Académique Documents
Professionnel Documents
Culture Documents
LGD10703 Engineering Science
Transféré par
Hafizuddin RazakCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
LGD10703 Engineering Science
Transféré par
Hafizuddin RazakDroits d'auteur :
Formats disponibles
JANUARY 2013
CONFIDENTIAL
SECTION A (Total: 60 marks)
INSTRUCTION: Answer ALL questions.
Please use the answer booklet provided.
Question 1
(a)
Equation p + a v2 = b is dimensionally correct. Unknown p and v represent pressure
and velocity respectively. Derive the dimensional analysis and determine S.I unit of
a.
(6 marks)
(b)
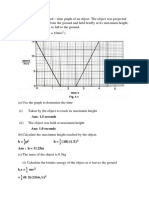
The velocity time graph is shown below in Figure 1 for an object travelling from one
point to another point within 8 seconds. Determine the accelerations at point A, B
and C. Then estimate the distance covered in 8 seconds.
Figure 1: Velocity Time Graph
(14 marks)
LGD10703 ENGINEERING SCIENCE
JANUARY 2013
CONFIDENTIAL
Question 2
(a)
State the theory of Newtons Law and formulae with an example each of them;
i.
ii.
Newtons First Law
Newtons Second Law
(6 marks)
(b)
Figure 2: Cable with pulley
A 7.5 kg object is placed on a frictionless horizontal table which is connected to a
cable that passes over a pulley and then is fastened to a hanging 12.5 kg object, as
in Figure 2.
i.
ii.
Draw free-body diagrams of both objects.
Analyse the acceleration of the two objects and the tension in the string.
(14 marks)
LGD10703 ENGINEERING SCIENCE
JANUARY 2013
CONFIDENTIAL
Question 3
(a)
Potential energy and kinetic energy are two forms of energy classified under the
Mechanical energy. Explain briefly their definitions with the aid of relevant formulae.
(6 marks)
(b)
A particle of mass m = 0.5 kg is released from point A and slides on the frictionless
track shown in Figure 3. Evaluate;
i.
the particle's speed at points B and C.
ii. the net work done by the gravitational force in moving the particle from A to C.
Figure 3: Frictionless track
(14 marks)
LGD10703 ENGINEERING SCIENCE
JANUARY 2013
CONFIDENTIAL
SECTION B (Total: 40 marks)
INSTRUCTION: Answer only TWO questions.
Please use the answer booklet provided.
Question 4
(a)
State two Pascals principle.
(4 marks)
(b)
A hydraulic lift is used to jack a 970-kg car 120 mm off the floor. The diameter of the
output piston is 180 mm, and the input force is 500 N shown in Figure 4. Determine;
i.
Area of the input piston.
ii. Work done in lifting the car 120 mm.
iii. If the input piston moves 150 mm in each stroke, determine the height the car move up for
each stroke?
Figure 4: Hydraulic Jack
(12 marks)
(c)
Draw a simple barometer and determine its standard Pressure in atmosphere.
[Hint: mercury=13.6 x 103 kg/m3 , g = 9.81ms-2].
(4 marks)
LGD10703 ENGINEERING SCIENCE
JANUARY 2013
CONFIDENTIAL
Question 5
(a)
Explain with sketch the relationship between pressure (P), volume (V) and
temperature (T) of an ideal gas law;
i. Boyles Law.
ii. Charless Law.
iii. Gay-Lussacs Law.
(6 marks)
(b)
A vertical cylinder of cross-sectional area A is fitted with a tight-fitting, frictionless
piston of mass m shown in Figure 5;
i.
If n moles of an ideal gas are in the cylinder at a temperature of T, determine the
height h at which the piston is in equilibrium under its own weight?
ii. Determine the value for h if n = 0.200 mol, T = 400 K, A = 0.008 00 m2, and
m = 20.0 kg.
(8 marks)
Figure 5: Frictionless Piston
(c)
If 18.75 mol of helium gas is at 10.0C and a gauge pressure of 0.350 atm. Analyse;
i.
ii.
the volume of the helium gas under these conditions.
the temperature if the gas is compressed to precisely half the volume at a gauge
pressure of 1.00 atm.[Hint: R=8.314J/(mol.K), P=1.013 x 105 N/m2]
(6 marks)
Question 6
(a)
With the aid of neat sketch, explain briefly the first law of thermodynamics.
