, Smith G. An adjunct mammary epithelial cell population in
parous females: its role in functional adaptation and tissue renewal.
Development 2002;129:137786.
[60] Marti A, Lazar H, Ritter P, Jaggi R. Transcription factor activities
and gene expression during mouse mammary gland involution. J
Mammary Gland Biol Neoplasia 1999;4:14552.
[61] Accorsi P, Pacioni B, Pezzi C, Forni M, Flint D, Seren E. Role
of prolactin, growth hormone and insulin-like growth factor 1
in mammary gland involution in the dairy cow. J Dairy Sci
2002;85:50713.
[62] Wilde C, Quarrie L, Tonner E, Flint D, Peaker M. Mammary
apoptosis. Livestock Prod Sci 1997;50:2937.
[63] Athie F, Bachman K, Head H, Hayen M, Wilcox C. Estrogen
administrated at final milk removal accelerates involution of bovine
mammary gland. J Dairy Sci 1996;79:226.
[64] Sandgren E, Schroeder J, Qui T, Palmiter R, Brinster R, Lee D.
Inhibition of mammary gland involution is associated with TGF
but not c-myc induced tumorigenesis in transgenic mice. Cancer
Res 1995;55:3927.
[65] Smith G, Sharp R, Kordon E, Jhappan C, Merlino G. Transforming
growth factor promotes mammary tumorigenesis through selective
survival and growth of secretory epithelial cells. Am J Pathol
1995;147:108196.
[66] Merlo G, Graus-Porta D, Cella N, Marte B, Taverna D, Hynes N.
Growth, differentiation and survival of HC11 mammary epithelial
cells: diverse effects of receptor tyrosine kinase-activating peptide
growth factors. Eur J Cell Biol 1996;70:97105.
[67] Amundadottir L, Nass S, Berchem G, Johnson M, Dickson R.
Cooperation of TGF and c-Myc mouse mammary tumorigenesis:
coordinated stimulation of growth and suppression of apoptosis.
Oncogene 1996;13:75765.
[68] Pinkas-Kramarski R, Alroy I, Yarden Y. Erb B receptors and
EGF-like ligands: cell lineage determination and oncogenesis
through combinatorial signaling. J Mammary Gland Biol Neoplasia
1997;2:97107.
[69] Troyer L, Lee D. Regulation of mouse mammary gland development
and tumorigenesis by the ERBB signaling network. J Mammary
Gland Biol Neoplasia 2001;6:721.
[70] Luetteke N, Qiu R, Fenton S, Troyer K, Riedel R, Chang A, et al.
Targeted inactivation of the EGF and amphiregulin genes reveals
distinct roles for EGF receptor ligands in mouse mammary gland
development. Development 1999;126:273950.
[71] Beardmore J, Richards R. Concentrations of epidermal growth factor
in mouse milk throughout lactation. J Endocrinol 1983;96:28792.
[72] Carpenter G. Epidermal growth factor is a major growth promoting
agent in human milk. Science 1980;210:1989.
[73] Varela L, Darcy K, Ip M. The epidermal growth factor receptor is
not required for tumor necrosis factor- action in normal mammary
epithelial cells. Endocrinology 1997;138:3891900.
158
I. Lamote et al. / Steroids 69 (2004) 145159
[74] Nass S, Li L, Amundadottir L, Furth P, Dickson R. Role for
Bcl-xl in the regulation of apoptosis by EGF and TGF1 in c-myc
overexpressing mammary epithelial cells. Biochem Biophys Res
Commun 1996;227:24856.
[75] Moorby C, Taylor J, Forsyth I. Transforming growth factor-:
receptor binding and action on DNA synthesis in the sheep
mammary gland. J Endocrinol 1995;144:16571.
[76] Paik S. Expression of IGF-I and IGF-II mRNA in breast tissue.
Breast Cancer Res Treat 1992;22:318.
[77] Evans-Storms R, Cidlowski J. Regulation of apoptosis by steroid
hormones. J Steroid Biochem Mol Biol 1995;53:18.
[78] Ronge H, Blum J, Clement C, Jans F, Leuenberger H, Binder H,
et al. Somatomedin C in dairy cows related to energy and protein
supply and to milk production. Anim Prod 1988;47:16583.
[79] Baumrucker C, Erondu N. Insulin-like growth factor (IGF) system
in the bovine mammary gland and milk. J Mammary Gland Biol
Neoplasia 2000;5:5364.
