Académique Documents
Professionnel Documents
Culture Documents
Manejo Melena 2004
Transféré par
Wilmer JimenezCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Manejo Melena 2004
Transféré par
Wilmer JimenezDroits d'auteur :
Formats disponibles
399
BEST PRACTICE
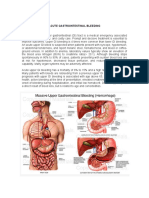
Management of haematemesis and melaena
K Palmer
...............................................................................................................................
Postgrad Med J 2004;80:399404. doi: 10.1136/pgmj.2003.017558
Acute upper gastrointestinal bleeding is a common medical
emergency which carries hospital mortality in excess of
10%. The most important causes are peptic ulcer and
varices. Varices are treated by endoscopic band ligation or
injection sclerotherapy and management of the underlying
liver disease. Ulcers with major stigmata are treated by
injection with dilute adrenaline, thrombin, or fibrin glue;
application of heat using the heater probe, multipolar
electrocoagulation, or Argon plasma coagulation; or
endoclips. Intravenous omeprazole reduces the risk of rebleeding in ulcer patients undergoing endoscopic therapy.
Repeat endoscopic therapy or operative surgery are
required if bleeding recurs.
...........................................................................
cute upper gastrointestinal haemorrhage is
responsible for about 25 000 admissions
each year to hospitals in the United
Kingdom. The incidence varies from approximately 50150 cases per 100 000 per year and is
highest in areas of social deprivation.
The mortality of patients admitted to hospital
because of acute gastrointestinal bleeding is
approximately 10%14% and has not changed
over half a century.1 In mitigation, case mix has
changed greatly over this time and patients are
now older and have greater medical disability
than was the case 50 years ago. Almost all deaths
occur in the elderly and there is a direct
relationship between the number and severity
of medical co-morbidities and mortality.
Key point
Deaths are almost entirely restricted to the
elderly and related to decompensation of
medical co-morbidity; this is particularly
relevant to the postoperative period in
patients undergoing urgent surgery.
.......................
Correspondence to:
Dr Kelvin Palmer,
Department of
Gastroenterology,
Western General Hospital,
Edinburgh EH4 2XU, UK;
kpalmer@golf5063.
freeserve.co.uk
Submitted
27 November 2003
Accepted 15 January 2004
.......................
CAUSES
Causes are listed in table 1.
The most important cause of major life
threatening acute gastrointestinal bleeding is
peptic ulcer. Significant haemorrhage is due to
erosion of an underlying artery and the magnitude of bleeding is related to the size of the
arterial defect and the diameter of the artery;
consequently bleeding from a large posterior
duodenal ulcer which may erode the gastroduodenal artery and high, lesser curve gastric ulcers
involving branches of the left gastric artery can
be particularly severe. The majority of cases
present with little or no history of dyspepsia,
while a history of aspirin or non-steroidal antiinflammatory drug (NSAID) consumption is
common.
Oesophagogastric varices are a less common
cause but because the patient often has other
features of decompensated cirrhosis and because
bleeding is often high volume the impact on
hospital resources is high. Prognosis is related to
the severity of liver disease rather than to the
magnitude of bleeding.
Mallory-Weiss tears are usually associated
with alcohol abuse but other causes of vomiting
including drugs (chemotherapy, digoxin toxicity,
etc), renal failure, or advanced malignancy may
be responsible. Bleeding usually stops spontaneously and endoscopic therapy only required in
rare severe cases.
Oesophagitis is a common finding in elderly
patients who present with coffee ground
haematemesis. Bleeding is never life threatening
and conservative supportive therapy combined
with the use of proton pump acid inhibitor drugs
is all that is necessary.
Gastritis, duodenitis, and gastroduodenal erosions are often linked to NSAID use and to
Helicobacter pylori infection. Circulatory support,
stopping NSAIDs, and H pylori eradication are
required.
A range of vascular anomalies may be responsible:
(1) Large or multiple arteriovenous malformations (AVMs) usually present with iron deficiency anaemia but occasionally cause major
acute haemorrhage. Most AVMs have no obvious
cause and present in elderly patients, but in
younger patients they are sometimes due to
hereditary haemorrhagic telangiectasia. Other
patients have valvular heart disease, or artificial
heart valves, and bleeding may be exacerbated by
anticoagulant drugs.
(2) Gastric antral vascular ectasia (GAVE) is
an uncommon vascular anomaly characterised
by linear, readily bleeding red streaks radiating
from the pyloris into the gastric antrum; it is
sometimes associated with liver disease.
(3) Portal hypertensive gastropathy is due to
venous congestion of the gastric mucosa from
portal hypertension.
Abbreviations: AVM, arteriovenous malformation;
GAVE, gastric antral vascular ectasia; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump
inhibitor
www.postgradmedj.com
400
Palmer
Table 1 Causes of acute upper gastrointestinal
bleeding
Cause
Frequency (%)
Peptic ulcer
Varices
Mallory-Weiss tear
Oesophagitis
Duodenitis/gastritis/erosions
Vascular
Tumours
Aortoduodenal fistula
3550
512
25
2030
1020
23
25
,1
(4) Dieulafoys lesion is an unusual cause of severe and
recurrent bleeding in which a superficial submucosal artery is
eroded by a small strategic ulcer. The commonest site is the
gastric fundus, although it can occur in the duodenum and
other parts of the stomach.
Oesophagogastric tumours are a relatively uncommon
cause of acute upper gastrointestinal haemorrhage. The
most important benign type is gastrointestinal stromal cell
tumour. Carcinomas and lymphomas of the stomach
tend to present with other upper gastrointestinal symptoms
and with iron deficiency anaemia rather than acute
bleeding.
Aortoduodenal fistula should be considered in patients
who present with major upper gastrointestinal bleeding
after aortic graft insertion. Bleeding occurs from the second
part of the duodenum, is massive, and may recur over hours
or days.
Small bowel or right sided colonic diseases sometimes
present as melaena and rarely as haematemesis. Colonoscopy, barium radiology, and enteroscopy may identify the
underlying tumour or vascular anomaly when upper gastrointestinal endoscopy fails to identify a bleeding source. In
young patients a bleeding Meckels diverticulum should be
considered.
