Académique Documents
Professionnel Documents
Culture Documents
15.enigmatic Variation PDF
Transféré par
Cristi Daniel NeagoeDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
15.enigmatic Variation PDF
Transféré par
Cristi Daniel NeagoeDroits d'auteur :
Formats disponibles
From www.bloodjournal.org by guest on July 29, 2015. For personal use only.
insideblood
22 DECEMBER 2011 I VOLUME 118, NUMBER 26
CLINICAL TRIALS
Comment on Gamis et al, page 6752
Enigmatic
variation
---------------------------------------------------------------------------------------------------------------Irene Roberts and Paresh Vyas
IMPERIAL COLLEGE LONDON; UNIVERSITY OF OXFORD
Variability in presentation and prognosis of transient myeloproliferative disorder
(TMD) in infants with Down syndrome (DS) has perplexed clinicians and scientists for decades and is explored in this issue of Blood by Gamis and colleagues from
the Childrens Oncology Group (COG).1
MD is a clonal myeloproliferation unique
to neonates with DS that is characterized
by increased circulating blast cells morphologically indistinguishable from those in DS
myeloid leukemia (DS-ML).2-4 Indeed, 20%
to 30% of infants with TMD subsequently
develop ML-DS; fortunately, in most cases
TMD spontaneously resolves without later
transformation.4 Although TMD is diagnosed
in 5% to 10% of neonates with DS, its exact
frequency is expected to be determined by
population-based studies currently in prog-
ress. Identification of N-terminal mutations in
the key megakaryocyte transcription factor
GATA1 in both ML-DS and TMD provided
important insight into their pathogenesis.5-8
Molecular, biologic, and clinical data indicate
that ML-DS is initiated before birth when
fetal liver hematopoietic stem and/or progenitor cells trisomic for chromosome 21 (Hsa21)
acquire GATA1 mutations.3,4,6,8,9 Cases with
paired ML-DS and TMD samples show the
same GATA1 mutation, indicating they are
clonally linked conditions.8 Thus, TMD and
Molecular, biologic, and clinical data, such as the study by Gamis et al,1 indicate that transient myeloproliferative disorder (TMD) and Down syndromeassociated acute myeloid leukemia (ML-DS) are initiated before birth
when fetal liver hematopoietic stem and/or progenitor cells trisomic for chromosome 21 acquire GATA1
mutations. TMD usually presents around birth with a spectrum of clinical features from life-threatening hepatic
fibrosis to asymptomatic leukocytosis. Although most cases of TMD spontaneously and permanently remit by
the age of 6 months, in 15% to 30% of cases additional genetic events lead to further expansion of the trisomic
GATA1-containing clone(s) resulting in ML-DS before the age of 5 years.
blood 2 2 D E C E M B E R 2 0 1 1 I V O L U M E 1 1 8 , N U M B E R 2 6
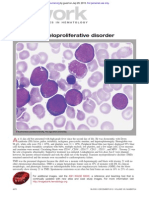
ML-DS provide a tractable model to investigate leukemia intiation and progression
(see figure). Nevertheless, many questions
remain to be answered before accumulating
molecular and clinical data can be assembled
into a model that accounts for the extraordinary variability in the natural history of these
conditions.
First, because TMD is only loosely defined
even in the World Health Organization classification,2 any DS or mosaic DS neonate could
have TMD because there are other causes of
circulating blasts. Thus, it is essential to accurately define TMD and establish useful diagnostic criteria. Second, because the clinical presentation of TMD varies from life-threatening
organ infiltration to asymptomatic leukocytosis,1,4 it is important to identify which factors
reliably predict TMD outcome and therefore
which patients are likely to benefit from treatment. Allied to this is the need to establish the
most effective treatment regimens for TMD.
While the study by Gamis and colleagues cannot answer all of these questions, it is one of
the largest prospective studies of the natural
history of clinically diagnosed TMD and provides valuable clinical information for hematologists and pediatricians.1 Strengths of the
study are the uniform, albeit broad, clinical
diagnostic criteria and treatment and monitoring guidelines. A limitation, as the authors
admit, is that the study was designed before
recognition of GATA1 mutations in TMD
which, given that TMD may be asymptomatic, prevents conclusions about the natural
history of the full spectrum of this enigmatic
disorder.
