Académique Documents
Professionnel Documents
Culture Documents
ME2151 Chp3
Transféré par
koiuy12Description originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
ME2151 Chp3
Transféré par
koiuy12Droits d'auteur :
Formats disponibles
CHAPTER 3
The mechanical and physical properties of a material are,
ATOMIC STRUCTURE
at the most fundamental level, determined by the
composition and structure of the material.
Composition refers to the types of elements that make up
3.1
THE ATOM
3.2
THE ELECTRONIC STRUCTURE OF ATOMS
3.3
THE PERIODIC TABLE
bonding and forces holding atoms together
3.4
PRIMARY BONDS
3.4.1 Ionic Bonding
3.4.2 Covalent Bonding
3.4.3 Metallic Bonding
as the ways in which atoms/molecules pack together (Chp 4).
3.5
the material and the proportions in which they exist.
The constituent elements will govern the nature of
(Chp. 3),
as well
3.1 THE ATOM
The atom is the smallest unit building block of all matter; it
SECONDARY BONDS
3.5.1 Van der Waals Bonding
3.5.2 Hydrogen Bonding
is the smallest quantity of an element that can take part in
a chemical reaction.
An atom may be thought of as a very small, dense nucleus
3.6
BONDING CHARACTER OF MATERIALS
3.7
BONDING FORCES AND ENERGIES
3.8
BONDING TYPE AND PROPERTIES
3.8.1 Thermal Expansion
3.8.2 Melting Point
3.8.3 Elastic Modulus
3.8.4 Hardness, Ductility and Toughness
surrounded by moving electrons (Fig. 3.1-1).
3-1
Fig. 3.1-1 Schematic
representation
of an atom.
3-2
3 . 2 T HE E LECTRONIC S TRUCTURE OF A TOMS
Each shell may be further divided into subshells s, p, d, f,
, each differing slightly in their energy levels
The electronic structure (arrangement of electrons) of
(Fig. 3.2-2 and
Table 3.2-1).
atoms dictates the nature of bonds and chemical reactions
between atoms, as well as electrical, thermal, magnetic
and optical properties of materials.
Fig. 3.2-2 Schematic
representation of the
relative energies of the
electrons for the various
shells and subshells.
Electrons are held to the nucleus by electrostatic (positivenegative) attraction, the force of which decreases with
distance from the nucleus. Electrons may be thought of as
being arranged in shells of increasing discrete energy
levels with increasing distances from the nucleus. Shells
are designated K, L, M, N, , (K being the closest to the
nucleus) (Fig. 3.2-1).
(a)
Electrons tend towards the lowest energy states (i.e.
(b)
greater stability). The electronic structure of an atom of
Fig. 3.2-1 Schematic representation of (a) the electron configuration; and
(b) the filled energy states for a sodium (Na) atom.
atomic number Z can be specified by putting one electron
in each of the Z lowest energy states.
3-3
3-4
The most stable (lowest energy) electron configuration is
that in which the electron states within the outermost shell
are completely filled; e.g., He, Ne, Ar (inert gases). Fullyfilled shells are considered closed; electrons in closed
shells do not readily enter into reactions.
Electrons occupying partially-filled outermost shells, known
as the valence electrons, are most likely to enter into
chemical reactions because their distance away from the
nucleus reduces their binding energy to the nucleus. In
addition, the closed inner shells shield the outermost
shells from the positive nuclear charge.
3.3 THE PERIODIC TABLE
Each element is identified by its atomic number, Z, which
is equal to the number of protons in the nucleus. Elements
are arranged in ascending atomic numbers (horizontal
rows) in chemically similar groups (vertical columns) in the
Periodic Table (Fig. 3.3-1).
Elements in the same column (i.e. with similar chemical
behaviour) in the Periodic Table have similar electronic
configurations in the outermost shells of their atoms.
3-5
3-6
3.4 PRIMARY BONDS
Atoms bond because there is a reduction in the overall
energy (i.e. greater stability) when the electrons amongst
the bonding atoms rearrange to achieve the stable
configuration of fully-filled outermost shells.