Table 3 Rockall score, re-bleeding, and mortality1
% Re-bleed
% Mortality
144
281
337
444
528
455
312
267
190
5
3
5
11
14
24
33
44
42
0
0
0.2
3
5
11
17
27
41
Key points
Risk assessment is based on both the severity of haemorrhage
and the general health of the patient.
The best risk assessment tool is the Rockall score,2
developed from a large prospective audit of patients who
were managed for acute upper gastrointestinal bleeding in
England. Multivariant analysis identified age, shock, medical
co-morbidity, and specific endoscopic findings as indepen-
A formal risk assessment should always be done. It
focuses the mind and identifies high risk patients, who
should be energetically resuscitated and monitored,
and low risk patients, who can be fast tracked
towards early discharge from hospital.
Risk assessment is essential for the audit process.
The Rockall scoring system1
Variable
Score 0
Score 1
Score 2
Score 3
Age (years)
,60
6079
.80
Shock
None
Pulse .100 beats/
min; normal blood
pressure
Pulse .100 beats/min;
systolic blood pressure
,100 mm Hg
Co-morbidity
None
Cardiac; gastrointestinal
cancer; other major
co-morbidity
Renal failure; liver
failure; disseminated
malignancy
Diagnosis
Mallory-Weiss tear;
no lesion, no SRH
All other
diagnoses
Malignancy of the upper
gastrointestinal tract
Major SRH
None or dark spots
Blood in the upper
gastrointestinal tract,
adherent blood clot,
visible or spurting vessel
SRH, stigmata of recent haemorrhage.
www.postgradmedj.com
No
0
1
2
3
4
5
6
7
.8
dent variables which predicted re-bleeding and death
(tables 2 and 3). Others have confirmed that the Rockall
score accurately predicts mortality but is less good at
predicting re-bleeding.3 A particular problem is that the
Rockall score depends upon knowledge of endoscopic
findings and while a modified score based upon the
remaining observations is sometimes used in clinical practice,
this has not been validated. Blatchford et al have developed
an entirely clinically based score which predicts outcome
without the need to undertake endoscopy, but this has yet to
be validated.4
Endoscopy provides very important prognostic information. The presence of blood within the upper gastrointestinal
tract, active spurting haemorrhage, and a non-bleeding
visible vessel are signs of poor prognosis. Active ulcer
bleeding implies an 80%90% risk of continuing haemorrhage or re-bleeding,5 while the visible vessel (representing
adherent blood clot or a pseudoaneurysm over the arterial
defect) is associated with a 50% chance of re-bleeding6 during
that hospital admission. Re-bleeding is associated with a 10fold increase in hospital mortality.
RISK ASSESSMENT
Table 2
Risk score
Management of haematemesis and melaena
401
The mortality of patients who bleed during the course of an
admission for other serious disease is particularly high,
approaching 40% in published series, compared with 10%
12% in patients who are admitted to hospital because of
gastrointestinal bleeding.1
Table 4
MANAGEMENT: RESUSCITATION
The principles of airway, breathing, and circulation apply.
Patients who present with major bleeding are frequently
elderly and have significant cardiorespiratory, renal, and
cerebrovascular co-morbidity. It is crucial that this is
recognised and supported since most deaths are due to
decompensation of general medical diseases precipitated
either by the bleed itself or postoperative complications
which are much more likely when medical co-morbidity is
present.7 Central venous pressure monitoring is useful in the
elderly and in patients with cardiac disease to optimise
decisions concerning fluid replacement. Intravenous fluids
should be given through a large cannula inserted in an
anticubital vein. Crystalloids (principally normal saline) are
used to normalise blood pressure and urine output; colloids
(such as gelefusin) are often employed in the presence of
major hypotension. Saline should be used with care in
patients with liver disease.
Blood transfusion is administered to patients who are
shocked and are actively bleeding. Blood is also transfused
when the haemoglobin concentration is less than 100 g/l. The
evidence base for this transfusion threshold is rather poor,
but it is known in the intensive care setting that a
haemoglobin concentration of less than 70 g/l has significant
adverse cardiac effects and it is reasonable to pre-empt this
by employing a value of 100 g/l in bleeding patients.
Appropriate monitoring includes measurement of pulse,
blood pressure, urine output (through an indwelling catheter), and central venous pressure. Actively bleeding, shocked
patients are managed in a high dependency environment.
Key points
N
N
N
Optimum resuscitation must be done before endoscopy
is undertaken. Endoscopy is dangerous in the haemodynamically compromised or hypoxic patient.
Take great care with sedation in the critically ill
patients. Unstable patients are best managed with the
help of an anaesthetist.
Patients must be supported by trained assistants at the
time of endoscopy.
ENDOSCOPY
Endoscopy is the primary diagnostic modality and is undertaken after optimum resuscitation has been achieved.
Endoscopy has three purposes:
(1) Establishment of an accurate diagnosis.
(2) Prognostic information (table 4) which influences flow of
the patient to the high dependency unit, general ward or,
in some very low risk patients, to immediate hospital
discharge.
(3) Most importantly, endoscopic therapy is used to stop
bleeding from specific disease processes (see below).
Endoscopy is best done in the great majority of cases
within 24 hours of admission, on the first available elective
Stigmata of recent haemorrhage8
Endoscopic finding
% Re-bleeding
Clean base
Flat spots
Oozing
Adherent blood clot
Non-bleeding visible vessel
Spurting vessel
3
7
10
33
50
80
list. Out of hours emergency endoscopy is only necessary in
a small minority of cases. There is a case for endoscopy to be
done in all patients within 24 hours, irrespective of the
apparent severity of the bleed since low risk patients can be
then safely discharged from hospital at an early stage. This
author believes that selected fit patients who have obviously
only sustained minor bleeds and have normal standard blood
tests do not merit endoscopy at any stage and can be
managed without hospital admission.
Key point
Endoscopy should only be done by practitioners who
are trained to apply endoscopic haemostatic therapy.
