Académique Documents
Professionnel Documents
Culture Documents
The Graduation Tower of Bad Kösen (Germany) Its History and The Formation of Thornstone
Transféré par
Anonymous pIpfPMrd3Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
The Graduation Tower of Bad Kösen (Germany) Its History and The Formation of Thornstone
Transféré par
Anonymous pIpfPMrd3Droits d'auteur :
Formats disponibles
The graduation tower of Bad Ksen (Germany)
its history and the formation of thornstone
H.-J. Engelhardt & L.E. von Borstel
DBE TECHNOLOGY GmbH, Eschenstrae 55, D-31224 Peine, Germany
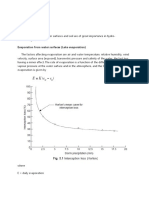
Graduation is a pre-concentration of low-saline brine up
to nearly sodium chloride saturation. The terms comes
from the graduation or marking of hydrometers according to the scale of Antoine Baum. The hydrometers
allow to determine the density of fluids. Graduation
towers made it possible to economically produce table
salt during the 18th and 19th century. The main element
of the towers are walls with blackthorn bundels. The
brine flows down on these bundels and drops are formed by the impact with the twigs and branches. The
technique guarantees high evaporation rates due to
the Kelvin effect that describes an increase of the vapor pressure (tendency to evaporate) with the surface
curvature of liquids (i.e. the fineness of drops) and
the increase of the air velocity on the surface of the
drops due to the fall of the drops.
Roofs protected the brine basins (cisterns) and the
blackthorn walls against rainfall.
Furthermore, graduation depends on the brine composition (Table 1) and the climatic conditions.
Table 1. Salt-components of the brines of Bad Ksen (Borlach shaft) and Bad Sulza (Saale-Unstrut region, Germany)
as well as of seawater in mg/l.
NaCl
KCl
MgCl2
MgSO4
CaCl2
CaSO4
Bad Ksen
41,739
1,040
3,704
Bad Ksen
42,865
1,075
3,536
Bad Sulza
103,413
534
1,532
380
5,249
Seawater
28,475
763
3,207
2,559
1,080
Figure 3. Diagram of the system NaCl-CaSO4-H2O with the
points of the brines in Bad Ksen and Bad Sulza. The arrows
mark the shift of the points during graduation.
Figure 6. Microscopic close-up view of gypsum crystals
(crossed polarisers).
According to the salt production quantity, about 50,000
tons of water evaporated annually. It was estimated that
a flat brine surface area of more than 100,000 m2 would
be necessary to reach this production rate. In comparison, surface area of the tower walls is about 7,500 m
and the water evaporation rate approx. 6,666 mm/a.
Changes of relative air humidity result in a rhythmic precipitation of carbonates and gypsum, however, at high
temperatures a crystallization of anhydrite would be possible. The precipitations are called thornstone. Changes
of the speed of water evaporation causes variations of
the growth rate of the minerals.
When the relative air humidity is lower than the water
activity of the brine, brine graduation starts. The extent
of the graduation (water evaporation) depends on
Figure 7. Incident, ultraviolet light microscopic close-up view
of gypsum crystals (parallel polarisers).
Crystal aggregates of similar visual appearance occur in
the anhydrite rocks of the Zechstein formation. The are
called thornstone aggregates.
the difference between the actual existing vapor
pressure and the saturated vapor pressure,
the wind velocity,
the air temperature,
the net solar radiation at the earth's furface, and
to a lower extent the air pressure.
Figure 4. Thornstone on a blackthorn twig. The gypsum
crystals are divergent oriented on the twigs. The picture width
is about 7.5 cm. The diameter of the thornstone at the upper
picture margin is 1.4 cm.
Figure 8. Microscopic close-up view of gypsum crystals with
incident, ultraviolet light and normal transmitted light (parallel
polarisers).
Figure 1. Relative air humidity, difference of the air pressure
from the standard atmosphere (1,013.25 hectopascals), and
wind velocity (May to July 2014).
Strontium and bromide concentrations of the brine fluctuates around 15 mg/l (Sr 2+) and 17 mg/l (Br ). The Srcontent of the carbonates and gypsum correlates with
the degree of evaporation and consequently with the
relative air humidity. The Sr-content of thornstone
samples reaches values up to about 0.2 wt.-% (2,000
g/g or ppm). Bromide remains in the solution and
would be fixed at a later stage together with chloride in
the structure of chlorides.
