Académique Documents
Professionnel Documents
Culture Documents
Antioxidant Activity and Cytotoxicity of Rang Chuet (Thunbergia Laurifolia Lindl.) Extracts
Transféré par
KhafidotulJanahCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Antioxidant Activity and Cytotoxicity of Rang Chuet (Thunbergia Laurifolia Lindl.) Extracts
Transféré par
KhafidotulJanahDroits d'auteur :
Formats disponibles
As. J. Food Ag-Ind.
2008, 1(02), 116-128
Asian Journal of
Food and Agro-Industry
ISSN 1906-3040
Available online at www.ajofai.info
Research Article
Antioxidant activity and cytotoxicity of Rang Chuet
(Thunbergia laurifolia Lindl.) extracts
Ratchadaporn Oonsivilai1, Mario G. Ferruzzi2 and Suwayd Ningsanond1
1
School of Food Technology, Suranaree University of Technology, 111 University Avenue, Muang,
Nakhon Ratchasima, Thailand, 30000
Department of Food Science, Purdue University, 745 Agricultural Mall Drive, West Lafayette,
IN 47907, USA
Author to whom correspondence should be addressed, email:
Paper originally presented at Food Innovation Asia 2007
Abstract: Thunbergia laurifolia Lindl. or Rang Chuet (RC) is widely described in Thai
traditional medicine for protection against dietary and environmental toxicants with little
substantiation. To better access the potential of RC as a medicinal plant, extracts were
prepared by infusion with water, ethanol and acetone.
Antioxidant activities and total phenolic content of RC extracts were evaluated using free
radical scavenging, ferric reducing antioxidant power assay (FRAP) and the FolinCiocalteu method. It was found that water extraction of phenolic compounds was the most
efficient ( 2433.9mg GAE/100g) compared to ethanol and acetone extraction which had
phenolic contents of 565 and 142.1mg GAE /100g, respectively. In addition, RC water
extract possessed the highest antioxidant activities using free radical scavenging at the
EC50 values of 0.13 mg GAE/mL, whereas ethanol and acetone extract showed EC50 at
0.26 and 0.61 mg GAE/mL respectively. Finally, the water extract also showed the
highest total antioxidant activity using FRAP assay at 0.93 mmol/g followed by ethanol
and acetone extracts (0.18 and 0.04 mmol/g).
Extracts were subsequently investigated for their cytotoxicity. Cytotoxicity of RC crude
extracts were investigated in L929, BHK(21)C13, HepG2 and Caco-2 cell lines. The
toxicity was indicated at high concentrations over 100 g/mL for all extracts, which would
be the index for further recommended concentration use.
Keywords: cytotoxicity, antioxidant activity, biochemistry, FARP
As. J. Food Ag-Ind. 2008, 1(02)
117
Introduction
Thunbergia laulifolia Lindl., commonly known in Thai as Rang Chuet (RC), belongs to
the botanical family of Acanthaceae. It has been used in Thailand as a natural remedy for
centuries. RC is commonly consumed as herbal tea. Various parts of RC are used, for
example, aqueous extracts of fresh leaves, dried leaves, dried root and bark as an antidote
for insecticide, ethyl alcohol, arsenic and strychnine poisoning; the dried root is also used
as anti-inflammatory and antipyretic agents (Thongsaard and Marsden., 2002). In addition,
it has been reported that RC leaves change body temperature of rats by centrally acting at
the thermo regulating centre and/or cause vasodilatation and thereby increase heat
dissipation (Chamreondararassame, 2003).
As Rang Chuet has been used in traditional medicine, researchers are interested in
studying the compounds in RC leaf extracts. Kanchanapoom et al. (2002) reported two
iridoid glucosides, 8-epi-grandiforic and 3-O- -glucopyranosyl-stibericoside isolated
from the aerial parts of Thunbergia laurifolia, along with seven known glucoside
compounds. The flowers of Thunbergia laurifolia were also reported to contain
Delphinidin
3:5-di-o--D-glucopyranoside,
apigenin
and
apigenin-7-o--Dglucopyranoside (Purnima and Gupta, 1978). In addition, the plant was reported to contain
flavonoids such as apigenin, casmosiin, delphinidin-3-5-di---D-glucoside and
chorogenic acid (Thongsaard and Marsden., 2002).
The pharmacological properties of Rang Chuet water crude extracts have been reported as
an antidote for insecticide poisoning, treatment for drug addiction, reducing toxicity of
insecticide (Folidol) and antimicrobial activity, as well as antioxidant activity (Tejasen
and Thongthapp, 1979; Rengyutthakan, 1980; Thongsaard and Marsden, 2002; Khunkitti
et al., 2003; Srida et al., 2002 ). It has also been reported that extracts of RC leaves have a
protective effect on ethanol-induced hepatotoxicity using hepatic lipid peroxidation,
blood ethanol concentration as well as hepatic alcohol dehydrogenase (ADH) and
aldehyde dehydrogenase (ALDH) as indicators (Chanawirat et al., 2000). Treatment of
alcoholism is also claimed using its aqueous extract. Alcohol and hexane extracts from
Thunbergia laurifolia also posses anti-inflammatory activity against carageenin-induced
paw edema in mice (Charumanee et al., 1998).