ENDOSCOPIC THERAPY
At least 80% of patients admitted to hospital because of acute
bleeding have an excellent prognosis; bleeding stops spontaneously and circulatory supportive therapy is all that is
required.
Endoscopic therapy is indicated in the following situations:
(1) Bleeding oesophageal varices.
(2) Peptic ulcer with major stigmata of recent haemorrhage
(active spurting bleeding, non-bleeding visible vessel or
non-adherent blood clot).
(3) Vascular malformations including actively bleeding
AVMs, GAVE, and the Dieulafoy malformation.
(4) Rarely for active bleeding from a Mallory-Weiss tear.
The evidence base of endoscopic therapy for non-variceal
therapy is principally based upon clinical trials for peptic
ulcer haemorrhage.8 Three types of direct endoscopic treatments have been evaluated; each is designed to seal the
arterial defect created by the ulcer. It is necessary to remove
as much overlying blood clot as possible during endoscopy (using washing devices and snares) in order that
therapy can be accurately given. This risks further active
bleeding but this can almost always be stopped by the
endoscopist.
Key point
The further treatment of oesophageal varices is a
special subject9 which is beyond the scope of this
article.
www.postgradmedj.com
402
Palmer
Injection
Direct injection of fluids into the bleeding ulcer using
disposable needles is technically straightforward. The efficacy
of therapy has been demonstrated by several clinical trials,
although mechanism are uncertain; tamponade, vasoconstriction from adrenaline, endarteritis after sclerosant, or
alcohol injection and a direct effect upon blood clot formation
from fibrin glue or thrombin may all be relevant.
The most widely used injection fluid is 1:10 000 adrenaline. This stops active bleeding in more than 90% of cases but
15%20% of cases will re-bleed.10 The addition of sclerosants
(polidocanol, STD, or ethanolamine) or alcohol does not
reduce the risk of re-bleeding11 and risks causing life
threatening necrosis of the injected area and should not be
used. Fibrin glue (a mixture of thrombin and fibrinogen) and
human thrombin are probably the most effective injection
materials and have few complications.12 13
Heat energy
Devices are applied directly to the bleeding point to cause
coagulation and thrombosis.
The heater probe is pushed firmly on to the bleeding lesion
to apply tamponade and deliver defined pulses of heat
energy. Clinical trials have shown the device to be as effective
and as safe as injection therapy.14 Multipolar coagulation
(BICAP), in which electrical energy is conducted between
multiple probes on the tip of an endoscopically positioned
catheter15 and the argon plasma coagulator16 have comparable efficacy.
Mechanical devices
Endoclips can be applied to visible vessel and although
they may be difficult deploy on to awkwardly positioned
ulcers, they may represent the best option for major bleeding
ulcers.17 It is known that arterial defects greater than 1 mm
in diameter do not usually respond to injection or thermal
therapies, while an adequately positioned clip can stop
bleeding from relatively large arteries.18
Combinations of endoscopic therapy
Although the exact modes of action of these endoscopic
therapies are largely speculative, it is clear that each achieve
haemostasis by different mechanisms and several groups
have examined the hypothesis that combinations of endoscopic therapies are better than single modalities.
Two large studies have compared the haemostatic effect
of combination endoscopic therapy comprising the heater
probe plus injection with injection alone.19 20 Neither study
showed significant advantage for combination treatment,
although there was a trend in one of these suggesting that
the combination was more effective in the subgroup of
patients treated for extremely severe active ulcer bleeding
(table 5).19
Complications of endoscopic therapy are remarkably
infrequent. The major concerns relate to aspiration pneumonia when endoscopy is protracted. Perforation and fibrous
structuring at the endoscopically treated point can occur,
particularly if sclerosants or alcohol are injected or if thermal
energy is applied to excess.
Table 5 Adrenaline plus heater probe v adrenaline
alone for peptic ulcer bleeding8
Outcome
All patients
Primary haemostasis
Re-bleed
Transfusion
(median units)
Surgery
Death
Subgroup with spurting
haemorrhage
Primary haemostasis
Re-bleed
Transfusion
(median units)
Surgery
Death
There is little to choose between any of the endoscopic
therapiesboth in terms of efficacy and complications.
www.postgradmedj.com
Adrenaline
alone (n)
136
135
5
3
134
131
12
2
8
8
32
14
7
25
31
2
4
25
6
5
2
Not stated
8*
Not stated
*p = 0.03.
Re-bleeding after endoscopic therapy
Primary haemostasis can be achieved in the great majority of
ulcer bleeding patients using endoscopic therapy but rebleeding occurs in 15%20% of cases, usually within the first
24 hours. This develops most often when the initial bleeding
episode was severe; thus shocked patients presenting with
active, spurting haemorrhage from large posterior duodenal
ulcers are the group most likely to re-bleed.21
Management following re-bleeding is often difficult and is,
to a large extent, based upon clinical judgment and local
expertise. Discussion between endoscopist and gastrointestinal surgeons is vital. In the majority of patients it is
appropriate to repeat the endoscopy and re-treat the bleeding
lesion. One important trial from Hong Kong showed that the
mortality and blood transfusion requirements of patients
who re-bled after initially successful endoscopic treatment
was similar whether they were treated by urgent operative
surgery or by repeat endoscopic therapy (table 6).22 If
adequate haemostasis is achieved by endoscopic re-treatment, an expectant policy is reasonable but further bleeding
is an absolute indication for operative intervention.
Key point
Optimum decision making in patients who re-bleed
demands close cooperation between gastrointestinal
physicians and surgeons.
Table 6 Repeat endoscopic therapy v surgery
for patients re-bleeding after initial endoscopic
treatment9
Outcome
Key point
Adrenaline +
heater probe (n)
Transfusion
(median units)
Number developing
complications
Death (30 days)
Endoscopic
treatment
(n = 48)
Surgery
(n = 44)
17*
Management of haematemesis and melaena
403
Table 7 Omeprazole v placebo for endoscopically
treated bleeding peptic ulcers11
Outcome
Re-bleeding (%)
Surgery (%)
Transfusion;
mean (SD)
Omeprazole
(n = 120)
Placebo
(n = 120)
p Value
8 (7)
3 (3)
2.7 (2.5)
27 (23)
9 (18)
3.5 (3.8)
,0.001
0.14
0.04
Key point
Remember to treat Helicobacter pylori and consider the
need for NSAIDs at the time of hospital discharge.