[80] Sharma B, Vandehaar M, Ames N. Expression of insulin-like growth
factor-1 in cows at different stages of lactation and in late lactation
cows treated with somatotropin. J Dairy Sci 1994;77:223241.
[81] Vega J, Gibson C, Skaar T, Hadsell D, Baumrucker C. Insulin-like
growth factor (IGF)-I and -II and IGF binding proteins in serum
and mammary secretions during the dry period and early lactation
in dairy cows. J Anim Sci 1991;69:253847.
[82] Rosfjord E, Dickson R. Growth factors, apoptosis, and survival
of mammary epithelial cells. J Mammary Gland Biol Neoplasia
1999;4:22937.
[83] LeRoith D, Neuenschwander S, Wood T, Henninghausen L.
Insulin-like growth factor-1 and insulin-like growth factor binding
protein-3 inhibit involution of the mammary gland following
lactation: studies in transgenic mice. Prog Growth Factor Res
1995;6:4336.
[84] Neuenschwander S, Schwartz A, Wood T, Roberts C, Hennighausen
L, LeRoith D. Involution of the lactating mammary gland is
inhibited by the IGF system in a transgenic model. J Clin Invest
1996;97:222532.
[85] Binoux M, Hossenlopp P. Insulin-like growth factor (IGF) and
IGF-binding proteins: comparison of human serum and lymph. J
Clin Endocrinol Metab 1988;67:50914.
[86] Baxtern R, Martin J. Binding proteins for the insulin-like growth
factors: structure, regulation and function. Prog Growth Factor Res
1989;1:4968.
[87] Bauman D, Vernon R. Effect of exogenous bovine somatotropin on
lactation. Ann Rev Nutr 1993;13:43761.
[88] Prosser C, Fleet I, Corps A, Froesch E, Heap R. Increase in milk
secretion and mammary blood flow by intra-arterial infusion of
insulin-like growth factor-I into milk of lactating goats. J Endocrinol
1990;126:43743.
[89] LeRoith D, Roberts C. The insulin-like growth factor system and
cancer. Cancer Lett 2003;195:12737.
[90] Varela L, Ip M. Tumor necrosis factor-: a multifunctional regulator
of mammary gland development. Endocrinology 1996;137:491524.
[91] Dollbaum C, Creasey A, Dairkee S, Hiller A, Rudolph A, Lin
L, et al. Specificity of tumor necrosis factor toxicity for human
mammary carcinomas relative to normal mammary epithelium and
correlation with response to doxorubicin. Proc Natl Acad Sci USA
1988;85:47404.
[92] Basolo F, Conaldi P, Fiore L, Calvo S, Toniolo A. Normal
breast epithelial-cells produce interleukin-6 and interleukin-8
together with tumor-necrosis-factor-defective IL6 expression in
mammary-carcinoma. Int J Cancer 1993;55:92630.
[93] Ip M, Shoemaker S, Darcy K. Regulation of rat mammary epithelial
cell proliferation and differentiation by tumor necrosis factor-.
Endocrinology 1992;130:283344.
[94] Miles D, Happerfield L, Naylor M, Bobrow L, Rubens R, Balkwill
F. Expression of tumor-necrosis-factor (TNF-) and its receptor in
benign and malignant breast-tissue. Int J Cancer 1994;56:77782.
[95] Pusztai L, Clover L, Cooper K, Starkey P, Lewis C, McGee J.
Expression of tumor-necrosis-factor- and its receptors in carcinoma
of the breast. Br J Cancer 1994;70:28992.
[96] Solari R, Kraehenbuhl J. Receptor-mediated transepithelial transport
of polymeric immunoglobulins. In: Neville M, Daniel C, editors.
The mammary gland. Development, regulation, and function. New
York: Plenum Press; 1987. p. 26998.
[97] Park Y, Fox L, Hamilton M, Davis W. Bovine mononuclear
leukocyte subpopulations in peripheral-blood and mammary-gland
secretions during lactation. J Dairy Sci 1992;75:9981006.
[98] Sordillo L, Pighetti G, Davis M. Enhanced production of bovine
tumor necrosis factor- during the periparturient period. Vet
Immunol Immunopathol 1995;49:26370.