In this work, the free radical scavenging activities of the plant extracts were followed via
their reaction with the stable DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical and their
ferric ions reducing antioxidant activity potential (FRAP) assay. In addition, cytotoxicity
of Rang Chuet crude extracts were investigated in L929, BHK(21)C13, HepG2 and Caco2
cell lines. Results from this study will provide a better understanding of the antioxidant
properties and cytotoxicity of this plant and possibly lead to the identification of plants
with high antioxidant activity for further investigation and development into value-added
food products and neutraceuticals. Furthermore, IC50 value of extracts will provide
indicators of safe level use for the future.
Materials and Methods
Plant materials
The medicinal plant Rang Chuet, Thunbergia laurifolia Lindl. (Acanthaceae) used in this
experiment was collected in December 2005 - Febuary 2006 from local areas in Nakhon
Ratchasima Province, Thailand. Leaves were air dried at 60oC for 6 h, after which they
were ground in a blender (National, MX-T2GN, Taiwan) to fine powder and stored in
As. J. Food Ag-Ind. 2008, 1(02)
118
vacuum packages at 4oC until use. Leaf powder such as this is typical of raw material
utilized for manufacture of extracts and/or herbal tea products from RC.
Chemicals and standards
All chemicals of analytical grade including: 1,1-diphenyl-2-picryhydrazyl free radical
(DPPH), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), Ferric, chloride-6-hydrate, Ferrous,
sulphate 7-hydrate, acetate buffer pH 4.6, Gallic acid, Folin-Ciocalteu phenol reagent and
anhydrous sodium carbonate (Na2CO3) were obtained from Sigma-Aldrich Co. (St. Louis,
USA). Solvents including acetone, ethanol, hydrochloric acid and methanol were
purchased from Mallinckrodt-Baker (Phillipsburg, NJ, USA). Cell culture, Media, MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], DMSO, Sorensens
Glycine buffer, were purchased from American Type Culture Collection; ATCC
(Manassas, VA, USA).
Preparation of RC Extracts
Extraction for antioxidant activity test and total phenolics
Leaf powder of about 100 mg was extracted with three 12 ml portions of boiling water
(100oC), ethanol and acetone in a shaking water bath at 25oC for 15 min. Centrifugation at
3000 g (Thermo IEC, Waltham, MA) was applied for 3 min between extraction three
times. The filtrates obtained after vacuum filtration were combined and the volumes were
adjusted to 50 ml with the same solvent. Aliquots of 2 ml were transferred to culture test
tubes (VWR, Wilmington, NC) and dried in a vacuum dryer (Rapid Vap Vacuum
Evaporation Systems, Labconco Corp., Kansas City, MO). Samples were stored at -20oC
until use.
Solvent extraction for cytotoxicity test
The powder (5g) was homogenized with water (200 ml), ethanol (200ml) and petroleum
ether (200 ml) and homogenate was extracted in a shaker at 55oC for 72 h. and then
filtered using Whatman No.1 paper. The filtrate was concentrated using vacuum
evaporator (BUCHI Rotavapor R-114, Switzerland) and kept at 4oC until use
(Kanchanapoom et al., 2002 with some modifications).
Supercritical Fluid Extraction for cytotoxicity test
Powder was sieved at 425 microns before extraction using supercritical fluid extraction. A
supercritical fluid extractor SFE was used for extraction. In this study, extraction was
performed by filling 8-ml volume extraction vessel with 3.0 g of the sieved leaf powder.
The leaf powder was then extracted with supercritical CO2 under 250 atm pressure and 50o
C temperature for 5 min static followed by 15 min dynamic. A Duraflow manual variable
restrictor was used in SFE system to collect the extracted analytes. The supercritical CO2
flow rate through the Duraflow restrictor was approximately 0.3-0.4 ml/min (compressed).
The analytes were collected in a 3 ml of petroleum ether (Yamini et al., 2002 with some
modification).
As. J. Food Ag-Ind. 2008, 1(02)
119
Total phenolics
Total soluble phenolic constituents of the extracts (water, ethanol and acetone) were
determined employing the method of Waterhouse (2002) using the Folin-Ciocalteu reagent
with gallic acid as standard. Twenty l of freshly prepared RC extract was added to a 1.5
ml cuvette, to which 1.58 m of dd water and 100 l Folin-Ciocalteu reagent was added.
The sample was thoroughly mixed and incubated for 5 min at room temperature.
Following incubation, 300 l of the NaCO3 (2% w/v) solution was added and the mixture
was allowed to stand at room temperature for 2 hr. Absorbance was measured at 765 nm.
Results were expressed as gallic acid equivalents.