Figure 5. Photograph of a thin section with gypsum crystals
(parallel polarisers), which grow on a wooden plan (lower
border of the image). The first layer consists of fine-grained
gypsum and organic material. The gypsum crystals, with a
length of 6 mm at maximum, contain layers of carbonates
and foreign particles.
Figure 2. Relative air humidity and water content of the air day-night differences. August 2014.
Due to the near constant composition of the brines,
investigations make it possible to prove meteorological
and geoscientific models of water evaporation and
mineral precipitation.
Nowadays, graduation towers are centres of recreation.
Water evaporation cools the air and the microclimate
with fresh, salty aerosols is used for therapeutic inhalations. In addition, the towers are technical monuments,
tourist attractions, and event locations. During the operation of a graduation tower, drops are blown away by
the wind. This disadavantage during the period of salt
production, is now an advantage for the inhalation therapy.
Vous aimerez peut-être aussi
- WaterResoucePart 1Document97 pagesWaterResoucePart 1Toyen Blake100% (3)
- I'm A Little SnowmanDocument2 pagesI'm A Little SnowmanLucianoPas encore d'évaluation
- 1135 PDFDocument464 pages1135 PDFsomu100% (1)
- Project Report On Water ResourcesDocument20 pagesProject Report On Water Resourcessymphonic8260% (15)
- Engineering - Hydraulics Manual (M 23-03)Document315 pagesEngineering - Hydraulics Manual (M 23-03)cementacioneshoyPas encore d'évaluation
- ATPL Met Study NotesDocument49 pagesATPL Met Study NotesPhil82% (11)
- Carbonation NBB Good ArticleDocument10 pagesCarbonation NBB Good ArticleDian PagePas encore d'évaluation
- Saltmaking PDFDocument4 pagesSaltmaking PDFDennis LiaoPas encore d'évaluation
- Corrosion Behavior of API 5L X65 Carbon Steel Under Supercritical and Liquid Carbon DioxideDocument8 pagesCorrosion Behavior of API 5L X65 Carbon Steel Under Supercritical and Liquid Carbon DioxideTariqPas encore d'évaluation
- Assessment of CO2 Injectivity During Sequestration in Depleted Gas Reservoirs PDFDocument19 pagesAssessment of CO2 Injectivity During Sequestration in Depleted Gas Reservoirs PDFsaladinayubi1234Pas encore d'évaluation
- The Graduation Tower of Bad Kösen (Germany) Its History and The Formation of ThornstoneDocument21 pagesThe Graduation Tower of Bad Kösen (Germany) Its History and The Formation of ThornstoneAnonymous pIpfPMrd3Pas encore d'évaluation
- Plug 0606Document15 pagesPlug 0606Yamal E Askoul TPas encore d'évaluation
- 51 Carbon Steel-Corrosion in Fresh Waters: A. IntroductionDocument16 pages51 Carbon Steel-Corrosion in Fresh Waters: A. IntroductionMohd Nizamuddin Mohamad NoorPas encore d'évaluation
- Abstraction From PrecipitationDocument6 pagesAbstraction From PrecipitationGerald Ordoñez Delos Reyes100% (1)
- Water Adsorption Desorption On Aluminum SurfaceDocument6 pagesWater Adsorption Desorption On Aluminum SurfacemakfirsefaPas encore d'évaluation
- Worthington2021 Article FactorsAffectingTheVariationOfDocument12 pagesWorthington2021 Article FactorsAffectingTheVariationOfJoséAndrésTrejoCorzoPas encore d'évaluation
- Advances in Water Resources: C.A. Aggelopoulos, M. Robin, E. Perfetti, O. VizikaDocument7 pagesAdvances in Water Resources: C.A. Aggelopoulos, M. Robin, E. Perfetti, O. VizikaTHANH HÙNG VÕPas encore d'évaluation
- VARIATION OF MICROSTRUCTURE WITH CARBONATION in Lime and Cement Blended PastesDocument32 pagesVARIATION OF MICROSTRUCTURE WITH CARBONATION in Lime and Cement Blended PastesSaurav BhattacharjeePas encore d'évaluation