[99] Atwood G, Ikeda M, Vonderhaar B. Involution of mouse mammary
glands in whole organ culture: a model for studying programmed
cell death. Biochem Biophys Res Commun 1995;207:8607.
[100] Motyl T, Gajkowska B, Wojewodzka U, Wareski P, Rekiel A, Ploszaj
T. Expression of apoptosis-related proteins in involuting mammary
gland of sow. Comp Biochem Physiol 2001;128:63546.
[101] Plath A, Einspanier R, Peters F, Sinowatz F, Schams D. Expression
of transforming growth factors and -1 messenger RNA in the
bovine mammary gland during different stages of development and
lactation. J Endocrinol 1997;155:50111.
[102] Wareski P, Motyl T, Ryniewicz Z, Orzechowski A, Gajkowska B,
Wojewodzka U, et al. Expression of apoptosis-related proteins in
mammary gland of goat. Small Rumin Res 2001;40:27989.
[103] Xie L, Law B, Aakre M, Edgerton M, Shyr Y, Bhowmick N,
et al. Transforming growth factor -regulated gene expression in
a mouse mammary gland epithelial cell line. Breast Cancer Res
2003;5:18798.
[104] Daniel C, Robinson S, Silberstein G. The transforming growth
factors in development and functional differentiation of the mouse
mammary gland. Bioactive Components Hum Milk 2001;501:61
70.
[105] Xu R, Doan Q, Regester G. Detection and characterisation of
transforming growth factor- in porcine colostrum. Biol Neonate
1999;75:5964.
[106] Robinson S, Roberts A, Daniel C. TGF- suppresses casein
synthesis in mouse mammary explants and may play a role in
controlling milk levels during pregnancy. J Cell Biol 1993;120:245
51.
[107] Schorr K, Li M, Bar-Peled U, Lewis A, Heredia A, Lewis B, et al.
Gain of Bcl-2 is more potent than Bax loss in regulating mammary
epithelial cell survival in vivo. Cancer Res 1999;59:25415.
[108] Hamelers I, Steenbergh P. Interactions between estrogens and
insulin-like growth factor signaling pathways in human breast tumor
cells. Endocr Relat Cancer 2003;10:33145.
[109] Huynh H, Nickerson T, Pollak M, Yang X. Regulation of insulin-like
growth factor I receptor expression by the pure antiestrogen ICI
182780. Clin Cancer Res 1996;2:203742.
[110] Perks C, Holly J. Insulin-like growth factor binding proteins
(IGFBPs) in breast cancer. J Mammary Gland Biol Neoplasia
2000;5:7584.
[111] Huff K, Knabbe C, Lindsey R, Kaufman D, Bronzert D,
Lippman M, et al. Multihormonal regulation of insulin-like growth
factor-I-related protein in MCF-7 human breast cancer cells. Mol
Endocrinol 1988;2:2008.
[112] Cohen F, Manni A, Glikman P, Bartholomew M, Demers L.
Interactions between growth factor secretion and polyamines in
MCF-7 breast cancer cells. Eur J Cancer 1990;26:6038.
[113] Yee D, Paik S, Lebovic G, Marcus R, Favoni R, Cullen K, et
al. Analysis of insulin-like growth factor I gene expression in
malignancy: evidence for a paracrine role in human breast cancer.
Mol Endocrinol 1989;3:50917.
[114] Van der Burg B, Rutteman G, Blankenstein M, De Laat S, Van
Zoelen E. Mitogenic stimulation of human breast cancer cells in
a growth factor-defined medium: synergistic action of insulin and
estrogen. J Cell Physiol 1988;134:1018.
I. Lamote et al. / Steroids 69 (2004) 145159
[115] Gebauer G, Jger W, Lang N. mRNA expression of components
of the insulin-like growth factor system in breast cancer cell
lines, tissues, and metastatic breast cancer cells. Anticancer Res
1998;18:11915.
[116] Hamelers I, van Schaik R, van Teeffelen H, Sussenbach J,
Steenbergh P. Synergistic proliferative action of insulin-like growth
factor I and 17 -estradiol in MCF-7S breast tumor cells. Exp Cell
Res 2002;273:10717.
[117] Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C.
Estrogen receptor rapidly activates the IGF-1 receptor pathway.
J Biol Chem 2000;275:1844753.