DPPH free radical scavenging assay
The DPPH free radical scavenging activity of RC extracts (acetone, ethanol and
water), BHT and ascorbic acid were determined using DU 800 Spectrophotometer
(Beckman Coulter, CA) according to the method described by Ferruzzi et al. (2002), in
terms of hydrogen donating or radical scavenging ability. Briefly, 0.1 mm solution of
DPPH in methanol was prepared. The initial absorbance of the DPPH in methanol was
measured at 515 nm and did not change throughout the period of assay. An aliquot (100
l) of an extract diluted at the concentration range of 0.01-0.15 mg GAE/ml was mixed
with 1.9 ml of methanolic DPPH solution. The change in absorbance at 515 nm was
measured at 15 min. The percentage of scavenging was calculated as the ratio of the
absorption of the sample relative to the control DPPH solution, without the extracts. The
BHT and ascorbic acid in MeOH solution were used as positive controls. The EC50 of the
extracts was calculated at the 15 min scavenging value using nonlinear regression of
Sigma Plot 9.1 (Systat Software Inc, Illinois). Inhibition of free radical DPPH (%) was
calculated according to the formula:
Inhibition % = (Ablank-Asample/Ablank) 100
Where Ablank is the absorbance of the control reaction (containing all reagents except the
test compound) and Asample is the absorbance of the tested compound.
Exact concentration providing 50% inhibition (IC50) was calculated from the graph plotted
of %inhibition against extract concentration. Tests were carried out in triplicate. The
synthetic antioxidant BHT and ascorbic acid were included in experiments as positive
controls (Schlesier et al., 2002).
Ferric-reducing antioxidant power (FRAP) assay
The method of Wong, et al.(2006) was utilized. Briefly, the FRAP reagent was prepared
from acetate buffer (pH 3.6), 10 mmol TPTZ solution in 40 mmol HCl and 20 mmol
ferrous chloride solution in the proportion of 10:1:1 (v/v), respectively. The FRAP reagent
was fresh and daily prepared and was warmed to 37oC in a water bath prior to use. The
extract of 50 l was added to 1.5 ml of the FRAP reagent. The absorbance of the reaction
mixture was then recorded at 593 nm after 4 min. The standard curve was constructed
using ferric sulphate solution (100-2000 m) and the results were expressed as mol
equivalents of ferric per g dry weight of plant materials. All measurements were taken in
triplicate and the mean values were calculated.
As. J. Food Ag-Ind. 2008, 1(02)
120
Cell culture
The target cells were L929 (mouse connective tissue ECACC Cat. No. 85011425),
BHK(21)C13 (baby hamster Syrian kidney ECACC Cat. No. 85011433), HepG2 (human
liver hepatocarcinoma ATCC Cat. No. HB-8065) and Caco2 (a human colon
adenocarcinoma ATCC Cat. No. HTB-37, ATCC, USA). The L929 cells were grown in
Dulbeccos Modified Eagles Medium (DMEM) supplemented with 10% fetal bovine
serum and 2mM L-glutamine. The BHK(21)C13 cells were grown in Glasgow Modified
Eagles Medium (GMEM) supplemented with 5% fetal bovine serum, 1.5 g/l tryptose
phosphate broth and 2mm L-glutamine. The HepG2 and Caco2 were grown in Minimun
Essential Medium (MEM) supplemented with 10% fetal bovine serum, 0.1mm MEM nonessential amino acid, 1.0 mm sodium pyruvate and 2 mm L-glutamine. All cells were
incubated at 37oC in a fully humidified incubator with 5% CO2 : 95% air atmosphere.
Cytotoxicity
L929, BHK(21)C13, HepG2 and Caco2 were seeded in a 96-well plate with 500 cells/well
for L929 and 2000 cells/well for the others, and incubated for 48 h. All the RC dried crude
extracts were dissolved in ethanol to make stock solution then diluted to various extract
concentrations in media from 1.56 ug/ml to 200 ug/ml. The extracts were added to the
wells and incubated for 24 h. The test samples were removed from the cell cultures and
the cells were re-incubated for a further 24 h in fresh medium and then tested with MTT.
Briefly, 50 l of MTT in PBS at 5 mg/mL was added to the medium in each well and the
cells were incubated for 4 h. Medium and MTT were then aspirated from the wells and
formazan solubilized with 200 l of DMSO and 25 l of Sorensens Glycine Buffer,
pH10.5. The optical density was read with a plate reader at a wavelength of 570 nm. The
data were analyzed with the SoftMax Program (Molecular Devices) to determine the IC50
for each extract sample. Two controls were set up, one with medium as reagent control
and the second with the ethanol 1% as solvent control.
A dose-response curve was derived from 8 concentrations in the test range of 200-1.56
g/ml using 4 wells per concentration to determine the mean of each point. Within each
experiment 2-dose response curves were obtained. Results of toxic compounds were
expressed as the concentration of sample required to kill 50% (IC50) of the cells compared
with controls.
Statistical analysis
Descriptive statistics including mean and standard error of mean (SEM) were calculated
for each extract antioxidant activity (n=3).