(6 marks)
LGD10703 ENGINEERING SCIENCE
JANUARY 2013
(b)
CONFIDENTIAL
Consider the following two-step process. Heat is allowed to flow out of an ideal gas at
constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas
expands at constant pressure, from a volume of 6.8 L to 9.3 L, where the
temperature reaches its original value shown in Figure 6. Analyse;
Figure 6: Two-step process
i.
ii.
iii.
the total work done by the gas in the process.
the change in internal energy of the gas in the process.
the total heat flow into or out of the gas.
(12 marks)
(c)
List two applications of thermodynamics.
(2 mark)
END OF QUESTION
Formulae
LGD10703 ENGINEERING SCIENCE
JANUARY 2013
CONFIDENTIAL
i )v u at
1
ii ) s ut at 2
2
2
2
iii )v u 2as
1 2
mv
2
v) P.E mgh
iv ) K .E
vi)Components :
Parallel mg sin
Perpendicu lar mg cos
vii) Ideal gas Law
PV nRT
viii )Trigonomet ry
Z
a ) sin y / z
b) cos x / z
c) tan y / x
X
Constant
i )Gravitional , g 9.81ms2
ii )Gas, R 8.314 J / mol.K
iii ) STP
(T 273K , P 1.0atm 1.013 105 Nm 2 101.3kPa)
iv ) Avogadro ' s, N A 6.02 1023 / mol
LGD10703 ENGINEERING SCIENCE
Vous aimerez peut-être aussi
- ExamDocument12 pagesExamapi-312236075Pas encore d'évaluation
- 2016 Specimen Paper 4 PDFDocument18 pages2016 Specimen Paper 4 PDFJKPas encore d'évaluation
- A6DEC19BMEADocument4 pagesA6DEC19BMEA2K19/EC/101 LOKESHPas encore d'évaluation
- BWM Final 2014 Draft 1Document17 pagesBWM Final 2014 Draft 1hero100% (1)
- Formatted MechDocument14 pagesFormatted MechVennela VennelaPas encore d'évaluation
- Thermo Chapter-1 CengelDocument3 pagesThermo Chapter-1 CengelHaardikGargPas encore d'évaluation
- JNTU Previous Paper Questions ThermodynamicsDocument61 pagesJNTU Previous Paper Questions ThermodynamicsVishnu MudireddyPas encore d'évaluation
- Ejercicios de Ecuaciones Diferenciales, Resueltos en MatlabDocument6 pagesEjercicios de Ecuaciones Diferenciales, Resueltos en MatlabCrezpo YzPas encore d'évaluation
- MAE 320 HW 1 Assignment and Pressure Transducer CalibrationDocument8 pagesMAE 320 HW 1 Assignment and Pressure Transducer CalibrationHaardikGargPas encore d'évaluation
- W12 1321 01 ADocument19 pagesW12 1321 01 ALucy SimmondsPas encore d'évaluation
- ETD Final Exam 4Document4 pagesETD Final Exam 4Bhargav Srinivas PadamataPas encore d'évaluation
- Thermodynamic Processes ExplainedDocument8 pagesThermodynamic Processes ExplainedAman BhuttaPas encore d'évaluation
- Dec 2014Document2 pagesDec 2014api-315791751Pas encore d'évaluation
- ThermodynamicsDocument14 pagesThermodynamicssarathPas encore d'évaluation
- Assignment 400523783 PDFDocument20 pagesAssignment 400523783 PDFsanju sharmaPas encore d'évaluation
- Class 11C - Physics Paper 2Document6 pagesClass 11C - Physics Paper 2St. Andrew's High School KarachiPas encore d'évaluation
- Engineering Mathematics: 1qllgiDocument10 pagesEngineering Mathematics: 1qllgiiwonttellyouthatPas encore d'évaluation
- Fluid Mechanics and Heat TransferDocument8 pagesFluid Mechanics and Heat Transfervenkat_nsnPas encore d'évaluation
- Hours: ExaminationDocument2 pagesHours: Examinationapi-279049687Pas encore d'évaluation
- 02-Thermodynamic Process (Practice Problem)Document8 pages02-Thermodynamic Process (Practice Problem)Aditya GuptaPas encore d'évaluation
- E.S. 313 Practice Exam I: Gravitational Acceleration Is Needed, Use Standard GravityDocument4 pagesE.S. 313 Practice Exam I: Gravitational Acceleration Is Needed, Use Standard GravityPraven IndranPas encore d'évaluation
- 11 - Heat and ThermodynamicsDocument6 pages11 - Heat and ThermodynamicsAman BhuttaPas encore d'évaluation