[118] Cardona-Gomez G, Mendez P, DonCarlos L, Azcoitia I,
Garcia-Segura L. Interactions of estrogens insulin-like growth
factor-I in the brain: implications for neuroprotection. Brain Res
Rev 2001;37:32034.
[119] Campbell R, Bhat-Nakshatri P, Patel N, Constantinidou D, Ali
S, Nakshatri H. Phosphatidyl 3-kinase/AKT-mediated activation of
estrogen receptor : a new model for anti-estrogen resistance. J
Biol Chem 2001;276:981724.
159
[120] Lee A, Weng C, Jackson J, Yee D. Activation of estrogen
receptor-mediated gene transcription by IGF-I in human breast
cancer cells. J Endocrinol 1997;152:3947.
[121] Ram P, Kiefer T, Silverman M, Song Y, Brown G, Hill S.
Estrogen receptor transactivation in MCF-7 breast cancer cells by
melatonin and growth factors. Mol Cell Endocrinol 1998;141:53
64.
[122] Stoica A, Saceda M, Fakhro A, Joyner M, Martin M. Role of
insulin-like growth factor-I in regulating estrogen receptor- gene
expression. J Cell Biochem 2000;76:60514.
[123] Vanderboom R, Sheffield L. Estrogen enhances epidermal growth
factor-induced DNA synthesis in mammary epithelial cells. J Cell
Physiol 1993;156:36772.
[124] Ankrapp D, Bennett J, Haslam S. Role of epidermal growth factor
in the acquisition of ovarian steroid hormone responsiveness in
the normal mouse mammary gland. J Cell Physiol 1998;174:251
60.
[125] Furth P. Mammary gland involution and apoptosis of mammary
epithelial cells. J Mammary Gland Biol Neoplasia 1999;4:1237.
Vous aimerez peut-être aussi
- 250 Conversation StartersDocument28 pages250 Conversation StartersmulePas encore d'évaluation
- Ddec VDocument30 pagesDdec Vllama100% (1)
- Homeopatia Vibracional RatesDocument45 pagesHomeopatia Vibracional RatesAugusto Bd100% (4)
- LEARNING ACTIVITY SHEET in Oral CommDocument4 pagesLEARNING ACTIVITY SHEET in Oral CommTinTin100% (1)
- SAMPLE Forklift Safety ProgramDocument5 pagesSAMPLE Forklift Safety ProgramSudiatmoko SupangkatPas encore d'évaluation
- Cesars WayDocument20 pagesCesars WayToni TursićPas encore d'évaluation
- Investigatory Project of BiologyDocument18 pagesInvestigatory Project of BiologyRanjana Singh87% (31)
- Zara Case StudyDocument26 pagesZara Case StudySeminarskiRadovi100% (2)
- Hormones and the Fetus: Volume 1: Production, Concentration and Metabolism During PregnancyD'EverandHormones and the Fetus: Volume 1: Production, Concentration and Metabolism During PregnancyPas encore d'évaluation
- Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human PrimatesDocument21 pagesPhysiologic Events of Embryo Implantation and Decidualization in Human and Non-Human PrimatesAnh Vũ Hồ Ngọc100% (1)
- Exercise and The Menstrual CycleDocument14 pagesExercise and The Menstrual CycleAndrew HaslettPas encore d'évaluation
- Molecular Mechanism of ImplantationDocument19 pagesMolecular Mechanism of ImplantationAbunu TilayePas encore d'évaluation
- Endocrinology of Milk ProductionDocument18 pagesEndocrinology of Milk Productionjuan carlos ardila fandiñoPas encore d'évaluation
- Progress in Research On Key Factors Regulating Lactation. Guo, H., Li, J., Wang, Y., Cao, X., LV, X., Yang, Z., & Chen, Z. (2023) .Document11 pagesProgress in Research On Key Factors Regulating Lactation. Guo, H., Li, J., Wang, Y., Cao, X., LV, X., Yang, Z., & Chen, Z. (2023) .Nxyz GxyzPas encore d'évaluation
- AbstractDocument22 pagesAbstractnayayathPas encore d'évaluation
- Goats Reproduction Estrual Cycle Estrous Synchronization Artificial InseminationDocument11 pagesGoats Reproduction Estrual Cycle Estrous Synchronization Artificial InseminationBayu Anke MardianPas encore d'évaluation