Hematologists usually encounter TMD
through consultation on abnormal hematologic findings. Importantly for diagnosis, most
neonates with TMD in the study were not
anemic and had platelet counts similar to DS
neonates without TMD.1 The principal hematologic abnormalities were leukocytosis (median
32.8 103/uL) and increased peripheral blood
blasts (median 25%). Interestingly 16% had
6723
From www.bloodjournal.org by guest on July 29, 2015. For personal use only.
mosaicism for Hsa21, confirming that phenotypically normal neonates with hematologic
findings consistent with TMD should be
screened for trisomy 21 because Gamis and colleagues study confirms others indicating they
are similarly at risk of ML-DS.1,4,10 More than
75% of infants underwent bone marrow examination. Because it is unclear what additional information was obtained, it could be argued that
marrow examination is unnecessary in infants
with trisomy 21 where clinical and hematologic features are typical of TMD, particularly
where GATA1 mutations confirm a (pre)leukemic clone.7,8,10
An important dilemma in TMD management is identifying which infants will benefit
from treatment and what treatment is most
effective in the short- and long-term. Gamis
and colleagues approached the first question
using prospectively defined criteria for the
presence of one or more life-threatening
symptoms (LTS), including hepatic dysfunction, hydrops fetalis, or blast count
( 100 000/L), as sole criteria for instituting treatment at the physicians discretion.1
Almost half of those with LTS treated according to the guidelines (13/29) succumbed to
TMD or treatment complications. By contrast, TMD resolved completely without
treatment in all patients without LTS, as
found previously where similar treatment
guidelines were used.4 These data support the
conclusion that neonates without LTS
(at diagnosis or while hematologic evidence of
TMD persists) can safely be monitored without treatment because their outcome is
favorable.
Previous reports confirmed here show that
TMD patients with high-risk features defined
as organ infiltration, especially hepatic, and/or
high leukocyte count ( 100,000/uL) have a
mortality of more than 30%.1,3,4 Klusmann et
als study showed that treatment of patients
with high-risk features (which also included
ascites, prematurity, and failure of remission
of TMD), improved survival in the first
months of life. Gamis and colleagues did not
find improved survival of TMD patients with
treatment (cytarabine 3.33 mg/kg/24 hours
by continuous infusion for 5 days), perhaps
because their guidelines permitted treatment
for less severe TMD (a single LTS) and/or
because cytarabine-related toxicity was high
(96% grade 3/4 toxicity). Although the main
aim of treating high-risk TMD is improvement in short-term survival, eradicating the
6724
(pre)leukemic clone(s) and consequent reduction in risk of later ML-DS is a potential longterm benefit. Unfortunately, no studies, including that of Gamis and colleagues, have yet
demonstrated a significant impact of treatment
on the likelihood of developing ML-DS.1,4
Thus, further studies are needed to refine
treatment intervention criteria for TMD and
the most effective treatment regimen for
short-term and long-term benefit.
The findings of Gamis et al are nevertheless helpful for clinicians caring for neonates
and children with DS and go some way to answering important clinical questions. However, many other questions remain: What is
the relationship between TMD, as clinically
defined, and the presence of GATA1 mutation(s)? Does the presence of GATA1 mutation(s) in the absence of typical clinical and
hematologic features constitute TMD and
does this carry the same risk of transformation
to ML-DS? Can patients without GATA1
mutations at birth develop ML-DS, and does
this share the same characteristic time window
of presentation ( 5 years of age) and immunophenotypic (typically megakaryoblastic)
and genetic features, characteristically seen in
patients with GATA1 mutations in the neonatal period? Answers to such questions continue to fascinate all interested in Hsa21 and
its many enigmatic links to leukemia.
Conflict-of-interest disclosure: The authors
declare no competing financial interests.
REFERENCES
1. Gamis AS, Alonzo TA, Gerbing RB, et al. Natural
history of clinically diagnosed transient myeloproliferative disorder in Down syndrome neonates: a report from
the Childrens Oncology Group study A2971. Blood. 2011;
118(26):6752-6759.
2. Hasle H, Niemeyer CM, Chessells JM, et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;
17(2):277-282.
3. Massey GV, Zipursky A, Chang MN, et al. A prospective study of the natural history of transient leukemia (TL)
in neonates with Down syndrome (DS): Childrens Oncology Group (COG) study POG-9481. Blood. 2006;107(12):
4606-4613.
4. Klusmann JH, Creutzig U, Zimmermann M, et al. Treatment and prognostic impact of transient leukemia in neonates
with Down syndrome. Blood. 2008;111(6): 2991-2998.