There are 3 types of primary bonds between atoms: ionic,
covalent and metallic.
Fig. 3.4-1 Ionic bonding in sodium chloride (NaCl).
Since electrostatic attraction is omnidirectional and long3.4.1
Ionic Bonding
ranged, ionic bonds are non-directional. The (usually)
Ionic bonding involves the transfer of electrons from the
nearly-empty outermost shell of one element to the nearly-
smaller positive ions are surrounded by as many negative
ions as possible and vice versa (Fig. 3.4-2).
full outermost shell of another element, resulting in
complete outermost shells in both (Fig. 3.4-1).
Ionic bonds form between elements located at the
horizontal extremities of the Periodic Table; e.g. between
Group I (metal) and Group VII elements (non-metal); e.g.
NaCl (Fig. 3.4-1).
Fig. 3.4-2 Ionic bonding is non-directional.
The bond arises from electrostatic (Coulombic) attraction
between the positive and negative ions (Fig. 3.4-1).
Ionic bonding is found in many ceramics; e.g. alumina
(Al2O3), titanium carbide (TiC), and zirconia (ZrO2).
3-7
3-8
3.4.2
Covalent Bonding
The bond arises from the electrostatic attraction between
the negatively-charged pair of shared electrons and the
Atoms with difficulty in gaining or losing electrons in their
two positively-charged atomic cores (Fig. 3.4-4).
outermost shells tend towards sharing of electrons in
pairs to complete their outermost shells; e.g. C, Si (Fig. 3.4-3).
Due to strong mutual repulsion, each negatively-charged
valence electron cloud assumes equilibrium positions as far
from one another as possible. Covalent bonds therefore
have a fixed directional relationship with one another
(Fig.
3.4-5).
Fig. 3.4-3 Covalent bonding in silicon.
Each pair of shared electrons orbit about both atomic
cores, mainly traversing the space between the two atomic
cores (Fig. 3.4-4).
Fig. 3.4-5 Directional covalent bonding in diamond.
Fig. 3.4-4 Electron orbital
penetrating two atomic cores.
Materials with covalent bonding include: semiconductors
(Si, Ge, GaAs), some ceramics (SiC, Si3N4), and diamond.
3-9
3-10
3.4.3
Metallic Bonding
Metallic bonding is confined to metals and near-metals
with few valence electrons in their outermost shells; e.g. in
Groups I, II and III of the Periodic Table.
Valence electrons, which are weakly-bound to the nucleus,
become easily detached and move freely around the ion
cores. These electrons are delocalized from their nucleus,
forming a randomly-moving sea of electrons surrounding
the ion cores.
The electrostatic attraction between the negativelycharged electron sea and the positive ion cores acts as a
glue that bonds the metallic structure. (Fig. 3.4-6).
Fig. 3.4-6 Schematic
illustration of metallic bonding
showing positive ion cores
immersed in a randomlymoving sea of negative
electrons.
Metallic bonds are non-directional.
3-11
3-12
3.5.2
3.5 SECONDARY BONDS
Secondary bonds are formed between molecules; no
change in the electronic structure is involved.
Hydrogen Bonding
Hydrogen bonding is a special type of van der Waals
bonding that involves the attraction between molecular
dipoles with hydrogen as a constituent.
Secondary bonds are weaker than primary bonds.
Covalent bonding between hydrogen and an element with
3.5.1
a nearly-full outermost shell concentrates the electron
Van der Waals Bonding
Van der Waals bonding involves the attraction between
atomic or molecular dipoles.
away from the hydrogen nucleus, leaving a positive charge
at the hydrogen end of molecule; this forms a permanent
dipole (Fig. 3.5-3).
A dipole arises when the centres of positive and negative
charge in an atom or molecule do not coincide.
The bond arises from electrostatic attraction between the
positive and negative ends of permanent dipoles (Fig. 3.5-3).
Due to the constant motion of
electrons, the negative charge is
sometimes
not
symmetric
with
Fig. 3.5-3 Hydrogen
bonding between water
molecules.
respect to the positive nucleus,
forming a temporary dipole.