- ACFrOgCu W0g9MxVK8YkEvfsqyGhjSstpPm3j2uJhpy4G69TRnw18zCsRC69oQlKhanJWV 6usc VqeS933XuRqaxNoOkxjXkvFwDAm6It15dFc0KjwYag EzHDH6St2KFBAy0eOIbDC WNeC9lpDocument18 pagesACFrOgCu W0g9MxVK8YkEvfsqyGhjSstpPm3j2uJhpy4G69TRnw18zCsRC69oQlKhanJWV 6usc VqeS933XuRqaxNoOkxjXkvFwDAm6It15dFc0KjwYag EzHDH6St2KFBAy0eOIbDC WNeC9lpUrvashi KhadePas encore d'évaluation
- ReproductionDocument10 pagesReproductionThiago SantosPas encore d'évaluation
- Scaramuzzi 2011 PDFDocument24 pagesScaramuzzi 2011 PDFKarol Mariam VelázquezPas encore d'évaluation
- Cold Spring Harb Perspect Biol-2010-Brisken-a003178Document17 pagesCold Spring Harb Perspect Biol-2010-Brisken-a003178wi bowoPas encore d'évaluation
- Placenta 2Document21 pagesPlacenta 2Nurba Enda Karina NasutionPas encore d'évaluation
- Biomarkers of Ovarian ReserveDocument15 pagesBiomarkers of Ovarian ReserveReni ReniPas encore d'évaluation
- Hormone Action in The Mammary Gland - C Brisken and B O Malley 2010Document15 pagesHormone Action in The Mammary Gland - C Brisken and B O Malley 2010tostonasPas encore d'évaluation
- Funcion Endocrina de La Placenta HumanaDocument30 pagesFuncion Endocrina de La Placenta HumanaSofia RossoPas encore d'évaluation
- Lonergan 2016Document9 pagesLonergan 2016Resita ReiitaPas encore d'évaluation
- Reproductive System: Dr. Dicky Moch. Rizal, Mkes, Spand Bag. Ilmu Faal, FK UgmDocument94 pagesReproductive System: Dr. Dicky Moch. Rizal, Mkes, Spand Bag. Ilmu Faal, FK UgmNi Made Dwiki AndriyaniPas encore d'évaluation
- Fisiología Del PartoDocument20 pagesFisiología Del PartoMatias Israel CandoPas encore d'évaluation
- J.theriogenology.2016.03.032 MetodologiaDocument6 pagesJ.theriogenology.2016.03.032 MetodologiaALEXA DANNAE MERLOS DIAZPas encore d'évaluation
- 02 - IRAOvulation Induction For TheDocument11 pages02 - IRAOvulation Induction For TheHartanto LiePas encore d'évaluation
- Fleming 2010Document4 pagesFleming 2010Ahmed GhanimPas encore d'évaluation
- Formação de Oocitos - ArtigoDocument18 pagesFormação de Oocitos - ArtigoTaynara BarreiraPas encore d'évaluation
- Physiological Control of Mammary Growth, Lactogenesis, and Lactation 1Document19 pagesPhysiological Control of Mammary Growth, Lactogenesis, and Lactation 1Tandyo TriasmoroPas encore d'évaluation
- Barker's Hypothesis. Adverse Events in Fetal Life Can Have Lifelong Effects On Health and DiseaseDocument4 pagesBarker's Hypothesis. Adverse Events in Fetal Life Can Have Lifelong Effects On Health and Diseasemary_fengPas encore d'évaluation
- UntitledDocument234 pagesUntitledMarco Antonio AlvarezPas encore d'évaluation
- MyomaDocument14 pagesMyomaAmanda ShermanPas encore d'évaluation
- Hormones and Embryonic Development: Advances in the BiosciencesD'EverandHormones and Embryonic Development: Advances in the BiosciencesG. RaspéPas encore d'évaluation
- Abstract Placenta AustráliaDocument111 pagesAbstract Placenta AustráliaMilena MoraesPas encore d'évaluation
- Physiological Breast Development and Involution - CompressedDocument7 pagesPhysiological Breast Development and Involution - CompressedmohamedhazemelfollPas encore d'évaluation
- The Ovarian ReserveDocument19 pagesThe Ovarian Reservepramod singhPas encore d'évaluation
- Myometrial ContractilityDocument9 pagesMyometrial ContractilityyeandunPas encore d'évaluation
- Mechanisms of Disease: I S E PDocument9 pagesMechanisms of Disease: I S E Plupiz717Pas encore d'évaluation