5. Wechsler J, Greene M, McDevitt MA, et al. Acquired
mutations in GATA1 in the megakaryoblastic leukemia of
Down syndrome. Nat Genet. 2002;32(1):148-152.
6. Rainis L, Bercovich D, Strehl S, et al. Mutations in
exon 2 of GATA1 are early events in megakaryocytic malignancies associated with trisomy 21. Blood. 2003;102(3):
981-986.
7. Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A.
GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood. 2003;101
(11):4301-4304.
8. Ahmed M, Sternberg A, Hall G, et al. Natural history
of GATA1 mutations in Down syndrome. Blood. 2004;103
(7):2480-2489.
9. Tunstall-Pedoe O, Roy A, Karadimitris A, et al. Abnormalities in the myeloid compartment in Down Syndrome fetal liver precede acquisition of GATA1 mutations.
Blood. 2008;112(12):4507-4511.
10. Alford KA, Reinhardt K, Garnett C, et al. Analysis of
GATA1 mutations in Down syndrome transient myeloproliferative disorder and myeloid leukemia. Blood. 2011;118 (8):
2222-2238.
IMMUNOBIOLOGY
Comment on Romer et al, page 6772
Predicting cytokine storms: its about
density
---------------------------------------------------------------------------------------------------------------Matthew J. Frigault and Carl H. June
ABRAMSON CANCER CENTER
Romer and colleagues in this issue of Blood report that the density of T cells during
culture increases sensitivity to the CD28 cosignaling agent TGN1412, providing
an improved means of predicting cytokine release syndrome (CRS).1
RS is a common clinical event with antibody therapies such as rituximab and
anti-CD3 antibody. After the infusion of the
CD28 superagonist TGN1412 all 6 healthy
recipients unexpectedly suffered a severe
form of CRS, often referred to as a cytokine
storm.2 The massive release of proinflammatory cytokines rapidly progressed to
severe multiorgan failure requiring advanced medical resuscitation. The events of
the March 2006 first-in-human trial of
TGN1412 have since sparked a fundamental
discussion regarding the failure of preclinical models to predict toxicities and how new
biologics are assessed before first-in-human
testing (see figure).
22 DECEMBER 2011 I VOLUME 118, NUMBER 26
blood
From www.bloodjournal.org by guest on July 29, 2015. For personal use only.
2011 118: 6723-6724
doi:10.1182/blood-2011-09-376145
Enigmatic variation
Irene Roberts and Paresh Vyas
Updated information and services can be found at:
http://www.bloodjournal.org/content/118/26/6723.full.html
Articles on similar topics can be found in the following Blood collections
Information about reproducing this article in parts or in its entirety may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests
Information about ordering reprints may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#reprints
Information about subscriptions and ASH membership may be found online at:
http://www.bloodjournal.org/site/subscriptions/index.xhtml
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society
of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Copyright 2011 by The American Society of Hematology; all rights reserved.
Vous aimerez peut-être aussi
- Cancer RenalDocument1 pageCancer RenalCristi Daniel NeagoePas encore d'évaluation
- Descargar e Imprimir Este Documento: Choose A FormatDocument1 pageDescargar e Imprimir Este Documento: Choose A FormatCristi Daniel NeagoePas encore d'évaluation
- IELTS - Exam Samples - Reading2Document28 pagesIELTS - Exam Samples - Reading2Hanin BziziPas encore d'évaluation
- English Grammar - Revision For IELTS Speaking TestDocument4 pagesEnglish Grammar - Revision For IELTS Speaking TestSilviu VornicescuPas encore d'évaluation
- Lessons From The TitanicDocument27 pagesLessons From The TitanicKevin KeefePas encore d'évaluation
- Check Your Vocabulary For Ielts - Answer KeyDocument22 pagesCheck Your Vocabulary For Ielts - Answer Keythutobe100% (2)