- Me 2353 - Finite Element AnalysisDocument4 pagesMe 2353 - Finite Element AnalysiscprabhakaranPas encore d'évaluation
- GATE Mechanical Engineering MCQ on Basic Concepts and Energy AnalysisDocument84 pagesGATE Mechanical Engineering MCQ on Basic Concepts and Energy AnalysisRajesh Verma75% (4)
- R059210304 ThermodynamicsDocument8 pagesR059210304 ThermodynamicsKushagra GuptaPas encore d'évaluation
- Process Dynamics and Control Exam QuestionsDocument9 pagesProcess Dynamics and Control Exam Questionsbhaskar537750% (2)
- Eme 21062010Document2 pagesEme 21062010Bhavesh PipaliyaPas encore d'évaluation
- Punjab Technical University: Applied Thermodynamics-IDocument2 pagesPunjab Technical University: Applied Thermodynamics-ItransendencePas encore d'évaluation
- 5054 MJ 2023 P21Document15 pages5054 MJ 2023 P21Raahin RahimPas encore d'évaluation
- Menoufiya University Faculty of Engineering Shebin El-KomDocument2 pagesMenoufiya University Faculty of Engineering Shebin El-KompaulaPas encore d'évaluation
- 9EH EoC Test 2013 No SolsDocument11 pages9EH EoC Test 2013 No SolsDanny EtievePas encore d'évaluation
- II B.tech I Sem Question Bank For r18Document30 pagesII B.tech I Sem Question Bank For r18saiharish634Pas encore d'évaluation
- End Semester Examination: Matunga, Mumbai-400 019Document3 pagesEnd Semester Examination: Matunga, Mumbai-400 019The CornerPas encore d'évaluation
- STPM 2014 p1 TrialDocument13 pagesSTPM 2014 p1 Trialshinichi_kesian6117Pas encore d'évaluation
- Physics Thermodynamics MCQDocument6 pagesPhysics Thermodynamics MCQkamilbismaPas encore d'évaluation
- Anglo-Chinese Sec 3 Physics PaperDocument16 pagesAnglo-Chinese Sec 3 Physics Papercjcsucks100% (1)
- HW4VM235SU2016Document2 pagesHW4VM235SU2016tony960129Pas encore d'évaluation
- Bgcse Double Award 2018 SolutionsDocument16 pagesBgcse Double Award 2018 SolutionsCrystal Machipisa100% (1)
- TEDocument107 pagesTEMohamed HenanshaPas encore d'évaluation
- Eng Therm and Fluid Mech E1 Level 4 Exam MAY 2021Document10 pagesEng Therm and Fluid Mech E1 Level 4 Exam MAY 2021Brody CrossPas encore d'évaluation
- Section A: Without DimplesDocument16 pagesSection A: Without DimplesLim WcPas encore d'évaluation
- Me6301 Engineering Thermodynamics May June 2014Document4 pagesMe6301 Engineering Thermodynamics May June 2014BIBIN CHIDAMBARANATHANPas encore d'évaluation
- Physics Paper Class 11thDocument10 pagesPhysics Paper Class 11thMahiPas encore d'évaluation
- Tutorial Chapter 02 - AnswerDocument8 pagesTutorial Chapter 02 - AnswerFateh Hakeem100% (4)
- Me6301 Engineering Thermodynamics May June 2013Document3 pagesMe6301 Engineering Thermodynamics May June 2013BIBIN CHIDAMBARANATHANPas encore d'évaluation
- Old Question-NePhO-2020Document6 pagesOld Question-NePhO-2020राम यादवPas encore d'évaluation
- GATE 2021 Aerospace EngineeringDocument20 pagesGATE 2021 Aerospace EngineeringCharana GsPas encore d'évaluation
- rr222102 Engineering ThermodynamicsDocument8 pagesrr222102 Engineering ThermodynamicsSRINIVASA RAO GANTAPas encore d'évaluation
- 5054 MJ 2023 P22Document16 pages5054 MJ 2023 P22Raahin RahimPas encore d'évaluation
- Vm235: Thermodynamics Homework 2: Assigned Tues May 24, 2016 Due Tues May 31 at The Start of ClassDocument3 pagesVm235: Thermodynamics Homework 2: Assigned Tues May 24, 2016 Due Tues May 31 at The Start of Classtony960129Pas encore d'évaluation
- Rr322105 High Speed AerodynamicsDocument8 pagesRr322105 High Speed Aerodynamicsgeddam06108825Pas encore d'évaluation
- GCE QuestionsDocument18 pagesGCE QuestionsBabatunde Victor JuniorPas encore d'évaluation