- Hormonal Control of Mammogenesis and Onset of Lactation in C o W S - A Review R. E. Erb Department of Animal SciencesDocument15 pagesHormonal Control of Mammogenesis and Onset of Lactation in C o W S - A Review R. E. Erb Department of Animal SciencesAh MagdyPas encore d'évaluation
- Biomarkers Related To Endometrial Receptivity and ImplantationDocument19 pagesBiomarkers Related To Endometrial Receptivity and ImplantationEndriana Gak Endut EndutPas encore d'évaluation
- Reproductive Biology and EndocrinologyDocument7 pagesReproductive Biology and EndocrinologyFabiola Ramos CavalieriPas encore d'évaluation
- Review Ontology and Endocrinology of The Reproductive System of Bulls From Fetus To MaturityDocument8 pagesReview Ontology and Endocrinology of The Reproductive System of Bulls From Fetus To MaturitySyahzidane YusufPas encore d'évaluation
- Leptin FullDocument12 pagesLeptin FullelenPas encore d'évaluation
- Pharma - Reproductive SystemDocument4 pagesPharma - Reproductive SystemJordann de GuzmanPas encore d'évaluation
- LactogenesisDocument4 pagesLactogenesisMaría Luz IglesiasPas encore d'évaluation
- GH MyomaDocument19 pagesGH MyomaEko RohartoPas encore d'évaluation
- Physiology of Labor AND Lactation: Ermin Rachmawati, MDDocument37 pagesPhysiology of Labor AND Lactation: Ermin Rachmawati, MDAulia OlvianaPas encore d'évaluation
- Embryology Data For AssignmentDocument33 pagesEmbryology Data For Assignmenttesfaye mekonnenPas encore d'évaluation
- Executive Summary of Hormonal PhysiologyDocument9 pagesExecutive Summary of Hormonal PhysiologyNailis Sa'adahPas encore d'évaluation
- MODULE 1-Lesson 2-3Document11 pagesMODULE 1-Lesson 2-3JOHN BRAINARD PEJOPas encore d'évaluation
- Hormonal Regulation of Implantation: Pinar H. Kodaman, MD, PHD, Hugh S. Taylor, MDDocument22 pagesHormonal Regulation of Implantation: Pinar H. Kodaman, MD, PHD, Hugh S. Taylor, MDnurul hidayahPas encore d'évaluation
- Uji "Toksisitas Perkembangan" Siprofloksasin Dan Studi Histologi Terhadap Mencit PutihDocument19 pagesUji "Toksisitas Perkembangan" Siprofloksasin Dan Studi Histologi Terhadap Mencit PutihwayanwiriawanPas encore d'évaluation
- Endocrinology of Ectopic PregnancyDocument16 pagesEndocrinology of Ectopic PregnancyJulian Andres CastroPas encore d'évaluation
- Ajpendo 00495 2015Document18 pagesAjpendo 00495 2015siskaPas encore d'évaluation
- Anatomy and Physiology GCSDocument7 pagesAnatomy and Physiology GCSSherry Ann FayePas encore d'évaluation
- FSHR and LHR in Granulosa CellsDocument13 pagesFSHR and LHR in Granulosa CellsBhupathi SukeshPas encore d'évaluation
- Review Article: The Role of Estrogen in The Pathophysiology of Tubal Ectopic PregnancyDocument10 pagesReview Article: The Role of Estrogen in The Pathophysiology of Tubal Ectopic PregnancySri AgustinaPas encore d'évaluation
- Reproduction System: ASIM ABDUL MAJID (11160161000032) AFRIANI AFIDAH (1116016100054)Document37 pagesReproduction System: ASIM ABDUL MAJID (11160161000032) AFRIANI AFIDAH (1116016100054)Afriani AfidahPas encore d'évaluation
- 2015 - ZHANG Et Al - Placental Adaptations in Growth RestrictionDocument30 pages2015 - ZHANG Et Al - Placental Adaptations in Growth RestrictionSamanta MonteiroPas encore d'évaluation
- Parto Na ÉguaDocument9 pagesParto Na ÉguaGabriel BeirãoPas encore d'évaluation
- Functions of Ovaries: Learning ObjectivesDocument9 pagesFunctions of Ovaries: Learning ObjectivesUloko ChristopherPas encore d'évaluation
- 1477 7827 5 6 PDFDocument14 pages1477 7827 5 6 PDFKaleb Rudy HartawanPas encore d'évaluation