SURGICAL INTERVENTION
Emergency surgery is done when endoscopic therapy
combined with pharmacological intervention fails to secure
permanent haemostasis:
DRUG THERAPY
Three groups of drugs have been used in an attempt to reduce
the risk of further bleeding in high risk patients:
(1) Acid suppressing drugs.
(2) Somatostatin and its analogue octreotide.
(3) Tranexamic acid.
Gastric acid lowering drugs
The stability of a blood clot is low in an acid environment and
powerful gastric acid suppressing drugs therefore have the
potential to optimise clot formation, thereby reducing the rebleeding risk. It is crucial that the gastric pH does not fall
below 6 and the only practical way that this can be achieved
is by constant infusion of a proton pump inhibitor (PPI).23
Only patients at high risk of re-bleeding should receive a PPI
infusion since the prognosis of the remainder, who comprise
the majority of cases, is good without their use. It follows that
patients with major stigmata of recent haemorrhage who
have undergone endoscopic therapy should be treated by
PPIs. Clinical trials have shown that an 80 mg bolus of
omeprazole followed by a 72 hour infusion of 8 mg/hour
significantly reduces the risk of re-bleeding and need for
emergency surgery. There is a trend for reduction in
mortality, which just fails to achieve statistical significance
(table 7).24
Key point
High dose intravenous proton pump inhibitor infusions
are indicated after endoscopic injection therapy has
been applied to ulcers with major stigmata. This is not
indicated in patients who lack these endoscopic
findings.
Somatostatin
This drug and its analogue octreotide are theoretically
attractive because they reduce mesenteric arterial flow and
suppress gastric acid secretion. A meta-analysis has shown
significant reduction in re-bleeding and need for emergency
surgery in somatostatin treated ulcer bleeding patients
compared with those receiving placebo infusions.25 The
quality of some of the trials is relatively weak and octreotide
appears ineffective. The efficacy of somatostatin in endoscopically treated patients has not been evaluated and the drug
is not widely used in clinical practice.
Tranexamic acid
This antifibrinolytic agent has the potential to improve the
stability of the clot and reduce the risk of re-bleeding.
Although one trial showed benefit in treated patients,26
tranexamic acid is not often used, possibly because of a fear
that its use could lead to the development of venous
thrombosis.
(1) Active bleeding which cannot be controlled by endoscopic therapy either because torrential haemorrhage
obscures the bleeding point or when active bleeding
continues despite successful application of endoscopic
therapy.
(2) Re-bleeding after initially successful endoscopic treatment. As previously discussed, it is reasonable to repeat
endoscopic therapy on one occasion after re-bleeding
providing local expertise is available and only after
discussion between endoscopist and surgeon.
The type of operation depends upon the site of the ulcer.
Bleeding duodenal ulcers are treated by under-running the
ulcer, sometimes with pyloroplasty. Gastric ulcers are treated
by partial gastrectomy of simple ulcer excision. Vagotomy is
no longer undertaken since PPIs abolish acid secretion.
SECONDARY PROPHYLAXIS
After haemostasis has been achieved it is important to
prevent late recurrent ulcer haemorrhage. H pylori eradication
virtually abolishes the risk of late re-bleeding. When a patient
needs for good reason to continue NSAID therapy the
following should be considered:
(1) Use the least toxic NSAID which controls the arthritic
symptoms.
(2) Co-prescribe a PPI with the NSAID.
(3) Consider use of a cyclo-oxygenase-2 specific antiinflammatory drug. These are associated with significantly fewer recurrent ulcer related adverse events than
occur with non-selective NSAIDs.
(4) Treatment in patients who have H pylori and need to
continue a NSAID remains controversial. Gastritis, which
is an inevitable consequence of H pylori infection, induces
prostaglandin production and this may protect the
gastroduodenal mucosa from the harmful effects of
NSAIDs. Current studies suggest, however, that the
magnitude of prostaglandin production is unlikely to
outweigh the deleterious effects of H pylori and that
eradication therapy is indicated in patients who have
developed ulcer bleeding, are H pylori positive, and
require long term NSAID therapy.
MULTIPLE CHOICE QUESTIONS (ANSWERS AT END
OF REFERENCES)
1. A 75 year old man taking warfarin following aortic valve
replacement presents with melaena. His systolic blood
pressure is 105 mm Hg, pulse 104, haemoglobin 90 g/l, and
an international normalised ratio of 9. The following is
appropriate management:
(A)
(B)
Vitamin K, fresh frozen plasma, and observe.
Emergency endoscopy with treatment if major stigmata
are present.
www.postgradmedj.com
404
(C)
(D)
(E)
Palmer
Fresh frozen plasma to optimize prothrombin time
followed by elective endoscopy but avoidance of
endoscopic therapy.
Fresh frozen plasma, vitamin K followed by therapeutic
endoscopy
Fresh frozen plasma followed by endoscopy within
24 hours. Endoscopic therapy given if major stigmata
present.
2. Patients presenting with major upper gastrointestinal
haemorrhage:
(A)
(B)
(C)
(D)
(E)
Should be routinely started on a proton pump inhibitor
drug infusion.
Are best managed in a high dependency unit.
May benefit from octreotide infusion.
Should have a nasogastric tube.
Are referred to a vascular surgeon if aortic grafting has
previously been undertaken.
3. Appropriate endoscopic therapy for peptic ulcer bleeding
may involve:
(A)
(B)
(C)
(D)
(E)
Injection of dilute adrenalineto stop acute bleeding
followed by ethanolamine or alcoholto prevent rebleeding.
Application of thermal energy using diathermy.
Vigorously removing blood clot which has adhered to
an underlying bleeding ulcer.
Routine re-endoscopy within 24 hours to ensure
haemostasis.
Application of clips.
4. Re-bleeding after initially successful endoscopic therapy
for peptic ulcer bleeding:
(A)
(B)
(C)
(D)
(E)
Is an indication for further endoscopy and it may be
reasonable to repeat endoscopic treatment.