Results and Discussion
Antioxidant activity measurement
The generation of radical oxidative species involves either radical processes or different
potential redox systems. The soluble properties of antioxidant compounds determine their
effective antioxidant activities in either aqueous or lipid systems (Sanchez-Gonzalez, et
al., 2005). Therefore, two aqueous-based models were chosen to assess the antioxidant
As. J. Food Ag-Ind. 2008, 1(02)
121
activity of the RC extracts, one measuring radical-scavenging activities and the other
measuring total reducing power.
Total phenolic contents
The phenolic contents of the aqueous, ethanol and acetone extracts of RC were tested
using the Folin-Ciocalteu reagent. The yield and total phenolics in RC extracts are
presented in Table 1. The phenolic contents of the water extract were found to have
highest phenolic contents (2433.9 mg GAE/100g) followed by ethanol (565 mg
GAE/100g), and acetone (142.1 mg GAE/100g). Comparing the efficiency of extraction
with boiling water, ethanol and acetone, the method using boiling water showed the
highest efficiency in the extraction of phenolic compounds. Therefore, RC water extract
would be expected to have the highest antioxidant activity. The acetone extracts gave the
highest yield of extract at 36.6% (g/g leaf powder x100) but this extract showed the lowest
phenolic contents.
Extraction method is also critical to the recovery of antioxidant phytochemicals. The
nature of both plant materials and the bioactive components should be considered in order
to achieve good extraction efficiency. Lipophilicity or hydrophilicity affects the solubility
of a phytochemical in the extracting solvent and, conversely, polarity of a solvent also has
an impact on the extraction efficiency. Many different extractions methods exist for
antioxidant phytochemicals, but most of them are based on solvent extraction using water,
organic solvent or liquefied gas, or combinations of these under different temperature and
pressure, although other methods such as physical press, filtration, stream distillation and
solid absorption have been used (Tsao and Deng, 2004).
Polar antioxidants such as phenolic acids and glycosides of many flavonoids are generally
extracted using water, alcohol or a mixture of water and alcohols. Our results (Table 1.)
are consistent with these previous notions. For antioxidants such as aglycones of some
flavonoids, non-aqueous solvents are used (Tsao and Deng, 2004). The efficiency of the
boiling water in extracting compounds producing antioxidant activity is higher than that of
methanol extract (Wong et al., 2006).
Table 1. Yield and total phenolics of Rang Chuet using various extraction solvents.
Solvent
Yields (g/g *100)
Total phenolic contents
(mg GAE/100g)
Water
32.6
2433.9 57.7
Ethanol
23.3
565.0 7.9
Acetone
36.6
142.1 10.6
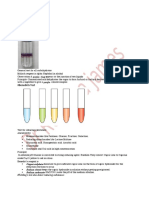
1,1-Diphenyl-2-picrylhydrazyl radical-scavenging
DPPH is a stable nitrogen-centered free radical. The colour changes from violet to yellow
upon reduction by either the process of hydrogen or electron-donation. Substances which
As. J. Food Ag-Ind. 2008, 1(02)
122
are able to perform this reduction can be considered as antioxidants and therefore radical
scavengers (Hinneburg et al., 2006). The hydrogen atoms or electrons donation ability of
the corresponding extract will be measured from the bleaching of violet colored methanol
solution of DPPH.
The studied extracts exhibited the scavenging activity of various strengths and were dose
dependent in all extracts. In addition, positive controls with BHT and ascorbic acid were
tested for their DPPH radical scavenging. The calculated EC50 for 15 min incubation time
are reported in Table 2.
Table 2. DPPH scavenging activity of RC extracts.
Rang Chuet Extracts
EC50
Water
Ethanol
Acetone
BHT
Ascorbic acid
0.129 0.01
0.261 0.04
0.607 0.06
0.278 0.04
0.103 0.02
(mgGAE/mL)
(mgGAE/mL)
(mgGAE/mL)
(mg/mL)
(mg/mL)
DPPH radical scavenging activity of water, ethanol, and acetone crude extracts of RC
revealed antioxidant potency when considering the EC50 values. A lower value of EC50
indicates a higher antioxidant activity. Water extract has the highest scavenging activity
amongst all extracts. Moreover, scavenging activity of water extract and ascorbic acid are
closely comparable and there appears to be high correlation between scavenging activity
and phenolic content of RC extracts.
The difference in antioxidant activities amongst the RC extracts is due to multiple factors,
including concentration of the extracts and qualitative profile of extracts. Water extract
had primarily components as apigenin and phenolic acids as caffeic acid and gallic acid as
well as acetone and ethanol had main components as chlorophyll derivatives and lutein
(Data not shown).
Antioxidant activity
The antioxidant potential of the RC extracts were estimated from their power to reduce the
TPTZ-Fe(III) complex to TPTZ-Fe(II) complex (FRAP assay), which is simple, fast and
reproducible (Wong et al., 2006). The FRAP is versatile and can be readily applied to both
aqueous and alcohol extracts of different plants. The results are expressed as mol ferrous
equivalents per gram of sample.