A dipole may induce a similar dipole in an adjacent atom
or molecule. The bond arises from weak electrostatic
attraction between the positive end of one dipole to the
negative end of the other dipole (Fig. 3.5-2).
Fig. 3.5-2 Van der Waals bonding
between two temporary dipoles.
3-13
Hydrogen bonds are stronger than van der Waals bonds
between fluctuating induced dipoles because the dipoles
involved in the bonding are permanent.
Hydrogen bonds are important in polymers and biological
molecules; e.g. cellulose, DNA.
3-14
Van der Waals bonding may be found between the layers
of covalently-bonded C atoms in graphite
between
chains
of
thermoplastic
(Fig. 3.5-4),
polymers;
polyethylene (PE), polyvinyl chloride (PVC)
(Fig.
and
e.g.
3.5-5),
polystyrene, and nylon.
3.6 BONDING CHARACTER OF MATERIALS
Bonding between atoms may involve more than one type
of primary bond and can also involve secondary bonds.
The transition from pure ionic to pure covalent bonding is
gradual and many ceramics display mixed ionic/covalent
bonding.
The transition metals (e.g. Fe, Cr, Mo, W, Pt) display mixed
metallic/covalent bonding because partially-filled inner
shells result in some covalent bonding.
Fig. 3.5-4 Structure of graphite showing covalent bonding between carbon atoms in
each layer, and van der Waals bonding between the layers.
Fig. 3.5-5 Atoms within each chain of polyvinyl chloride (PVC) are covalently bonded,
while van der Waals (hydrogen) bonding exists between the chains.
3-15
Fig. 3.6-1 Tetrahedron
representing the relative
contribution of different
bond types to the four
fundamental categories of
engineering materials.
3-16
3.7 BONDING FORCES AND ENERGIES
Equilibrium spacing (bond-length), ro, occurs when
When two atoms are brought close together, an attractive
force, FA, is set up, which increases with decreasing atomic
separation (Fig. 3.7-1a).
At short separations, a repulsive force, FR, arises due to the
resistance of the closed inner shells to mutual penetration
(Fig. 3.7-1a).
FN = FA + FR = 0
Potential energy due to the interaction between the atoms,
UN, is such that:
or
FN =
dUN
dr
UN =
#"FN dr
! When r = r0, potential energy is a minimum (Fig. 3.7-1b):
dU N %'
$ dr ''
#
&
"
FN = $$
=0
r=r0
! Mechanical model of a solid: a solid may be envisaged as
an array of particles (atoms) with each particle linked to its
neighbours by springs (bonds) (Fig. 3.7-2).
Fig. 3.7-1 The variation of (a)
forces and (b) energies with
interatomic separations. C and K
in FA and FR are constants,
and m < n.
(a)
3-17
(b)
Fig. 3.7-2 Mechanical model of a solid showing (a) atoms linked by atomic bonds that
behave like springs, and (b) the variation in forces and energies with atomic separation.
3-18
The energy-separation curve is also known as the potential
By convention, the potential energy is set at zero when the
well; it represents the potential energy of an atom due to
interatomic separation is infinite (i.e. r = !), since the
the influence of its neighbouring atom. The bottom of the
atoms would have zero interaction (Fig. 3.7-4).
well (minimum of the curve) corresponds to the total
energy of the atom at absolute zero (0 Kelvin). At this
temperature, the atom has potential energy only, without
Fig. 3.7-4 By convention,
potential energy is set at
zero for r = !.
[Note that mathematically,
any kinetic energy.
Above 0 K, the atom also possesses kinetic energy, which
must be added above the potential energy curve
(Fig. 3.7-3a).
(Fig.3.7-3b),
" N
As the temperature increases, the atom may be seen as
moving up the potential well
F dr also leads to
negative potential energy.]
vibrating back and
forth the distance dictated by the width of the well at any
particular temperature.
If the temperature of the solid rises such that the total
energy becomes greater than zero (i.e. interatomic
separation approaches infinity), the material exists in the
gaseous state (Fig. 3.7-4).