- Thomas Adam Peter GrüblDocument12 pagesThomas Adam Peter GrüblPanagiotis StamatisPas encore d'évaluation
- Presentation MartinsDocument29 pagesPresentation MartinsAlexPas encore d'évaluation
- 1 Universidad Autónoma de Nuevo León, UANL, Facultad de Ingeniería Mecánica y Eléctrica, Av. Universidad S/N, CDDocument43 pages1 Universidad Autónoma de Nuevo León, UANL, Facultad de Ingeniería Mecánica y Eléctrica, Av. Universidad S/N, CDyolima alvarezPas encore d'évaluation
- Loss On IgnitionDocument7 pagesLoss On IgnitionkktttPas encore d'évaluation
- Flow-Assurance Challenges in Gas-Storage Schemes in Depleted ReservoirsDocument3 pagesFlow-Assurance Challenges in Gas-Storage Schemes in Depleted ReservoirsbibiPas encore d'évaluation
- Asteman Influence of Water Vapor and Flow Rate On The High-Temperature OxidatioDocument16 pagesAsteman Influence of Water Vapor and Flow Rate On The High-Temperature OxidatioAbigail GuerreroPas encore d'évaluation
- Dublin-Pointing MortarDocument8 pagesDublin-Pointing MortarDionys Van GemertPas encore d'évaluation
- Document PreviewDocument3 pagesDocument PreviewbahranPas encore d'évaluation
- Hydrology 05Document7 pagesHydrology 05Mica L. SanoPas encore d'évaluation
- LTpart 2Document7 pagesLTpart 2Lin Xian XingPas encore d'évaluation
- REPORT (New Thesis)Document10 pagesREPORT (New Thesis)Marzhan ZhandildinaPas encore d'évaluation
- Freezing and Thawing Effect On ConcreteDocument7 pagesFreezing and Thawing Effect On ConcreteS PraveenkumarPas encore d'évaluation
- Properties of Drilling Fluids - Jude LichyDocument12 pagesProperties of Drilling Fluids - Jude LichyJude LichyPas encore d'évaluation
- Ogst 200053Document10 pagesOgst 200053Carmel UyumbuPas encore d'évaluation
- EvaporationDocument9 pagesEvaporationEdgar Cyrelle EscritorPas encore d'évaluation
- EvaporationDocument9 pagesEvaporationEdgar Cyrelle EscritorPas encore d'évaluation
- Khaled Zohdy, Maha Abdel Kareem and Hussein Abdel-AalDocument3 pagesKhaled Zohdy, Maha Abdel Kareem and Hussein Abdel-AalAmar BenAmarPas encore d'évaluation
- Solutions For Reducing Dissolved Hydrogen Sulphide in The Black Sea by Electrochemical OxidationDocument8 pagesSolutions For Reducing Dissolved Hydrogen Sulphide in The Black Sea by Electrochemical Oxidation10 000 de cartiPas encore d'évaluation
- EVAPORATIONDocument13 pagesEVAPORATIONGuevara, Blez M.Pas encore d'évaluation
- Two-Step Water Splitting by Cerium Oxide-Based RedDocument7 pagesTwo-Step Water Splitting by Cerium Oxide-Based RedVojtech VolozsnaiPas encore d'évaluation
- Probset 3 (Mass)Document2 pagesProbset 3 (Mass)Ralph EvidentePas encore d'évaluation
- Gas Fron Green SandDocument13 pagesGas Fron Green Sandjose.figueroa@foseco.comPas encore d'évaluation
- Olds Et Al 2014 Minimisation of Acid Mine Drainage Generation in Waste Rock DumpsDocument10 pagesOlds Et Al 2014 Minimisation of Acid Mine Drainage Generation in Waste Rock DumpsDaniel PrimantoPas encore d'évaluation
- Geologic Storage of Greenhouse Gases: Multiphase and Non-Isothermal Effects, and Implications For Leakage BehaviorDocument5 pagesGeologic Storage of Greenhouse Gases: Multiphase and Non-Isothermal Effects, and Implications For Leakage BehaviordeepmetalPas encore d'évaluation
- Pages From GS 06 50701 Multiphase - Pipelines - Pipe RoughnessDocument1 pagePages From GS 06 50701 Multiphase - Pipelines - Pipe RoughnessRama SPas encore d'évaluation
- WRE Assignment 4 Soln QTN 1 3 13 OCIDocument10 pagesWRE Assignment 4 Soln QTN 1 3 13 OCIOCARE IVANPas encore d'évaluation