Table 3 shows the antioxidant activity of RC extracts including positive controls of BHT.
For the RC extracts, the antioxidant activity ranged from 0.044 to 0.928 mmol Fe(II)/g.
Water extract showed the highest antioxidant activity (0.928 mmol Fe(II)/g), followed by
the ethanol extract (0.079 mmol Fe(II)/g) and the acetone extract (0.044 mmol Fe(II)/g).
Wong et al. (2006) classified categories of medicinal plants based on their antioxidant
activities: extremely high (> 500 mol Fe(II)/g), high (100 500 mol Fe(II)/g), medium
(10 100 mol Fe(II)/g ), and low (<10 mol Fe(II)/g). Under this classification, the
As. J. Food Ag-Ind. 2008, 1(02)
123
water extract exhibited extremely high antioxidant activity, while ethanol and acetone
extracts showed medium antioxidant activity. In this study, RC was extracted with boiling
water similar to the traditional Thai method used for medicinal herbs. The other samples
were extracted with a more modern method, using ethanol and acetone.
Table 3. Antioxidant activity of water, ethanol and acetone RC extracts using FRAP
assay.
Rang Chuet Extracts
Water
Ethanol
Acetone
BHT
Total antioxidant activity (mmol/g)
0.928 0.050
0.079 0.002
0.044 0.005
1.421 0.593
It should be added that the leaves of RC are available at a low cost and thus provide an
economic source of potential natural antioxidants for use as food supplements or food
additives. The results combined with phytochemical profiling showed that RC leaves
contain phenolic acid as gallic acid, caffeic acid, protocatechuic acid, other than that it
contains flavonoid as apigenin and apigenin glucosides (Data not shown). Caffeic acid has
been reported to have antioxidant activity (Son and Lewis, 2002) and this phenolic acid
was found in all extracts, being found the highest in the water extract (142.1 mg/100g),
thus related to the highest antioxidant activities by both radical scavenging and FRAP
assay. Interestingly, it was found that gallic acid is a component in the water extract which
related to the highest antioxidant activity. Wong, et al. (2006) reported that gallic acid
isolated from Chinese medicinal plants had shown strong DPPH radical scavenging
activities. However, when compared to standards in FRAP assay, BHT (1.421 mmol
Fe(II)/g), ascorbic acid ( 119.5 mmol Fe(II)/g) and trolox (7.2 mmol Fe(II)/g), all RC
extracts showed relatively modest total antioxidant activity.
Antioxidant activity can be explained as reductants, and inactivation of oxidants by
reductants can be described as redox reactions in which one reaction species (oxidant) is
reduced at the expense of the oxidation of another antioxidant. The FRAP assay measures
the antioxidant effect of any substance in the reaction medium as reducing ability. In this
assay, the antioxidant activity is determined on the basis of the ability of reduce ferric to
ferrous. In the DPPH assay, antioxidants reduce the free radical 2,2-diphenyl-1picrylhydrazyl, which has an absorption max at 515 nm (Wong, et al, 2006).
Water extract showed the highest antioxidant activity both by DPPH and FRAP assay. It
could be explained that water extract had the ability to reduce both radicals and ferric ions
and also better reduce radicals. Also, all extracts showed antioxidant activity differently,
depending on the components in each extract. That the water extract showed the highest
antioxidant activity might be because the main constituents are phenolic acids and
flavonoids and flavonoids glucosides. Previous researcher reported that flavonoids and
phenolic acids are the source of antioxidant activity in plants (Cook and Samman, 1996).
The ethanol extract and acetone extract which showed less antioxidant activity was
composed of chlorophylls, chlorophyll derivatives and luteins. It could be explained that
chlorophylls and chlorophyll derivatives exhibited low antioxidant activity. In addition,
the luteins identified in ethanol and acetone extract were in very low amount thus did not
demonstrate antioxidant activity.
As. J. Food Ag-Ind. 2008, 1(02)
124
Hundreds of different polyphenols have been identified in food. The two main types of
polyphenols are flavonoids and phenolics. As antioxidants, polyphenols may protect cell
constituents against oxidative damage and therefore limit the risk of various degenerative
diseases associated with oxidative stress. The phenolic groups in polyphenols can accept
an electron to form relatively stable phenoxyl radicals, thereby disrupting chain oxidation
reactions in cellular components (Scalbert, et al., 2005).
Flavonoids are potent antioxidants, free radical scavengers and metal chelators and inhibit
lipid peroxidation. The structural requirement for the antioxidant and free radical
scavenging functions of flavonoids include hydroxyl carbon in position three, double
bonds between position two and three, a carbonyl group in carbon position four and
polyhydroxylation of the A and B aromatic rings (Cook and Samman, 1996). The radical
scavenging activities of flavonoids were highly controlled by the number and
configuration of phenolic hydroxyl groups in the molecules and also influenced by
glycosylation and configuration of other substituents. The flavonoids without any
hydroxyl group (e.g., trans-chalcone, flavone, flavanone, and isoflavone) had no radical
scavenging capacity (Cai, et al., 2006).