However, the energy-separation curve does not provide a
basis for discussing the liquid state, since the phase change
from solid to liquid or from liquid to gas requires a supply
of energy (latent heat) that is unrelated to a rise in
(a)
temperature.
(b)
Fig. 3.7-3 (a) Potential and kinetic energy components of the total energy of an atom.
(b) The atom moves up the potential well as temperature increases.
[Note that in these diagrams, the potential energy is set at zero when r=r0;
the usual convention is to set potential energy to zero when r = !.]
3-19
3-20
3.8 BONDING TYPE AND PROPERTIES
Materials that are ionically and/or covalently bonded; e.g.
The type of bonding in a material determines several
ceramics, tend to have deep and narrow wells, and thus,
properties, some of which may be related to the shape of
good thermal stability. Metals experience slightly greater
the force-separation and energy-separation curves.
expansion. Materials containing secondary bonding, e.g.
polymers, have very large coefficients of expansion.
3.8.1
T he rm al Expansion, !
As temperature rises above 0 K, kinetic energy results in
the atom shifting up the potential well. Due to the
asymmetrical shape of the well, the mean position of the
atom changes with temperature and the atoms tend to
move apart as temperature increases (Fig. 3.8-1a).
Thermal expansion becomes an important consideration
when there are temperature changes during service.
Nonuniform dimensional changes, as well as expansion or
contraction that is constrained, would generate stresses,
and may lead to distortion or cracking. Low expansion
coefficients are generally preferred in such applications.
For a material with a deep and narrow well, the change in
interatomic distance for a given rise in temperature (i.e.
Assemblies containing two or more different materials
would require careful matching of expansion coefficients.
the thermal expansion coefficient) is smaller than that for a
material with a broader, shallower well (Fig. 3.8-1b).
3.8.2
M e l ti n g Poi n t
The melting point is a measure of bond strength, given by
the depth of the potential well. The deeper the well, the
greater the thermal energy required to reach levels where
bonding energy is low (liquid) or zero (gas).
Materials with primary bonds (ionic, covalent, metallic)
T3 > T2 > T1
(a)
generally have deep wells and high melting points, while
(b)
secondary bonding gives rise to low melting points.
Fig. 3.8-1 (a) Increase in interatomic separation with temperature.
(b) Comparison of the energy-separation curves for two materials: !A < !B.
3-21
3-22
3.8.3
Elastic Modulus
(see also Sec. 2.2.1)
The elastic modulus (Youngs modulus) is a measure of a
materials stiffness or resistance to elastic deformation. It is
related to the slope of the force-separation curve near the
equilibrium separation distance, r0; the steeper the slope,
the greater the force required to move the atoms from
their equilibrium positions, and the higher the modulus
(the stiffer the material) (Fig. 3.8-2).
Fig. 3.8-3 Mechanical model of a solid where the spring stiffness
is a reflection of the elastic modulus.
Note that atomic bonds, like springs, have breaking points
(Fig. 3.8-4).
Fig. 3.8-2 A steep
gradient indicates a
high elastic modulus.
If the atoms are pulled with a force greater than
the peak on the force-separation curve, i.e. Fmax, the atomic
bond breaks and the attractive force between the atoms
will fall to zero as they separate further and further until
they no longer have an effect on each other. (See also Sec. 3.8.2)
In the mechanical model of a solid
(Fig. 3.8-3),
a higher elastic
modulus may be thought of as having stiffer springs
linking the particles, resisting their movement.
Materials containing primary bonds (ionic, covalent,
metallic) tend to have high stiffness, while those with
secondary bonds are less rigid.
3-23
Fig. 3.8-4 Atomic bond strength is measured by the peak of the force-separation curve
when a tensile force greater than Fmax is applied, the atomic bond breaks.
3-24
3.8.4
Hardness, Ductility and Toughness
Although hardness, ductility and toughness are different
In metals, the sea of electrons shield the positive ion cores
from one another and the ion cores can slip without
regard to electrical-charge constraints
(Fig.
3.8-5b).