- IELTS General Training ReadingDocument2 pagesIELTS General Training ReadingCristi Daniel NeagoePas encore d'évaluation
- Medicina AsDocument1 pageMedicina AsCristi Daniel NeagoePas encore d'évaluation
- Ielts Academic Reading Bone Stretching in ChinaDocument6 pagesIelts Academic Reading Bone Stretching in ChinaVeasna ChannPas encore d'évaluation
- IELTS Academic ReadingDocument3 pagesIELTS Academic ReadingbalajivaidhyanathaPas encore d'évaluation
- English - How To Prepare For IELTS PDFDocument7 pagesEnglish - How To Prepare For IELTS PDFmrcpianPas encore d'évaluation
- IELTS - Exam Samples - Reading2Document28 pagesIELTS - Exam Samples - Reading2Hanin BziziPas encore d'évaluation
- 21.down Syndrome and GATA1 Mutations in Transient Abnormal Myeloproliferative Disorder - Mutation Classes Correlate With Progression To Myeloid LeukemiaDocument9 pages21.down Syndrome and GATA1 Mutations in Transient Abnormal Myeloproliferative Disorder - Mutation Classes Correlate With Progression To Myeloid LeukemiaCristi Daniel NeagoePas encore d'évaluation
- CAE ReadingDocument2 pagesCAE ReadingAbidien Frenzoid'zPas encore d'évaluation
- 17.analysis of GATA1 Mutations in Down Syndrome Transient Myeloproliferative Disorder and Myeloid Leukemia PDFDocument18 pages17.analysis of GATA1 Mutations in Down Syndrome Transient Myeloproliferative Disorder and Myeloid Leukemia PDFCristi Daniel NeagoePas encore d'évaluation
- 18.acute Megakaryoblastic Leukemia Without GATA1 Mutation After Transient Myeloproliferative Disorder in An Infant Without Down Syndrome PDFDocument4 pages18.acute Megakaryoblastic Leukemia Without GATA1 Mutation After Transient Myeloproliferative Disorder in An Infant Without Down Syndrome PDFCristi Daniel NeagoePas encore d'évaluation
- 16.natural History of Transient Myeloproliferative Disorder Clinically Diagnosed in Down Syndrome Neonates - A Report From The Children's Oncology Group Study A2971 PDFDocument9 pages16.natural History of Transient Myeloproliferative Disorder Clinically Diagnosed in Down Syndrome Neonates - A Report From The Children's Oncology Group Study A2971 PDFCristi Daniel NeagoePas encore d'évaluation
- 19.myeloid Proliferation in A Newborn With Down Syndrome PDFDocument2 pages19.myeloid Proliferation in A Newborn With Down Syndrome PDFCristi Daniel NeagoePas encore d'évaluation
- 14.increased Dosage of The Chromosome 21 Ortholog Dyrk1a Promotes Megakaryoblastic Leukemia in A Murine Model of Down Syndrome PDFDocument15 pages14.increased Dosage of The Chromosome 21 Ortholog Dyrk1a Promotes Megakaryoblastic Leukemia in A Murine Model of Down Syndrome PDFCristi Daniel NeagoePas encore d'évaluation
- 13.transient Myeloproliferative Disorder in Down's SyndromeDocument3 pages13.transient Myeloproliferative Disorder in Down's SyndromeCristi Daniel NeagoePas encore d'évaluation
- 06.development of Acute Megakaryoblastic Leukemia in Down Syndrome Is Associated With Sequential Epigenetic ChangesDocument12 pages06.development of Acute Megakaryoblastic Leukemia in Down Syndrome Is Associated With Sequential Epigenetic ChangesCristi Daniel NeagoePas encore d'évaluation
- 12.the Impact of Trisomy 21 On Early Human HematopoiesisDocument2 pages12.the Impact of Trisomy 21 On Early Human HematopoiesisCristi Daniel NeagoePas encore d'évaluation
- Valencia e Senay 2Document31 pagesValencia e Senay 2Cristi Daniel NeagoePas encore d'évaluation
- 11.transient Myeloproliferative DisorderDocument2 pages11.transient Myeloproliferative DisorderCristi Daniel NeagoePas encore d'évaluation
- 04.analysis of GATA1 Mutations and Leukemogenesis in Newborns With Down SyndromeDocument9 pages04.analysis of GATA1 Mutations and Leukemogenesis in Newborns With Down SyndromeCristi Daniel NeagoePas encore d'évaluation
- 42629260Document11 pages42629260Cristi Daniel NeagoePas encore d'évaluation