Is more likely in the case of posterior duodenal ulcers
than those at other sites.
Is indicated by the continued presence of black stool at
day 5.
Is less likely in the presence of an intravenous proton
pump inhibitor drug infusion.
Is associated with a tenfold increased mortality.
5. Endoscopic injection of 1:1000 adrenaline is indicated:
(A)
(B)
(C)
(D)
(E)
In all patients found at endoscopy to have peptic
ulcers.
For the Dieulafoy malformation.
When an ulcer has stopped bleeding, but contains a
protuberant blood vessel.
For bleeding oesophageal varices.
For the management of gastric antral vascular ectasia.
www.postgradmedj.com
REFERENCES
1 Rockall TA, Logan RFA, Devlin HB, et al. Incidence of and mortality from acute
upper gastrointestinal haemorrhage. BMJ 1995;311:2226.
2 Rockall TA, Logan RFA, Devlin HB, et al. Risk assessment after acute upper
gastrointestinal haemorrhage. Gut 1996;38:31621.
3 Vreeburg EM, Terwee CB, Snel P, et al. Validation of the Rockall scoring
system in upper gastrointestinal bleeding. Gut 1999;44:3315.
4 Blatchford O, Murray WR, Blatchford M. A risk score to predict need for
treatment for upper gastrointestinal haemorrhage. Lancet
2000;356:131821.
5 Bornman PC, Theodorou N, Shuttleworth D, et al. Importance of hypovolaemic
shock and endoscopic signs in predicting recurrent haemorrhage from peptic
ulceration: a prospective evaluation. BMJ 1985;291:2459.
6 Griffiths WJ, Neuman DA, Welsh DA, et al. The visible vessel as an indicator
of uncontrolled or recurrent gastrointestinal haemorrhage. N Engl J Med
1979;300:141113.
7 British Society of Gastroenterology Endoscopy Section. Non-variceal upper
gastrointestinal haemorrhage guidelines. Gut 2002;51(suppl IV).
8 Church NC, Palmer KR. Acute non-variceal gastrointestinal hemorrhage:
treatment. In: McDonald J, Burroughs A, Feagan B, eds. Evidence based
gastroenterology and hepatology. London: BMJ Books, 1999:11839.
9 Jalan R, Hayes PC. UK guidelines on the management of variceal
haemorrhage in cirrhotic patients. Gut 2000;46(suppl 11).
10 Chung SCS, Leung JWC, Steele RJC. Endoscopic injection of adrenaline for
actively bleeding ulcers; a randomised trial. BMJ 1988;296:16313.
11 Choudari CP, Palmer KR. Endoscopic injection therapy for bleeding peptic
ulcer: a comparison of adrenaline alone with adrenaline plus ethanolamine
oleate. Gut 1994;35:60810.
12 Rutgerts P, Rauws E, Wara P, et al. Randomized trial of single and repeated
fibrin glue compared with injection of polidocanol in treatment of bleeding
peptic ulcer. Lancet 1997;350:6926.
13 Kubba AK, Murphy W, Palmer KR. Endoscopic injection for bleeding peptic
ulcer: a comparison of adrenaline with adrenaline plus human thrombin.
Gastroenterology 1996;111:6238.
14 Fullarton GM, Birnie GG, MacDonald A, et al. Controlled trial of heater probe
in bleeding peptic ulcers. Br J Surg 1989;76:5414.
15 Laine L. Multipolar electrocoagulation in the treatment of active upper
gastrointestinal haemorrhage. A prospective controlled trial. N Engl J Med
1987;316:161317.
16 Cipolletta L, Bianco MA, Rotondano G, et al. Prospective comparison of argon
plasma coagulator and heater probe in the endoscopic treatment of major
peptic ulcer bleeding. Gastrointest Endosc 1998;48:1915.
17 Cipolletta L, Bianco MA, Marmo R, et al. Endoclips verses heater probe in
preventing recurrent bleeding from peptic ulcer: a prospective and
randomized trial. Gastrointest Endosc 2001;53:14751.
18 Swain CP, Storey DW, Bown DG. Nature of the visible vessel in recurrently
bleeding gastric ulcers. Gastroenterology 1986;90:595606.
19 Chung SCS, Lau JY, Sung JJ. Randomized comparison between adrenaline
injection alone and adrenaline injection plus heat probe treatment for actively
bleeding peptic ulcers. BMJ 1997;314:130711.
20 Church NI, Dallal HJ, Masson J, et al. A randomized trial comparing heater
probe plus thrombin with heater probe plus placebo for bleeding peptic ulcer.
Gastroenterology 2003;125:396404.
21 Villanueva C, Balanzo J, Espinos JC. Prediction of therapeutic failure in
patients with bleeding peptic ulcers treated with endoscopic injection. Dig Dis
Sci 1993;28:206370.
22 Lau JYW, Sung JJY, Kim HS, et al. Endoscopic retreatment compared with
surgery in patients with recurrent bleeding after initial endoscopic control of
bleeding ulcers. N Engl J Med 1999;340:7516.
23 Patchett SE, Enright I, Afdal N, et al. Clot lysis by gastric juice; an in-vitro
study. Gut 1989;30:17047.
24 Lau JYW, Sung JY, Lee KK, et al. Effect of intravenous omeprazole on
recurrent bleeding after endoscopic treatment of bleeding peptic ulcers.
N Engl J Med 2000;343:31016.
25 Imperale TF, Birgisson S. Somatostatin or octreotide compares with H2
antagonists and placebo in the management of acute non-variceal upper
gastrointestinal haemorrhage: a meta-analysis. Ann Intern Med
1997;127:106271.
26 Barer D, Ogilvie A, Henry D, et al. Cimetidine and tanexamic acid in the
treatment of acute upper gastrointestinal tract bleeding. N Engl J Med
1983;308:15715.