The
mechanical properties, they may all be related to a
delocalized nature of the electrons also allows that the ions
materials ability to deform plastically (permanently) under
to slide past one another without interrupting the inter-
an externally applied stress. The plastic deformation of a
atomic bonding. Metals are relatively ductile and tough.
material requires atoms to slip and slide past one another.
In ionic solids, a displacement of the ions might lead to like
charges moving into adjacent positions
(Fig. 3.8-5a),
causing
repulsion. In covalent solids, strong mutual repulsion
between the negatively-charged electron clouds limits
atomic movement. Ionic and covalent solids are very hard,
In conclusion, understanding the chemical composition
(type of atoms), the nature of atomic bonding and the
property trends of various classes of materials enables us to
select and design materials more efficiently.
TABLE 3.8-1
but have low ductility and toughness.
Uo = depth of energy-separation curve at equilibrium separation, ro (bonding energy)
Ey = Elastic modulus (Youngs modulus)
! = coefficient of thermal expansion
Fig. 3.8-5 (a) Slip in ionic solids lead to repulsion of like charges.
(b) Ionic cores in metals are shielded by the electron sea.
3-25
3-26
Vous aimerez peut-être aussi
- Dental Biomaterial Book (61-71)Document146 pagesDental Biomaterial Book (61-71)Abdelruhman SobhyPas encore d'évaluation
- Chemical Bonding NotesDocument6 pagesChemical Bonding NotesAyesha Awan100% (1)
- Presentation of Bonding in SolidsDocument14 pagesPresentation of Bonding in SolidsRohit BiswasPas encore d'évaluation
- Chemical Bonding and Molecular Structure PDFDocument56 pagesChemical Bonding and Molecular Structure PDFAbhishek AgrahariPas encore d'évaluation
- Atomic Structure and BondingDocument15 pagesAtomic Structure and BondingPaidamoyo MunhengaPas encore d'évaluation
- Lecture 2 - Atomic Structure and BondingDocument29 pagesLecture 2 - Atomic Structure and BondingMercylin MakamburePas encore d'évaluation
- Enlaces InteratómicosDocument40 pagesEnlaces InteratómicosNAYELI VIVEROS CRUZPas encore d'évaluation
- MLN 02Document18 pagesMLN 02Anand K. MouryaPas encore d'évaluation
- Material Bonding Character Example: Primary Interatomic Bonds Ionic BondingDocument4 pagesMaterial Bonding Character Example: Primary Interatomic Bonds Ionic BondingCsir RimlaPas encore d'évaluation
- Atomic BondingDocument80 pagesAtomic BondingChan Chan M100% (1)
- AQA Chemistry A-Level: 3.1.3: Bonding and StructuresDocument10 pagesAQA Chemistry A-Level: 3.1.3: Bonding and StructuresAshleigh NcubePas encore d'évaluation
- Structure of Atoms and Molecules: Bohr's Atomic ModelDocument4 pagesStructure of Atoms and Molecules: Bohr's Atomic ModelChristianPas encore d'évaluation
- Material Science NPTELDocument146 pagesMaterial Science NPTELOnkar RatheePas encore d'évaluation
- Periodic Table and PeriodicityDocument11 pagesPeriodic Table and Periodicityakeemoluwadamilare623Pas encore d'évaluation
- 31 - Metallurgy - For - Non-Metallurgist 10Document1 page31 - Metallurgy - For - Non-Metallurgist 10Sridhar PranatharthiharanPas encore d'évaluation
- Solid State ch-3Document17 pagesSolid State ch-3Abrish HaremPas encore d'évaluation