- Mathematics Mm2B Unit Mechanics 2BDocument8 pagesMathematics Mm2B Unit Mechanics 2BAhmed NurulPas encore d'évaluation
- Solving Partial Differential Equation Applications with PDE2DD'EverandSolving Partial Differential Equation Applications with PDE2DPas encore d'évaluation
- A Modern Course in Statistical PhysicsD'EverandA Modern Course in Statistical PhysicsÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportD'EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportPas encore d'évaluation
- Universiti Kuala Lumpur Malaysian Institute of Marine Engineering TechnologyDocument7 pagesUniversiti Kuala Lumpur Malaysian Institute of Marine Engineering TechnologyHafizuddin RazakPas encore d'évaluation
- Aqua Fresh Water Generator IntroDocument2 pagesAqua Fresh Water Generator IntroHafizuddin RazakPas encore d'évaluation
- Community service event helps local communityDocument1 pageCommunity service event helps local communityHafizuddin RazakPas encore d'évaluation
- Understanding Stress and Strength in MaterialsDocument29 pagesUnderstanding Stress and Strength in MaterialsHafizuddin RazakPas encore d'évaluation
- AIR COMPRESSORS: EFFICIENT GAS PRESSURIZATIONDocument11 pagesAIR COMPRESSORS: EFFICIENT GAS PRESSURIZATIONHafizuddin RazakPas encore d'évaluation
- Xial OAD: Principle of Superposition Axial - Force / Stress Diagram StrainDocument48 pagesXial OAD: Principle of Superposition Axial - Force / Stress Diagram StrainHafizuddin RazakPas encore d'évaluation
- Chapter 7 Steering GearDocument80 pagesChapter 7 Steering GearHafizuddin Razak100% (2)
- Air Compressor Overall - Component DiagramDocument18 pagesAir Compressor Overall - Component DiagramHafizuddin RazakPas encore d'évaluation
- Energy Analysis of Closed Systems: MAE 320-Chapter 4Document9 pagesEnergy Analysis of Closed Systems: MAE 320-Chapter 4Hafizuddin RazakPas encore d'évaluation
- WBB10102 TechnopreneurshipDocument4 pagesWBB10102 TechnopreneurshipHafizuddin RazakPas encore d'évaluation
- 2 - Charpy - ImpactDocument6 pages2 - Charpy - ImpactMuzamil Hasan0% (1)
- Energy Analysis of Closed Systems: MAE 320-Chapter 4Document9 pagesEnergy Analysis of Closed Systems: MAE 320-Chapter 4Hafizuddin RazakPas encore d'évaluation
- ThermodynamicsDocument1 pageThermodynamicsHafizuddin RazakPas encore d'évaluation
- Chapter 2 Integration of Tangent Power and Multiple AngleDocument3 pagesChapter 2 Integration of Tangent Power and Multiple AngleHafizuddin RazakPas encore d'évaluation
- Differentiation High Order and ImplicitDocument5 pagesDifferentiation High Order and ImplicitHafizuddin RazakPas encore d'évaluation
- Magnetic Particle InspectionDocument3 pagesMagnetic Particle InspectionHafizuddin RazakPas encore d'évaluation
- Rotational & Circular Motion ProblemsDocument5 pagesRotational & Circular Motion ProblemsHafizuddin RazakPas encore d'évaluation
- Fiberglass Fatigue TestingDocument5 pagesFiberglass Fatigue TestingHafizuddin RazakPas encore d'évaluation
- Calculation of Displacement, LWT and DWTDocument18 pagesCalculation of Displacement, LWT and DWTM.Neuer91% (45)
- Fire Fighting and SafetyDocument58 pagesFire Fighting and SafetyHafizuddin RazakPas encore d'évaluation
- ThermodynamicsDocument1 pageThermodynamicsHafizuddin RazakPas encore d'évaluation
- Impact Toughness Testing of SteelsDocument12 pagesImpact Toughness Testing of SteelsHafizuddin RazakPas encore d'évaluation
- Magnetic Particle InspectionDocument3 pagesMagnetic Particle InspectionHafizuddin RazakPas encore d'évaluation
- Ship StabilityDocument40 pagesShip Stabilityapi-1977578367% (3)