- Spe 193123-MSDocument18 pagesSpe 193123-MSbayuPas encore d'évaluation
- Sea Level RiseDocument18 pagesSea Level RiseomaransPas encore d'évaluation
- OTC 14031 Dynamic Hydrate Changes With TimeDocument15 pagesOTC 14031 Dynamic Hydrate Changes With Timethanhvinh710Pas encore d'évaluation
- EvaporationDocument8 pagesEvaporationEdgar Cyrelle EscritorPas encore d'évaluation
- Deep BlowDocument9 pagesDeep BlowJanimatorPas encore d'évaluation
- EvaporationDocument115 pagesEvaporationGuevara, Blez M.Pas encore d'évaluation
- The Effect of Water-Vapor Content and Gas Flow RateDocument22 pagesThe Effect of Water-Vapor Content and Gas Flow RateFrancois BornmanPas encore d'évaluation
- Changes in Stratospheric OzoneDocument2 pagesChanges in Stratospheric OzoneANMOLPas encore d'évaluation
- Carbonation of Flyash ConcretDocument6 pagesCarbonation of Flyash ConcretAnshul SoniPas encore d'évaluation
- Farelas - Effect of CO2 Phase - H2O and SO2Document24 pagesFarelas - Effect of CO2 Phase - H2O and SO2Andy ChoPas encore d'évaluation
- Fluid Flow and Bubble Behaviour in The Aluminium Electrolysis CellDocument6 pagesFluid Flow and Bubble Behaviour in The Aluminium Electrolysis Cellfahrgeruste3961Pas encore d'évaluation
- Advances in SteelmakingDocument190 pagesAdvances in Steelmakingrajkumar baskeyPas encore d'évaluation
- TP48 Materials For Pumping Seawater and Media With High Chloride Content G.Pini and J.Weber Sulzer Technical Review PDFDocument10 pagesTP48 Materials For Pumping Seawater and Media With High Chloride Content G.Pini and J.Weber Sulzer Technical Review PDFOmar GRPas encore d'évaluation
- Lime Water ConsolidationDocument2 pagesLime Water ConsolidationMyra Elisse M. Anit100% (1)
- Wendy R. Panero, Laura Robin Benedetti and Raymond Jeanloz - Transport of Water Into The Lower Mantle: Role of StishoviteDocument9 pagesWendy R. Panero, Laura Robin Benedetti and Raymond Jeanloz - Transport of Water Into The Lower Mantle: Role of StishoviteDrebuioPas encore d'évaluation
- Analysis of Development of Carbonation and Surface Wear of The Concrete: A Case Study in Ship Lock 1 of The Transposition System of Tucuruı DamDocument8 pagesAnalysis of Development of Carbonation and Surface Wear of The Concrete: A Case Study in Ship Lock 1 of The Transposition System of Tucuruı DamCarlos Augusto Sánchez RondónPas encore d'évaluation
- Potential For Real-Time Monitoring and Control of Dissolved Oxygen in The Injection Water Treatment ProcessDocument8 pagesPotential For Real-Time Monitoring and Control of Dissolved Oxygen in The Injection Water Treatment ProcessMinhquang NgoPas encore d'évaluation
- Solution - CVEN 3304 Workshop 2 - 2016Document8 pagesSolution - CVEN 3304 Workshop 2 - 2016StephClHPas encore d'évaluation
- Sweet Corrosion in PipelineDocument8 pagesSweet Corrosion in PipelinedgkmurtiPas encore d'évaluation
- Unit-3 DMDocument55 pagesUnit-3 DMManasa DasariPas encore d'évaluation
- Hydrogeochemistry Fundamentals and Advances, Environmental Analysis of GroundwaterD'EverandHydrogeochemistry Fundamentals and Advances, Environmental Analysis of GroundwaterPas encore d'évaluation
- BGE TECHNOLOGY GMBH News 2 2019 - Sealing and Injection Measures at The Asse II MineDocument2 pagesBGE TECHNOLOGY GMBH News 2 2019 - Sealing and Injection Measures at The Asse II MineAnonymous pIpfPMrd3Pas encore d'évaluation
- Investigation of Construction Materials - Overview of Consistency Test MethodsDocument1 pageInvestigation of Construction Materials - Overview of Consistency Test MethodsAnonymous pIpfPMrd3Pas encore d'évaluation