- Interatomic Forces: What Kind of Force Holds The Atoms Together in A Solid?Document25 pagesInteratomic Forces: What Kind of Force Holds The Atoms Together in A Solid?lianghoo94Pas encore d'évaluation
- Metallic Bonding 2Document7 pagesMetallic Bonding 2GoshikhanPas encore d'évaluation
- 1.3. BondingDocument10 pages1.3. BondingdodoPas encore d'évaluation
- S9Q2T2L2 Types of Chemical BondingDocument37 pagesS9Q2T2L2 Types of Chemical BondingMark Kevin Cagande EscletoPas encore d'évaluation
- Metallic BondingDocument3 pagesMetallic BondingBern BilazonPas encore d'évaluation
- Handbook No.1Document125 pagesHandbook No.1Dhinakar AnnaduraiPas encore d'évaluation
- 3 2 Physical PropertiesDocument4 pages3 2 Physical PropertiesNguyenHoangMinhDucPas encore d'évaluation
- Applied PhysicsDocument178 pagesApplied PhysicsYadavalli SanthoshPas encore d'évaluation
- Lec 2Document43 pagesLec 2SaadFarooqPas encore d'évaluation
- Metallic Bonding - 1 - Free Electron ModelDocument21 pagesMetallic Bonding - 1 - Free Electron Modelsherin joyPas encore d'évaluation
- Chemical BondingDocument44 pagesChemical Bondingjas_ong_man_ling1996Pas encore d'évaluation
- Lecture 2Document10 pagesLecture 2siphosakhemdunge114Pas encore d'évaluation
- Atomic Theory - Part 1Document13 pagesAtomic Theory - Part 1satish_trivediPas encore d'évaluation
- An Overview of The Properties of Elements in The Periodic Table The Key Atomic PropertiesDocument8 pagesAn Overview of The Properties of Elements in The Periodic Table The Key Atomic PropertiesMichelle MalabananPas encore d'évaluation
- Interatomic Forces: What Kind of Force Holds The Atoms Together in A Solid?Document36 pagesInteratomic Forces: What Kind of Force Holds The Atoms Together in A Solid?aditya_2013Pas encore d'évaluation
- Atomic Structure and BondingDocument26 pagesAtomic Structure and Bondingfieldsher_kdhPas encore d'évaluation
- Atomic Bonding (Metallic, Ionic, Covalent, and Van Der Waals Bonds)Document5 pagesAtomic Bonding (Metallic, Ionic, Covalent, and Van Der Waals Bonds)Aris YusepPas encore d'évaluation
- Manufacturing Processes Ch.2Document37 pagesManufacturing Processes Ch.2Hani BanatPas encore d'évaluation
- Introduction To Chemical BondingDocument20 pagesIntroduction To Chemical BondingDe AktivedPas encore d'évaluation
- Assignment 1 Atomic StructureDocument9 pagesAssignment 1 Atomic StructureAnonymousPas encore d'évaluation
- Band Theory of SolidsDocument75 pagesBand Theory of Solidsmk.manishkhatreePas encore d'évaluation
- Chemical Bonding (Engineering Chemistry Lecture)Document13 pagesChemical Bonding (Engineering Chemistry Lecture)kiana Jessica MonroePas encore d'évaluation
- CHAPTER 3-METALLIC BONDING-SbH-L5Document21 pagesCHAPTER 3-METALLIC BONDING-SbH-L5ezanaPas encore d'évaluation
- CeramicsDocument103 pagesCeramicsTamil SelvanPas encore d'évaluation
- 01-Metallic BondingDocument2 pages01-Metallic BondingNkemzi Elias NzetengenlePas encore d'évaluation
- Metallic BondDocument10 pagesMetallic BondAbhishek NayakPas encore d'évaluation
- Chemical Bonding: Lewis Dot Carbon Hydrogen OxygenDocument17 pagesChemical Bonding: Lewis Dot Carbon Hydrogen OxygenSheila Mae AramanPas encore d'évaluation
- As Topic 4 Notes - Bonding & PeriodicityDocument8 pagesAs Topic 4 Notes - Bonding & PeriodicityJoyce LimPas encore d'évaluation