- Business Case PresentationDocument27 pagesBusiness Case Presentationapi-253435256Pas encore d'évaluation
- Breaking NewsDocument149 pagesBreaking NewstigerlightPas encore d'évaluation
- Iphoneos 31Document159 pagesIphoneos 31Ivan VeBoPas encore d'évaluation
- Money Laundering in Online Trading RegulationDocument8 pagesMoney Laundering in Online Trading RegulationSiti Rabiah MagfirohPas encore d'évaluation
- Ovr IbDocument27 pagesOvr IbAriel CaresPas encore d'évaluation
- I Will Be Here TABSDocument7 pagesI Will Be Here TABSEric JaoPas encore d'évaluation
- Gabinete STS Activity1Document2 pagesGabinete STS Activity1Anthony GabinetePas encore d'évaluation
- Copula and Multivariate Dependencies: Eric MarsdenDocument48 pagesCopula and Multivariate Dependencies: Eric MarsdenJeampierr Jiménez CheroPas encore d'évaluation
- United States Bankruptcy Court Southern District of New YorkDocument21 pagesUnited States Bankruptcy Court Southern District of New YorkChapter 11 DocketsPas encore d'évaluation
- Equilibruim of Forces and How Three Forces Meet at A PointDocument32 pagesEquilibruim of Forces and How Three Forces Meet at A PointSherif Yehia Al MaraghyPas encore d'évaluation
- Techniques in Selecting and Organizing InformationDocument3 pagesTechniques in Selecting and Organizing InformationMylen Noel Elgincolin ManlapazPas encore d'évaluation
- Template WFP-Expenditure Form 2024Document22 pagesTemplate WFP-Expenditure Form 2024Joey Simba Jr.Pas encore d'évaluation
- Computer Networks Transmission Media: Dr. Mohammad AdlyDocument14 pagesComputer Networks Transmission Media: Dr. Mohammad AdlyRichthofen Flies Bf109Pas encore d'évaluation
- What Is A Problem?: Method + Answer SolutionDocument17 pagesWhat Is A Problem?: Method + Answer SolutionShailaMae VillegasPas encore d'évaluation
- Bad DayDocument3 pagesBad DayLink YouPas encore d'évaluation
- A Reconfigurable Wing For Biomimetic AircraftDocument12 pagesA Reconfigurable Wing For Biomimetic AircraftMoses DevaprasannaPas encore d'évaluation
- There Is There Are Exercise 1Document3 pagesThere Is There Are Exercise 1Chindy AriestaPas encore d'évaluation
- LIST OF ENROLLED MEMBERS OF SAHIWAL CHAMBER OF COMMERCEDocument126 pagesLIST OF ENROLLED MEMBERS OF SAHIWAL CHAMBER OF COMMERCEBASIT Ali KhanPas encore d'évaluation
- EA Linear RegressionDocument3 pagesEA Linear RegressionJosh RamosPas encore d'évaluation
- Pulse Width ModulationDocument13 pagesPulse Width Modulationhimanshu jainPas encore d'évaluation
- Reading Comprehension Exercise, May 3rdDocument3 pagesReading Comprehension Exercise, May 3rdPalupi Salwa BerliantiPas encore d'évaluation
- CS709 HandoutsDocument117 pagesCS709 HandoutsalexPas encore d'évaluation
- 100 Training Games - Kroehnert, GaryDocument180 pages100 Training Games - Kroehnert, GarywindsorccPas encore d'évaluation
- White Box Testing Techniques: Ratna SanyalDocument23 pagesWhite Box Testing Techniques: Ratna SanyalYogesh MundhraPas encore d'évaluation
- Endangered EcosystemDocument11 pagesEndangered EcosystemNur SyahirahPas encore d'évaluation
- Technical specifications for JR3 multi-axis force-torque sensor modelsDocument1 pageTechnical specifications for JR3 multi-axis force-torque sensor modelsSAN JUAN BAUTISTAPas encore d'évaluation
- Physioex 9.0 Exercise 1 Act 1Document5 pagesPhysioex 9.0 Exercise 1 Act 1Adela LhuzPas encore d'évaluation
- Assignment - Final TestDocument3 pagesAssignment - Final TestbahilashPas encore d'évaluation
- United-nations-Organization-uno Solved MCQs (Set-4)Document8 pagesUnited-nations-Organization-uno Solved MCQs (Set-4)SãñÂt SûRÿá MishraPas encore d'évaluation