- 10.naturally Occurring Oncogenic GATA1 Mutants With Internal Deletions in Transient Abnormal Myelopoiesis in Down SyndromeDocument5 pages10.naturally Occurring Oncogenic GATA1 Mutants With Internal Deletions in Transient Abnormal Myelopoiesis in Down SyndromeCristi Daniel NeagoePas encore d'évaluation
- Dokdo eDocument6 pagesDokdo eyoungbjpPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- 110 TOP SURGERY Multiple Choice Questions and Answers PDF - Medical Multiple Choice Questions PDFDocument11 pages110 TOP SURGERY Multiple Choice Questions and Answers PDF - Medical Multiple Choice Questions PDFaziz0% (1)
- Severe Gastric ImpactionDocument4 pagesSevere Gastric ImpactionNanda Ayu Cindy KashiwabaraPas encore d'évaluation
- The Privileges and Duties of Registered Medical Practitioners - PDF 27777748Document6 pagesThe Privileges and Duties of Registered Medical Practitioners - PDF 27777748innyPas encore d'évaluation
- Antipsychotic Agents: Conventional vs AtypicalDocument18 pagesAntipsychotic Agents: Conventional vs AtypicalmengakuPas encore d'évaluation
- Alarp Risk Assessment SampleDocument7 pagesAlarp Risk Assessment SampleCriticalEyePas encore d'évaluation
- Hypertension: Rojina Bhurtel Lecturer MmihsDocument36 pagesHypertension: Rojina Bhurtel Lecturer MmihsRojina Bhurtel100% (2)
- Bu SuryaniDocument68 pagesBu SuryaniMaulana SaputraPas encore d'évaluation
- Records of Patient Medical CheckupsDocument237 pagesRecords of Patient Medical CheckupsPanca WirawanPas encore d'évaluation
- CPV/CCV Ag (3 Lines) : VcheckDocument2 pagesCPV/CCV Ag (3 Lines) : VcheckFoamfab WattikaPas encore d'évaluation
- CBTDocument21 pagesCBTsavvy_as_98Pas encore d'évaluation
- MidazolamDocument18 pagesMidazolamHarnugrahanto AankPas encore d'évaluation
- Ecg InterpretationDocument3 pagesEcg Interpretationman0billi0% (1)
- Birtcher 774 ESU - User and Service Manual PDFDocument39 pagesBirtcher 774 ESU - User and Service Manual PDFLuis Fernando Garcia SPas encore d'évaluation
- FIP PharmabridgeDocument2 pagesFIP PharmabridgeInternational Pharmaceutical Students' Federation (IPSF)Pas encore d'évaluation
- The Abc of Handover: E.D. Registrar A: Areas AllocationDocument1 pageThe Abc of Handover: E.D. Registrar A: Areas AllocationDiahNiatiPas encore d'évaluation
- Safety Data Sheet: Product Name: MOBILGEAR 600 XP 320Document11 pagesSafety Data Sheet: Product Name: MOBILGEAR 600 XP 320RupeshBabu SankariyaPas encore d'évaluation
- CefepimeDocument2 pagesCefepimeMae Ann Bueno CastillonPas encore d'évaluation
- Youtsey Kristen Cover LetterDocument1 pageYoutsey Kristen Cover Letterapi-457850399Pas encore d'évaluation
- Malaysia - Kontrak - Pusat - Ubat-Ubatan - KKM - 20.03.12Document21 pagesMalaysia - Kontrak - Pusat - Ubat-Ubatan - KKM - 20.03.12Anuj Mairh0% (1)
- Prevalence of thyroid dysfunctionDocument32 pagesPrevalence of thyroid dysfunctiondalip kumarPas encore d'évaluation
- 11 16 16Document22 pages11 16 16WoodsPas encore d'évaluation
- You Can Grow Your IntelligenceDocument6 pagesYou Can Grow Your IntelligenceSoniaPas encore d'évaluation
- Hospitals Penalized for Refusing Emergency CareDocument6 pagesHospitals Penalized for Refusing Emergency CareApril Isidro100% (1)
- 05 - SPSF3 04 B2 PDFDocument20 pages05 - SPSF3 04 B2 PDFCikgu RoshailaPas encore d'évaluation
- Pharyngitis Laryngitis TonsillitisDocument10 pagesPharyngitis Laryngitis Tonsillitisapi-457923289Pas encore d'évaluation
- AutohalerDocument51 pagesAutohalerLinto JohnPas encore d'évaluation
- Packable Composite PropertiesDocument23 pagesPackable Composite PropertiesmegamarwaPas encore d'évaluation
- 11th Zoology EM Practical NotesDocument11 pages11th Zoology EM Practical NotesBhavana Gopinath100% (1)
- Create Consent Form (39 charactersDocument3 pagesCreate Consent Form (39 charactersJan Diel100% (2)
- Anderson2008 Levofloxasin A ReviewDocument31 pagesAnderson2008 Levofloxasin A ReviewFazdrah AssyuaraPas encore d'évaluation