- Chapter 3Document55 pagesChapter 3Imperial PlayzPas encore d'évaluation
- CeramicsDocument10 pagesCeramicsMayank GaurPas encore d'évaluation
- Chapter 2Document22 pagesChapter 2mehrunnisaqaisar111Pas encore d'évaluation
- Inorganic Chemistry: The Pattern of First Ionisation Energies Across Period 3Document43 pagesInorganic Chemistry: The Pattern of First Ionisation Energies Across Period 3Themba NyagomoPas encore d'évaluation
- F321 PeriodicityDocument3 pagesF321 PeriodicityDoc_CrocPas encore d'évaluation
- Lecture #2. Contact Between Solids. Solid Surfaces. Roughness of Surfaces. Characterization of Surface TopographyDocument10 pagesLecture #2. Contact Between Solids. Solid Surfaces. Roughness of Surfaces. Characterization of Surface TopographyAbdulla CamalPas encore d'évaluation
- Pert 13 Gaya IntermolekulerDocument138 pagesPert 13 Gaya IntermolekulerNatasyaxoPas encore d'évaluation
- 2023 Grade 11 Learner Support DocumentDocument131 pages2023 Grade 11 Learner Support DocumentanathinothandoPas encore d'évaluation
- Topic 4 Bonding 4.1to 4.5 14.1to 14.2Document156 pagesTopic 4 Bonding 4.1to 4.5 14.1to 14.2SujithPas encore d'évaluation
- Molecular Polarity: Science, Grade 12, Physical ScienceDocument51 pagesMolecular Polarity: Science, Grade 12, Physical ScienceReign MayorPas encore d'évaluation
- MSM R19 - Unit-1Document62 pagesMSM R19 - Unit-1Madheswaran DharmapuriPas encore d'évaluation
- Introduction To Chemical BondingDocument5 pagesIntroduction To Chemical BondingMini PGPas encore d'évaluation
- Chemical Bonding: Understanding The Forces that Hold Molecules Together.D'EverandChemical Bonding: Understanding The Forces that Hold Molecules Together.Pas encore d'évaluation
- Corrosion: 8.1 Electrochemical Reactions 8.2 Forms of CorrosionDocument8 pagesCorrosion: 8.1 Electrochemical Reactions 8.2 Forms of Corrosionkoiuy12Pas encore d'évaluation
- ME2151 Chp7Document15 pagesME2151 Chp7koiuy12Pas encore d'évaluation
- Mechanical Properties: Echanical RopertiesDocument14 pagesMechanical Properties: Echanical Ropertieskoiuy12Pas encore d'évaluation
- ME2101 - Design Against fatigue-2012-2x2-landscape-B+WDocument22 pagesME2101 - Design Against fatigue-2012-2x2-landscape-B+Wkoiuy12Pas encore d'évaluation
- Me2143 Me2143eDocument18 pagesMe2143 Me2143ekoiuy12Pas encore d'évaluation
- CS1010E Lecture 9: Problem Solving With One Dimensional ArraysDocument29 pagesCS1010E Lecture 9: Problem Solving With One Dimensional Arrayskoiuy12Pas encore d'évaluation
- L3 - FunctionsDocument42 pagesL3 - Functionskoiuy12Pas encore d'évaluation
- Problem: Euclidean Distance: StructuresDocument6 pagesProblem: Euclidean Distance: Structureskoiuy12Pas encore d'évaluation
- Problem Statement #1: ArraysDocument9 pagesProblem Statement #1: Arrayskoiuy12Pas encore d'évaluation
- And Henry A. Nasrallah 2008) Suggest That As Many As 83%Document2 pagesAnd Henry A. Nasrallah 2008) Suggest That As Many As 83%koiuy12Pas encore d'évaluation
- L2 - Basic C ElementsDocument46 pagesL2 - Basic C Elementskoiuy12Pas encore d'évaluation
- L1 - IntroductionDocument34 pagesL1 - Introductionkoiuy12Pas encore d'évaluation
- Serma LonDocument1 pageSerma LonIrwanzPas encore d'évaluation