The results from the antioxidant assays show that all extracts can act as radical scavengers
to a certain extent. Water extract showed the highest activity in the iron reduction assay
and DPPH assay. Yet, the EC50 values for these extracts were still lower than those of the
tested reference antioxidant, ascorbic acid and BHT. From these results, it could be
suggested that RC extracts could be a natural source for antioxidants in food, but the
amount required to produce antioxidant activity similar to standard use antioxidants could
be large.
Cytotoxicity
Over the last few decades several in vitro assays using mammalian cell cultures have been
developed thus avoiding the excessive use of laboratory animals which is expensive, time
consuming and often involves ethical problems. Cell culture systems can be more
sensitive and more reproducible than tests involving intact animals (Cetin and Bullerman,
2005).
The cytotoxicity of RC extracts were evaluated in BHK, L929, Hep G2 and Caco-2 cell
lines with the MTT assay (Mossman, 1983). The IC50 value for Thunbergia laurifolia is
shown in Table 4. In addition, there was no difference between the two controls of
medium and ethanol 1%, with no toxicity to the tested cell lines.
As. J. Food Ag-Ind. 2008, 1(02)
125
Table 4. IC50 values for Thunbergia laurifolia extracts (water, petroleum ether,
ethanol and SFE).
Sample
Vehicle
control
Water crude
extract
Petroleum
ether crude
extract
Ethanol crude
extract
SFE
L929
67711
IC50 (ug/mL)
BHK(21)C13
HepG2
68913
529972
Caco2
299327
182+8
>200
>200
147+15
>200
103+1
140+4
117+9
>200
1342
>200
ND
145+4
118+4
154+1
120
The results showed that the IC50s were affected differently in each cell line. The
petroleum ether extract showed the highest cytotoxicity toward BHK(21)C13 cell line at
concentration of 103+1 g/mL. The water extract showed the lowest cytotoxicity toward
BHK(21)C13 and HepG2 cell lines at concentration over 200 g/ml. Also, petroleum
ether and ethanol extracts showed low cytotoxicity toward L929 at concentration over 200
g/ml. However, the toxicity for all extracts was indicated at high concentration of 200
ug/ml, which would be the index for further recommended concentration being applied.
All RC extracts exhibited an extremely high value of IC50 (>100 g/ml) against all cell
lines tested indicating low cytotoxic to the cells (Okonogi et al., 2006). When compared
amongst tested cell lines, BHK(21)C13 cells were found to be sensitive to the cytotoxic
effect of RC extracts including petroleum ether, ethanol and SFE extracts, followed by
Caco-2 and HepG2 after 24-h exposure, whereas L929 cells showed low response to RC
extracts toxicity. The highest cytotoxicity of BHK(21)C13 cells, baby hamster Syrian
kidney, toward RC extracts (Petroleum ether, ethanol, and SFE) could lead to
understanding that these RC extracts affect kidney cells when concentration at specific
values was applied. In petroleum ether extract, cytotoxicity may be caused from the
solvent itself and components in extracts which are from non-polar extraction. The use of
large amounts of organic solvents poses health and safety risks (Tsao and Deng, 2004).
The water extracts showed low cytotoxicity (> 200 g/ml) toward BHK(21)C13 and
HepG2 cell lines which leads to the suggestion to use this kind of RC extract due to its
low toxicity to kidney cells and human liver cell lines. Water extraction is a non-toxic
method for extraction and also the components extracted could be polyphenol parts
including flavonoids and phenolic acids which are generally extracted using water (Tsao
and Deng, 2004). Quite oppositely, the water extract showed high cytotoxicity at IC50 of
147 g/ml toward Caco-2 cells, which leads to the suggestion that water extract, even
though it exhibits low toxicity towards kidney and liver cells, is moderately toxic toward
intestinal cells. For Caco-2 cells, the cytotoxicity of RC extracts at IC50 of 117, 120, and
147 for SFE, water and petroleum ether extracts respectively. SFE extracts show high
cytotoxicity toward human intestinal cells and this suggests careful use is required with
As. J. Food Ag-Ind. 2008, 1(02)
126
specific concentration of this extract. When compared between the cell lines tested, L929
cells showed the lowest cytotoxicity from all RC extracts, which might be due to the type
of cell lines (connective tissue) and if compared between types of RC extracts, the water
extract had the lowest cytotoxicity for all cell lines tested.
Okonogi, et al. (2006) studied antioxidant capacities and cytotoxicities of certain fruit
peels in Caco-2 cells and peripheral blood mononuclear cells and reported that the peels of
rambutan may be considered potentially useful as a source of natural antioxidants for food
or drug products because of their high antioxidant activity and non-toxic properties to
normal cells (IC 50 > 100 ug/ml).