- The Impact of Age and BMI On Oocyte Maturation and Embryo DevelopmentDocument14 pagesThe Impact of Age and BMI On Oocyte Maturation and Embryo DevelopmentFarah S KadhimPas encore d'évaluation
- First Page PDFDocument1 pageFirst Page PDFAndrian SirghiPas encore d'évaluation
- Best S and Nocella, III (Eds.) - Igniting A Revolution - Voices in Defense of The Earth PDFDocument455 pagesBest S and Nocella, III (Eds.) - Igniting A Revolution - Voices in Defense of The Earth PDFRune Skjold LarsenPas encore d'évaluation
- California Academy For Lilminius (Cal) : Lesson PlanDocument4 pagesCalifornia Academy For Lilminius (Cal) : Lesson Plandarryl franciscoPas encore d'évaluation
- FORM Module IpsDocument10 pagesFORM Module IpsRizalPas encore d'évaluation
- Christine Remembered That Today Is The Birthday of Her BossDocument1 pageChristine Remembered That Today Is The Birthday of Her BossA.Pas encore d'évaluation
- Transformational Leadership in The UmcDocument17 pagesTransformational Leadership in The Umcapi-202352366Pas encore d'évaluation
- Chapter 34 Esip For FinalDocument35 pagesChapter 34 Esip For FinalJeaniel BorlingPas encore d'évaluation
- Tutorial Letter 101/0/2022: Foundations in Applied English Language Studies ENG1502 Year ModuleDocument17 pagesTutorial Letter 101/0/2022: Foundations in Applied English Language Studies ENG1502 Year ModuleFan ele100% (1)
- PC2000-8 Spec SheetDocument20 pagesPC2000-8 Spec SheetNeeraj ChauhanPas encore d'évaluation
- IRAQ Reproductive Maternal, Newborn, Child and Adolescent HealthDocument32 pagesIRAQ Reproductive Maternal, Newborn, Child and Adolescent HealthbejarhasanPas encore d'évaluation
- Bach Polonaise G Min BWV 119 A4Document1 pageBach Polonaise G Min BWV 119 A4vincenzovaiaPas encore d'évaluation
- Malnutrition Case StudyDocument3 pagesMalnutrition Case Studyapi-622273373Pas encore d'évaluation
- Menu Planning in HospitalDocument4 pagesMenu Planning in HospitalERva Soelkarnaen100% (1)
- Thermal ComfortDocument50 pagesThermal ComfortSSPas encore d'évaluation
- Ashfaque Ahmed-The SAP Materials Management Handbook-Auerbach Publications, CRC Press (2014)Document36 pagesAshfaque Ahmed-The SAP Materials Management Handbook-Auerbach Publications, CRC Press (2014)surajnayak77Pas encore d'évaluation
- Lesson 7Document6 pagesLesson 7Jeya Plays YTPas encore d'évaluation
- This Study Resource Was: MCV4U Exam ReviewDocument9 pagesThis Study Resource Was: MCV4U Exam ReviewNathan WaltonPas encore d'évaluation
- FacebookDocument2 pagesFacebookAbhijeet SingarePas encore d'évaluation
- Anansi and His Six Sons An African MythDocument3 pagesAnansi and His Six Sons An African MythShar Nur JeanPas encore d'évaluation
- Philips fwt9200Document37 pagesPhilips fwt9200Alex BrazPas encore d'évaluation
- A Software Architecture For The Control of Biomaterials MaintenanceDocument4 pagesA Software Architecture For The Control of Biomaterials MaintenanceCristian ȘtefanPas encore d'évaluation
- Ip TunnelingDocument15 pagesIp TunnelingBon Tran HongPas encore d'évaluation
- Algorithm Design: Figure 1. Architecture Diagram For Greykite Library's Main Forecasting Algorithm, SilverkiteDocument3 pagesAlgorithm Design: Figure 1. Architecture Diagram For Greykite Library's Main Forecasting Algorithm, Silverkitesiper34606Pas encore d'évaluation
- 1939 - Hammer - Terrain Corrections For Gravimeter StationsDocument11 pages1939 - Hammer - Terrain Corrections For Gravimeter Stationslinapgeo09100% (1)