- Engelhardt 39th Cement Concrete Science Conference Bath - Properties of Magnesiabinder SystemsDocument23 pagesEngelhardt 39th Cement Concrete Science Conference Bath - Properties of Magnesiabinder SystemsAnonymous pIpfPMrd3Pas encore d'évaluation
- Camera Inspections of Boreholes - Borehole Video InspectionsDocument2 pagesCamera Inspections of Boreholes - Borehole Video InspectionsAnonymous pIpfPMrd3Pas encore d'évaluation
- 2019-02-14b RWM Workshop 1 Backfill Integrated Project Konrad BackupDocument78 pages2019-02-14b RWM Workshop 1 Backfill Integrated Project Konrad BackupAnonymous pIpfPMrd3Pas encore d'évaluation
- Bge Technology News 1-2019Document4 pagesBge Technology News 1-2019Anonymous pIpfPMrd3Pas encore d'évaluation
- Bge Technology News 1 2019Document3 pagesBge Technology News 1 2019Anonymous pIpfPMrd3Pas encore d'évaluation
- Study On The Availability of Cement Suitable For Disposal PurposesDocument3 pagesStudy On The Availability of Cement Suitable For Disposal PurposesAnonymous pIpfPMrd3Pas encore d'évaluation
- Logistic Simulation of Underground Operation of Future Belgian Geological Repository IHLRW 2017 Conference Charlotte North CarolineDocument23 pagesLogistic Simulation of Underground Operation of Future Belgian Geological Repository IHLRW 2017 Conference Charlotte North CarolineAnonymous pIpfPMrd3Pas encore d'évaluation
- 2019-02-14 RWM Workshop 1 Backfill Integrated Project KonradDocument61 pages2019-02-14 RWM Workshop 1 Backfill Integrated Project KonradAnonymous pIpfPMrd3Pas encore d'évaluation
- Reference Backfill Material For The Disposal Galleries in The Current Belgian Reference ConceptDocument4 pagesReference Backfill Material For The Disposal Galleries in The Current Belgian Reference ConceptAnonymous pIpfPMrd3Pas encore d'évaluation
- Reference Backfill Material For The Disposal Galleries in The Current Belgian Reference ConceptDocument4 pagesReference Backfill Material For The Disposal Galleries in The Current Belgian Reference ConceptAnonymous pIpfPMrd3Pas encore d'évaluation
- 2019-02-14 RWM Workshop 1 Backfill Integrated Project KonradDocument61 pages2019-02-14 RWM Workshop 1 Backfill Integrated Project KonradAnonymous pIpfPMrd3Pas encore d'évaluation
- Study On The Availability of Cement Suitable For Disposal PurposesDocument3 pagesStudy On The Availability of Cement Suitable For Disposal PurposesAnonymous pIpfPMrd3Pas encore d'évaluation
- Feasibility Studies For An HLW Repository in Belgian Clay FormationsDocument4 pagesFeasibility Studies For An HLW Repository in Belgian Clay FormationsAnonymous pIpfPMrd3Pas encore d'évaluation
- BGE Technology News 2 2008Document3 pagesBGE Technology News 2 2008Anonymous pIpfPMrd3Pas encore d'évaluation
- BGE Technology News 2 2008Document3 pagesBGE Technology News 2 2008Anonymous pIpfPMrd3Pas encore d'évaluation
- (2018!05!09) A Briug China Backfill Material Transport PlacementDocument38 pages(2018!05!09) A Briug China Backfill Material Transport PlacementAnonymous pIpfPMrd3Pas encore d'évaluation
- Backfilling of Geological Disposal Facilities - Development of Optimized Backfill Material - WM2018 Conference, Phoenix, ArizonaDocument17 pagesBackfilling of Geological Disposal Facilities - Development of Optimized Backfill Material - WM2018 Conference, Phoenix, ArizonaAnonymous pIpfPMrd3Pas encore d'évaluation
- DBE TECHNOLOGY GMBH, Calender 2016, Kalender 2016, JahreskalenderDocument14 pagesDBE TECHNOLOGY GMBH, Calender 2016, Kalender 2016, JahreskalenderAnonymous pIpfPMrd3Pas encore d'évaluation