Conclusion
The antioxidant activity and total phenolic contents of RC extracts were evaluated. The
RC extracts, in general, showed high antioxidant activities and phenolic content
particularly in the water extract, followed by ethanol and acetone extract respectively.
Relatively, the antioxidant activity by DPPH and FRAP assay were both highest in water
extract at 0.129 mg GAE/ml and 0.928 mmol Fe (II)/g respectively. In addition, the
antioxidant activity values of water extract was less than the positive control (BHT), one
fold by DPPH assay and 1.6 fold in FRAP assay. Moreover, there is a relationship
between antioxidant activities and total phenolic contents in all extracts. High total
phenolic contents showed high antioxidant activity in all extracts. It could be concluded
that polyphenol compounds contribute to antioxidant activity in RC extracts.
In addition, the RC crude extracts indicated toxicity to L929, BHK (21)C13, HepG2 and
Caco2 over the tested concentration ranges of 200 to 1.56 ug/ml. The IC50 could be
summarized as low cytotoxicity at concentrations over 100 ug/ml for all crude extracts.
Acknowledgment
This research work was supported by the Personnel Development Project, Commission on
Higher Education, Thailand under a consortium program with the Food Science
Department, Purdue University, USA.
References
1.
Cai, Y.Z., Sunb, M., Xingc, J., Luod, O., and Corkea, H.(2006). Structure radical
scavenging activity relationships of phenolic compounds from traditional Chinese
medicinal plants. Life Sciences. 78 (25): 2872 2888.
2.
Cetin, Y. and Bullerman, L.B.( 2005). Cytotoxicity of Fusarium mycotoxins
tomammalian cell cultures as determined by the MTT bioassay. Food and
Chemical Toxicology. 43(5): 755 764.
3.
Chamreondararassame, B.(2003). The effects of Rangjert leaves on body
temperature. Faculty of Pharmacy, Chiang Mai University, Chiang Mai, Thailand.
4.
Chanawirat, A., Toshulkao, C., Temcharoen, P., Glinsukon, T.(2000). Protective
effect of Thunbergia laurifolia extract on ethanol-induced hepatotaxicity in
As. J. Food Ag-Ind. 2008, 1(02)
127
mice. Thesis, Faculty of Graduate Studies, Mahidol University, Bangkok,
Thailand.
5.
Charumanee, S., Vejabhikul, S., Taesotikul, T., Netsingha, W., Sirisaad, P.,
Leelapornpisit, P.(1998). Development of topical anti-inflammatory
preparations. From Phase I Research Report. Faculty of Pharmacy, Chiang Mai
University, Chiang Mai, Thailand.
6.
Cook, N.C. and Samman, S.(1996). Flavonoids-chemistry, metabolism
cardioprotective effects and dietary sources. Nutrition Biochemistry. 7: 66 76.
7.
Ferruzzi, M.G., Bohm, V., Courtney, P.D., and Schwartz, S.J.(2002).
Antioxidant and antimutagenic activity of dietary chlorophyll derivatives
determined by radical scavenging and bacterial reverse mutagenesis assays. J.
Food Sci. 67(7):2589-2595.
8.
Hinneburg, I., Damien Dorman, H.J., and Hiltunen, R.(2006). Antioxidant
activities of extracts from selected culinary herbs and spices. Food Chem. 97:
122-129.
9.
Kanchanapoom, T., Kasai, R., and Yamasaki, K.(2002). Iridoid glucosides from
Thunbergia laurifolia. Phytochemistry 60: 769-771.
10.
Khunkitti, W., Taweechaisupapong, S., Aromdee, A., and Pese, M.(2003).
Antimicrobial activity of Thunbergia laurifolia crude extract. 3rd World
Congress on Medicinal Plants and Aromatic Plants for Human Welfare, 3-7
February 2003, Chiang Mai, Thailand.
11.
Mosmann, T.(1983). Rapid colorimetric assay for cellular growth and survival:
application to proliferation and cytotoxicity assay. J. Immunol. Methods.
65 (1-2): 55-63.
12.
Okonogia, S.,
Duangrata, C., Anuchpreedab, S.,
Tachakittirungroda,
S., and Chowwanapoonpohna, S.(2006). Comparison of antioxidant capacities and
cytotoxicities of certain fruit peels. Food Chemistry. Article in Press.
13.
Purnima and
Gupta, P.C. (1978). Colouring matters from the flowers of
Thunbergia laurifolia. J. Indian Chem. Soc. LV, June.
14.
Ruengyutthakan, W.(1980). The pharmacological studies of Rang Chuet leaves.
MSc. Thesis. Chiang Mai University. Thailand.
15.
Sanchez-Gonzalez, I., Jimenez-Escrig, A. and Saura-Calixto, F. (2005). In vitro
antioxidant activity of coffees brewed using different procedures (Italian,
espresso and filter). Food Chemistry, 90: 133 139.
16.
Scalbert, A., Manach, C., Morand, C., and Remesy, C.(2005). Dietary polyphenols
and
the prevention of disease. Critical Reviews in Food and Nutrition. 45:
287306.