ANSWERS
1. E; 2. B and E; 3. B, C, and E; 4. A, B, D, and E; 5. C.
Vous aimerez peut-être aussi
- Uppergastrointestinalbleeding: Marcie Feinman,, Elliott R. HautDocument11 pagesUppergastrointestinalbleeding: Marcie Feinman,, Elliott R. HautjosePas encore d'évaluation
- Mallory Weiss Syndrome - StatPearls - NCBI BookshelfDocument9 pagesMallory Weiss Syndrome - StatPearls - NCBI BookshelfDaniela EstradaPas encore d'évaluation
- Gi BleedingDocument6 pagesGi BleedingVicPas encore d'évaluation
- Upper Gastrointestinal Bleeding.Document13 pagesUpper Gastrointestinal Bleeding.Gieselle Scott100% (1)
- Overview of Mallory - Weiss SyndromeDocument3 pagesOverview of Mallory - Weiss SyndromeLeslyAgredaNavarroPas encore d'évaluation
- IM Essentials QuestionsDocument12 pagesIM Essentials QuestionsAakash Shah100% (1)
- Data Interpretation For Medical StudentDocument18 pagesData Interpretation For Medical StudentWee K WeiPas encore d'évaluation
- Gastrointestinal Haemorrhage ABC of The Upper Gastrointestinal Tract: UpperDocument4 pagesGastrointestinal Haemorrhage ABC of The Upper Gastrointestinal Tract: UpperFernando AtenciaPas encore d'évaluation
- Abdominal ImagingDocument6 pagesAbdominal Imagingmihaela2786Pas encore d'évaluation
- HP Jan05 BleedDocument5 pagesHP Jan05 BleedSyahanim IsmailPas encore d'évaluation
- Anemia Ec Perdarahan Saluran CernaDocument6 pagesAnemia Ec Perdarahan Saluran CernaSiti Nur RachmaniPas encore d'évaluation
- Portal Vein Thrombosis: ReviewDocument9 pagesPortal Vein Thrombosis: ReviewMahmoud AbouelsoudPas encore d'évaluation
- Cirhossis, Diagnosis, Management, PreventionDocument7 pagesCirhossis, Diagnosis, Management, PreventiondgumelarPas encore d'évaluation
- Lecture 3. Bleeding Disorders Part 1Document31 pagesLecture 3. Bleeding Disorders Part 1Kekelwa Mutumwenu Snr100% (1)
- Acute Gastrointestinal Bleeding Upper GI BleedingDocument18 pagesAcute Gastrointestinal Bleeding Upper GI BleedingAldwin AyuyangPas encore d'évaluation
- Surgical Management of Portal HypertensionDocument9 pagesSurgical Management of Portal HypertensionFaisal Al-alemPas encore d'évaluation
- GI Bleeding (Text)Document11 pagesGI Bleeding (Text)Hart ElettPas encore d'évaluation
- Quick Review/Pearl Sheet: These Are in Random Order To Help You Prepare For You NBME ExamDocument19 pagesQuick Review/Pearl Sheet: These Are in Random Order To Help You Prepare For You NBME ExamWyoXPat100% (11)
- Cirrhosis and Portal Hypertension in Pregnancy: Michelle A. Russell and Sabrina D. CraigoDocument10 pagesCirrhosis and Portal Hypertension in Pregnancy: Michelle A. Russell and Sabrina D. CraigoMutianaUmminyaKhanzaPas encore d'évaluation
- 2009 Pearl SheetDocument19 pages2009 Pearl Sheetmikez100% (1)
- Mallory-Weiss Tear Diagnosis and Treatment in Alcoholic MaleDocument4 pagesMallory-Weiss Tear Diagnosis and Treatment in Alcoholic MaleSherwin SyPas encore d'évaluation
- Usmle World Step 2 NotesDocument241 pagesUsmle World Step 2 NotesAdnan MallickPas encore d'évaluation
- Role of Nurse Practitioners in The Management of Cirrhotic PatientsDocument6 pagesRole of Nurse Practitioners in The Management of Cirrhotic Patientsleti komaliaPas encore d'évaluation
- Diagnosis of Gastrointestinal Bleeding in AdultsDocument8 pagesDiagnosis of Gastrointestinal Bleeding in AdultsSaeed Al-YafeiPas encore d'évaluation
- Management of End-Stage Liver DiseaseDocument34 pagesManagement of End-Stage Liver DiseaseAldo IbarraPas encore d'évaluation
- Clinical Haematology 201 256Document38 pagesClinical Haematology 201 256Ahmed El AlfyPas encore d'évaluation
- Upper GI BleedDocument8 pagesUpper GI BleedbbyesPas encore d'évaluation
- Anticoagulation in The Cirrhotic PatientDocument13 pagesAnticoagulation in The Cirrhotic PatientPeeyoosh RaiPas encore d'évaluation
- Non-Variceal Upper Gastrointestinal Haemorrhage: GuidelinesDocument6 pagesNon-Variceal Upper Gastrointestinal Haemorrhage: GuidelinesJoash JosephPas encore d'évaluation
- Case Abdominal Pain in A 49-Year-OldDocument6 pagesCase Abdominal Pain in A 49-Year-OldPutri AmeliaPas encore d'évaluation
- Portal HypertensionDocument13 pagesPortal HypertensionCiprian BoesanPas encore d'évaluation
- शल्यतन्त्र Paper II, Part BDocument79 pagesशल्यतन्त्र Paper II, Part BAnil DasPas encore d'évaluation
- Esophageal Varices: Pathophysiology, Approach, and Clinical DilemmasDocument2 pagesEsophageal Varices: Pathophysiology, Approach, and Clinical Dilemmaskaychi zPas encore d'évaluation
- Referat DR Ty 2Document8 pagesReferat DR Ty 2Muhamad Amar'sPas encore d'évaluation
- Dr. S.P. Hewawasam (MD) Consultant Gastroenterologist/Senior Lecturer in PhysiologyDocument33 pagesDr. S.P. Hewawasam (MD) Consultant Gastroenterologist/Senior Lecturer in PhysiologyAjung SatriadiPas encore d'évaluation
- Upper GI Bleeding Causes, Signs, Risk Factors & ManagementDocument46 pagesUpper GI Bleeding Causes, Signs, Risk Factors & ManagementRashed ShatnawiPas encore d'évaluation