- R. Meredith, U.S. Patent 2,274,631Document5 pagesR. Meredith, U.S. Patent 2,274,631Erivaldo ConstantPas encore d'évaluation
- Chap 14 PDFDocument22 pagesChap 14 PDFnelson escuderoPas encore d'évaluation
- Guia de Aditivos para Resolucao de ProblemasDocument20 pagesGuia de Aditivos para Resolucao de ProblemasFabiano DesangiacomoPas encore d'évaluation
- BecherDocument7 pagesBechervalholPas encore d'évaluation
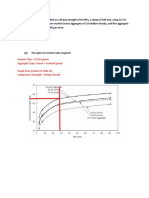
- Cement Class 52.5R (Given) Aggregate Type: Coarse Crushed (Given) Graph From (Lecture 8, Slide 22) Compressive Strength 55mpa (Found)Document11 pagesCement Class 52.5R (Given) Aggregate Type: Coarse Crushed (Given) Graph From (Lecture 8, Slide 22) Compressive Strength 55mpa (Found)Shael BridgelalPas encore d'évaluation
- Mullion Transom Connector BS-EN-1991-1-1 V2.0Document2 pagesMullion Transom Connector BS-EN-1991-1-1 V2.0Giri DharanPas encore d'évaluation
- Worksheet Ch1Document36 pagesWorksheet Ch1Shazia FarheenPas encore d'évaluation
- Moisture and Fire Resistant Gypsum Board (WRFR)Document3 pagesMoisture and Fire Resistant Gypsum Board (WRFR)burakPas encore d'évaluation
- Tianjin Soright Technology Product CatalogueDocument7 pagesTianjin Soright Technology Product Cataloguecacalot93Pas encore d'évaluation
- Double Wall Corrugated (DWC) HDPE Pipe PDFDocument3 pagesDouble Wall Corrugated (DWC) HDPE Pipe PDFSumukh Mahekar0% (1)
- Lab2 TecnoDocument38 pagesLab2 TecnoSophia Del VallePas encore d'évaluation
- Understanding Sour Service Performace of Coiled TubingDocument12 pagesUnderstanding Sour Service Performace of Coiled TubingMubeenPas encore d'évaluation
- Normal ResistivityDocument1 pageNormal ResistivityShahzad KhanPas encore d'évaluation
- 2013 Alkane Tutorial (Solutions)Document7 pages2013 Alkane Tutorial (Solutions)Pinzhen ChenPas encore d'évaluation
- Science6 - q1 - Mod1les3 - Factors Affecting Solubility - EDITEDDocument10 pagesScience6 - q1 - Mod1les3 - Factors Affecting Solubility - EDITEDGene-Beth Cacho Garce50% (2)
- Advantages of ForgingDocument20 pagesAdvantages of ForgingPramod DhaigudePas encore d'évaluation
- Chem Project Kanishka KhamkarDocument10 pagesChem Project Kanishka KhamkarKanishka P KhamkarPas encore d'évaluation
- Cal OSHA Handbook 2022Document92 pagesCal OSHA Handbook 2022Russell SadlerPas encore d'évaluation
- Jis B 2291-1994Document9 pagesJis B 2291-1994Ilka RaffaelliPas encore d'évaluation
- The Italian Art of TunnelingDocument9 pagesThe Italian Art of TunnelingGihartoPas encore d'évaluation
- C Mastic: Main ApplicationDocument1 pageC Mastic: Main ApplicationHung Mai Van100% (2)
- PCL BiomedicalDocument6 pagesPCL BiomedicalCynthia CastroPas encore d'évaluation
- Qualcast Trucks Catalog2015Document188 pagesQualcast Trucks Catalog2015Edwin Javier Garavito100% (2)
- MSDS DECON Beton InstanDocument5 pagesMSDS DECON Beton InstanMuhammad RidwanPas encore d'évaluation
- Vessel Nozzle PDFDocument30 pagesVessel Nozzle PDFEugenia LorenzaPas encore d'évaluation
- Unit-5 PTDocument136 pagesUnit-5 PTTanay NaikPas encore d'évaluation
- Jsa Jis G 3117Document15 pagesJsa Jis G 3117farhad0% (1)
- Aluminum 1100 InformationDocument1 pageAluminum 1100 InformationaxatpgmePas encore d'évaluation
- CorrosionDocument12 pagesCorrosiontabaPas encore d'évaluation