- DBE TECHNOLOGY GMBH, Calender 2016, Kalender 2016, JahreskalenderDocument14 pagesDBE TECHNOLOGY GMBH, Calender 2016, Kalender 2016, JahreskalenderAnonymous pIpfPMrd3Pas encore d'évaluation
- Injection of Sodium Silicate Solutions Into Rock Salt - Long-Term BehaviourDocument1 pageInjection of Sodium Silicate Solutions Into Rock Salt - Long-Term BehaviourAnonymous pIpfPMrd3100% (1)
- Investigation of The Lanthanide Content of Talc - and Koenenite-Containing Samples of The German Zechstein BasinDocument1 pageInvestigation of The Lanthanide Content of Talc - and Koenenite-Containing Samples of The German Zechstein BasinAnonymous pIpfPMrd3Pas encore d'évaluation
- (PARGAD) Weather and Climatic Elements of Philippine ArchipelagoDocument28 pages(PARGAD) Weather and Climatic Elements of Philippine ArchipelagoTrisha Cristi Ramos AlmoniaPas encore d'évaluation
- PRECIPITATIONDocument8 pagesPRECIPITATIONAljon LanguingPas encore d'évaluation
- Semi Detailed Lesson Plan Factors Affecting Climate ChangeDocument5 pagesSemi Detailed Lesson Plan Factors Affecting Climate ChangeLeah Beth CañedoPas encore d'évaluation
- RIVER Action Plan SubarnrekhaDocument56 pagesRIVER Action Plan Subarnrekha9450969009Pas encore d'évaluation
- 1 s2.0 S0048969718337732 MainDocument15 pages1 s2.0 S0048969718337732 Mainngan ping pingPas encore d'évaluation
- CE3141 Module 2021-22Document85 pagesCE3141 Module 2021-22Asus LaptopPas encore d'évaluation
- Final End 2-Mathayom 1 ScienceDocument8 pagesFinal End 2-Mathayom 1 ScienceAbdullohPas encore d'évaluation
- Dixit & Tandon - Hydroclimatic Variability On The Indian Subcontinent in The Past Millennium: Review and AssessmentDocument15 pagesDixit & Tandon - Hydroclimatic Variability On The Indian Subcontinent in The Past Millennium: Review and AssessmentEric M GurevitchPas encore d'évaluation
- Lec 6 Analysis of PrecipitationDocument43 pagesLec 6 Analysis of PrecipitationumairPas encore d'évaluation
- Riparian HouseDocument30 pagesRiparian HouseManas MalpekarPas encore d'évaluation
- English Class: 1. See The Vocabulary On Page 36, Search For The Meaning and Make Sentences With ThemDocument3 pagesEnglish Class: 1. See The Vocabulary On Page 36, Search For The Meaning and Make Sentences With ThemCarolyn BáezPas encore d'évaluation
- UMB Meteorological SensorsDocument26 pagesUMB Meteorological SensorsManish NarkhedePas encore d'évaluation
- Worksheet 2.3: Biomes: ActivityDocument4 pagesWorksheet 2.3: Biomes: Activityapi-305791685Pas encore d'évaluation
- A. Soal Pilihan GandaDocument10 pagesA. Soal Pilihan GandaMAS Al HIdayah WarungkondangPas encore d'évaluation
- Ps-1 Unit 3Document21 pagesPs-1 Unit 3Prathap VuyyuruPas encore d'évaluation
- WEATHER VocabularyDocument3 pagesWEATHER VocabularyMari CarmenPas encore d'évaluation
- Geog 150 Chap 6 SummaryDocument20 pagesGeog 150 Chap 6 SummaryScion McKinleyPas encore d'évaluation
- Water 12 01906 v2Document19 pagesWater 12 01906 v2DRACO SpadilePas encore d'évaluation
- PTE Speaking Practice Test 3Document8 pagesPTE Speaking Practice Test 3siddhantspearheadPas encore d'évaluation
- The Role of Clouds in The Climate SystemDocument27 pagesThe Role of Clouds in The Climate Systemyzz36520Pas encore d'évaluation
- Climatic Regions of IndiaDocument15 pagesClimatic Regions of IndiaAmeyavikram MahalingashettyPas encore d'évaluation
- Weather Systems in The CaribbeanDocument5 pagesWeather Systems in The CaribbeanAnthonyPas encore d'évaluation
- Jama Masjid BadaunDocument27 pagesJama Masjid BadaunAman KhanPas encore d'évaluation
- Dike Calculation PDFDocument4 pagesDike Calculation PDFarini_aristia_sPas encore d'évaluation