- Diagnostic Approach To Pleural Effusion in AdultsDocument10 pagesDiagnostic Approach To Pleural Effusion in AdultsBRONKO500Pas encore d'évaluation
- All About Pleural EffusionDocument6 pagesAll About Pleural EffusionTantin KristantoPas encore d'évaluation
- Case Alcohol Abuse and Unusual Abdominal Pain in A 49-Year-OldDocument7 pagesCase Alcohol Abuse and Unusual Abdominal Pain in A 49-Year-OldPutri AmeliaPas encore d'évaluation
- Step by Step: Clinical Problem-SolvingDocument5 pagesStep by Step: Clinical Problem-SolvingMirin ApuniusPas encore d'évaluation
- Gastrointestinal Bleeding Risk Factors and ManagementDocument17 pagesGastrointestinal Bleeding Risk Factors and ManagementMaría José GalvisPas encore d'évaluation
- BMJ l536 FullDocument15 pagesBMJ l536 Fulldendy17018Pas encore d'évaluation
- Oncologic Mechanical Emergencies 2014 Emergency Medicine Clinics of North AmericaDocument14 pagesOncologic Mechanical Emergencies 2014 Emergency Medicine Clinics of North AmericamarcosjuniormutucaPas encore d'évaluation
- Pe in HospitalDocument5 pagesPe in HospitalAbdiaziz WalhadPas encore d'évaluation
- GIT Portal HypertensionDocument24 pagesGIT Portal HypertensionDr.P.NatarajanPas encore d'évaluation
- Diagnosis and Management of Upper Gastrointestinal Bleeding PDFDocument10 pagesDiagnosis and Management of Upper Gastrointestinal Bleeding PDFKetut Suwadiaya P AdnyanaPas encore d'évaluation
- Pulmonary Embolism Is A Common and Potentially Lethal ConditionDocument13 pagesPulmonary Embolism Is A Common and Potentially Lethal ConditionJasleen KaurPas encore d'évaluation
- Mrcs Upper GitDocument59 pagesMrcs Upper GitAdebisiPas encore d'évaluation
- Esophageal varicesDocument12 pagesEsophageal varicesgemergencycarePas encore d'évaluation
- Hematemesis PDFDocument7 pagesHematemesis PDFkevin_jawanPas encore d'évaluation
- Gallbladder EmpyemaDocument17 pagesGallbladder EmpyemaYayut 18Pas encore d'évaluation
- Preoptimisation of Haemoglobin: General Anaesthesia Tutorial 389Document5 pagesPreoptimisation of Haemoglobin: General Anaesthesia Tutorial 389bobbykrishPas encore d'évaluation
- Cecum Dieulafoy's Lesion Presenting With Recurrent Lower Gastrointestinal Bleeding: Case ReportDocument5 pagesCecum Dieulafoy's Lesion Presenting With Recurrent Lower Gastrointestinal Bleeding: Case ReportSabrina JonesPas encore d'évaluation
- Angio Displa SiaDocument4 pagesAngio Displa SiaBelaFawziaPas encore d'évaluation
- Management of Acute Upper Gastrointestinal BleedingDocument15 pagesManagement of Acute Upper Gastrointestinal BleedingShiva ValeskaPas encore d'évaluation
- Practical Hemostasis and ThrombosisD'EverandPractical Hemostasis and ThrombosisNigel S. KeyPas encore d'évaluation
- Epoc Gold 2004Document41 pagesEpoc Gold 2004Wilmer JimenezPas encore d'évaluation
- Emp CopdDocument20 pagesEmp CopdWilmer JimenezPas encore d'évaluation
- American Family Physician Dec 15, 2004 70, 12 Proquest Medical LibraryDocument3 pagesAmerican Family Physician Dec 15, 2004 70, 12 Proquest Medical LibraryWilmer JimenezPas encore d'évaluation
- Anaphylaxis, Urticaria, and Angioedema 2013Document13 pagesAnaphylaxis, Urticaria, and Angioedema 2013Memento MoriPas encore d'évaluation
- Emp AsthmaDocument28 pagesEmp AsthmaWilmer JimenezPas encore d'évaluation
- Neck Trauma Dont Put Your Neck On The LineDocument28 pagesNeck Trauma Dont Put Your Neck On The LineMnauel Prado MedianoPas encore d'évaluation
- Endocrine EmergenciesDocument10 pagesEndocrine EmergenciesWilmer JimenezPas encore d'évaluation
- Epidemiology of Acute Lung InjuryDocument9 pagesEpidemiology of Acute Lung InjuryWilmer JimenezPas encore d'évaluation
- Me of Acute Respiratory Failure in Intensive Care Unit PatientsEpidemiology and OutcoDocument4 pagesMe of Acute Respiratory Failure in Intensive Care Unit PatientsEpidemiology and OutcoWilmer JimenezPas encore d'évaluation
- Acutely Decompensated Heart FailureDocument28 pagesAcutely Decompensated Heart FailureSabyasachi MukhopadhyayPas encore d'évaluation
- Emp SyncopeDocument20 pagesEmp SyncopeWilmer JimenezPas encore d'évaluation
- Alveolar Recruitment in Acute Lung InjuryDocument11 pagesAlveolar Recruitment in Acute Lung InjuryWilmer JimenezPas encore d'évaluation
- Emp TiaDocument20 pagesEmp TiaWilmer JimenezPas encore d'évaluation
- Emp PharyngitisDocument24 pagesEmp PharyngitisWilmer JimenezPas encore d'évaluation
- Emp Gastrointestinal BleedingDocument20 pagesEmp Gastrointestinal BleedingWilmer JimenezPas encore d'évaluation
- Emp DiarreaDocument20 pagesEmp DiarreaWilmer JimenezPas encore d'évaluation
- Advances in The Understanding of Acute Respiratory Distress SyndromeDocument7 pagesAdvances in The Understanding of Acute Respiratory Distress SyndromeWilmer JimenezPas encore d'évaluation
- Alveolar Recruitment in Acute Lung InjuryDocument11 pagesAlveolar Recruitment in Acute Lung InjuryWilmer JimenezPas encore d'évaluation
- Cardio Guidelines PDFDocument36 pagesCardio Guidelines PDFoana_meetyPas encore d'évaluation
- Adult Psychiatric PatientDocument21 pagesAdult Psychiatric PatientWilmer JimenezPas encore d'évaluation
- Emp Chest PainDocument32 pagesEmp Chest PainWilmer JimenezPas encore d'évaluation
- Secuencia de Intubación RápidaDocument16 pagesSecuencia de Intubación Rápidaalis_maPas encore d'évaluation
- Acute Respiratory Distress SyndromeDocument7 pagesAcute Respiratory Distress SyndromeWilmer JimenezPas encore d'évaluation
- Acp - ArdsDocument12 pagesAcp - Ardsapi-3765169Pas encore d'évaluation
- Acute Lung Injury in Patients With Subarachnoid HemorrhageDocument7 pagesAcute Lung Injury in Patients With Subarachnoid HemorrhageWilmer JimenezPas encore d'évaluation
- Acute cholecystitis review: early surgery indicatedDocument5 pagesAcute cholecystitis review: early surgery indicatedElsa SimangunsongPas encore d'évaluation
- ABC of Interventional CardiologyDocument3 pagesABC of Interventional CardiologyBeny RiliantoPas encore d'évaluation
- New Guidelines For Potassium Replacement in Clinical PracticeDocument18 pagesNew Guidelines For Potassium Replacement in Clinical PracticeWilmer JimenezPas encore d'évaluation
- MigraineDocument14 pagesMigraineWilmer JimenezPas encore d'évaluation
- Advanced Technologies of CDQ Plant Advanced Technologies of CDQ PlantDocument12 pagesAdvanced Technologies of CDQ Plant Advanced Technologies of CDQ Plant조기현Pas encore d'évaluation
- BASIC IMMUNOLOGY TERMSDocument2 pagesBASIC IMMUNOLOGY TERMSAnnicoldjohn LariozaPas encore d'évaluation
- Evolis User ManualDocument28 pagesEvolis User ManualIonmadalin1000Pas encore d'évaluation
- Genius+ Ba Cu en 1113Document72 pagesGenius+ Ba Cu en 1113AlbertPas encore d'évaluation
- Grade 9 Unit 1 - Part 1: Square RootsDocument20 pagesGrade 9 Unit 1 - Part 1: Square RootsWilson ZhangPas encore d'évaluation
- Guía Fallas para Ricoh Aficio 220Document31 pagesGuía Fallas para Ricoh Aficio 220desechablePas encore d'évaluation
- 2tak Vs 4takDocument3 pages2tak Vs 4takTaufiq AlhakimPas encore d'évaluation
- 2206 - Stamina Monograph - 0 PDFDocument3 pages2206 - Stamina Monograph - 0 PDFMhuez Iz Brave'sPas encore d'évaluation
- WHO Blocks Nanosilver Shipments to Treat Ebola in AfricaDocument2 pagesWHO Blocks Nanosilver Shipments to Treat Ebola in AfricaTamas ZefferPas encore d'évaluation
- WIP CaseStudyDocument3 pagesWIP CaseStudypaul porrasPas encore d'évaluation
- Shri Durga Chalisa 1Document25 pagesShri Durga Chalisa 1gsameeriitdPas encore d'évaluation
- Xoro Hrs 8540 HD Sat ReceiverDocument33 pagesXoro Hrs 8540 HD Sat ReceiverPinoPas encore d'évaluation
- Radiesse Pálpebras Gox-8-E2633Document7 pagesRadiesse Pálpebras Gox-8-E2633Camila CrosaraPas encore d'évaluation
- Wire Rope: - Bright - 6 X 19 - Fibre CoreDocument8 pagesWire Rope: - Bright - 6 X 19 - Fibre CoreQuynh NguyenPas encore d'évaluation
- Syllabus - 2nd Paper NTCDocument2 pagesSyllabus - 2nd Paper NTCajesharyalPas encore d'évaluation
- CSSBI Tablas de Carga Perfiles PDFDocument60 pagesCSSBI Tablas de Carga Perfiles PDFRamón RocaPas encore d'évaluation
- Project Information for 2x660 MW Lube Oil PumpsDocument93 pagesProject Information for 2x660 MW Lube Oil PumpsghostamirPas encore d'évaluation
- Personal Care Na Hair GuideDocument8 pagesPersonal Care Na Hair GuideIsabellaPas encore d'évaluation
- 25f8e d64fDocument6 pages25f8e d64fapi-233604231Pas encore d'évaluation
- SEFT Islamic care effect on hypertension patients' blood pressureDocument12 pagesSEFT Islamic care effect on hypertension patients' blood pressureSopian HadiPas encore d'évaluation
- Discrete Variable Probability Distribution FunctionsDocument47 pagesDiscrete Variable Probability Distribution FunctionsJanine CayabyabPas encore d'évaluation
- Nsf-Ansi 55 PDFDocument56 pagesNsf-Ansi 55 PDFJawwad AhmedPas encore d'évaluation
- LearnEnglish Video Zone How These Women Changed Science ForeverDocument3 pagesLearnEnglish Video Zone How These Women Changed Science ForeverDaniella MensatoPas encore d'évaluation
- Boutique Olive Oil Machines Catalogue ENG5Document33 pagesBoutique Olive Oil Machines Catalogue ENG5Younesse EL BraiPas encore d'évaluation
- Basicline BL 21t9stDocument28 pagesBasicline BL 21t9stgabriel6276Pas encore d'évaluation
- Surface Roughness Measurement - MitutoyoDocument2 pagesSurface Roughness Measurement - MitutoyoSelvaraj BalasundramPas encore d'évaluation
- Unit-I EsDocument53 pagesUnit-I Eschethan.naik24Pas encore d'évaluation
- Chinkon Kishin - Origens Shintoístas Do Okiyome e Do Espiritismo Na MahikariDocument2 pagesChinkon Kishin - Origens Shintoístas Do Okiyome e Do Espiritismo Na MahikariGauthier Alex Freitas de Abreu0% (1)
- Alternator NotesDocument24 pagesAlternator Notesarunima arunimaPas encore d'évaluation
- Equations 2Document8 pagesEquations 2Patrick ValdezPas encore d'évaluation