As. J. Food Ag-Ind. 2008, 1(02)
128
17.
Schlesier, K, Harwat, M., Bohm, V., and Bitsch, R.(2002). Assessment of
antioxidant activity by using different in vitro methods. Free Radical
Research. 36 (2): 177187.
18.
Srida, C., Hankete, J., Khunkitti, W, Aromdee, C., and Pese, M.(2002).
Antioxidant activity of Thunbergia laurifolia ethanolic extract. Thai J. Pharm.
Sci. Vol. 26.
19.
Tsao, R. and Deng, Z.(2004). Separation procedures for naturally occurring
antioxidant phytochemicals. J. Chromatog. B. 812: 85 99.
20.
Tejasen, P. and Thongthapp, C.(1979). The study of the insecticide antitoxicity of
Thunbergia laurifolia Linn. Chiang Mai. Bull. 19, pp. 105-114.
21.
Thongsaard, W. and Marsden, C.A.(2002). A herbal medicine used in the
treatment of addiction mimics the action of amphetamine on in vitro rats trial
dopamine release. Neuroscience Letters. 329(2): 129-132.
22.
Wong, C. C., Li, H., Cheng, K., and Chen, F.(2006). A systemic survey of
antioxidant activity of 30 Chinese medicinal plants using the ferric reducing
antioxidant power assay. Food Chem. 97: 705 711.
23.
Yamini, Y., Asghari-Khiavi, M., and Bahramifar, M.(2002). Effects of different
parameters on supercritical fluid extraction of steroid drugs, from spike
matrices and tablets. Talanta, 58 (5): 1003-1010.
Vous aimerez peut-être aussi
- Preparation of K (Cu (C O) ) .2H ODocument3 pagesPreparation of K (Cu (C O) ) .2H Omick100% (1)
- Determination of Phosphate in Sugarcane JuicesDocument8 pagesDetermination of Phosphate in Sugarcane Juicessha2salaPas encore d'évaluation
- Estimation of Different Extracts of Amaranthus Viridis L For Invitro Antioxidant ActivityDocument6 pagesEstimation of Different Extracts of Amaranthus Viridis L For Invitro Antioxidant ActivityiajpsPas encore d'évaluation
- Rule of ThumbDocument5 pagesRule of ThumbAnGahBasHa100% (1)
- IX News: Water Demineralisation. Ion Exchange and Reverse Osmosis: Competitors or Associates ?Document8 pagesIX News: Water Demineralisation. Ion Exchange and Reverse Osmosis: Competitors or Associates ?nermeen ahmed100% (1)
- Study of Common Food AdulterantsDocument20 pagesStudy of Common Food AdulterantsChaitanya PantulaPas encore d'évaluation
- Moles Workbook Y10Document8 pagesMoles Workbook Y10api-125934329100% (1)
- Huang Et Al 2005 PDFDocument8 pagesHuang Et Al 2005 PDFYoga Bririan JatiPas encore d'évaluation
- Contents of Wetland Medicinal Plants in Taiwan: Antioxidant Properties and Total PhenolicDocument12 pagesContents of Wetland Medicinal Plants in Taiwan: Antioxidant Properties and Total Phenolicvaishali shuklaPas encore d'évaluation
- Antioxidant Activity of Pandanus Amaryllifolius Leaf and Root Extract and Its Application in Topical EmulsionDocument7 pagesAntioxidant Activity of Pandanus Amaryllifolius Leaf and Root Extract and Its Application in Topical EmulsionprashanthiarunPas encore d'évaluation
- Zanthoxylum Alatum: A Commonly UsedDocument13 pagesZanthoxylum Alatum: A Commonly UsedThiago AlmeidaPas encore d'évaluation
- Effects of Temperature and Solvent On Antioxidant Properties of Curry Leaf (Murraya Koenigii L.)Document5 pagesEffects of Temperature and Solvent On Antioxidant Properties of Curry Leaf (Murraya Koenigii L.)Moolam RaoPas encore d'évaluation
- Structural Features and Immunological Activity of A Polysaccharide From Dioscorea Opposita Thunb RootsDocument7 pagesStructural Features and Immunological Activity of A Polysaccharide From Dioscorea Opposita Thunb RootsFrontiersPas encore d'évaluation
- Artocarpus LakoochaDocument16 pagesArtocarpus LakoochaMoney ManPas encore d'évaluation
- tmp4359 TMPDocument12 pagestmp4359 TMPFrontiersPas encore d'évaluation
- Aloe VeraDocument0 pageAloe VeraSyahrul FarhanahPas encore d'évaluation
- 036 PandeDocument6 pages036 PandeabdurrahmanassudaisPas encore d'évaluation
- Antioxidant & Antimicrobial Activities of Cocos Nucifera Linn. (Arecaceae) Endocarp ExtractsDocument8 pagesAntioxidant & Antimicrobial Activities of Cocos Nucifera Linn. (Arecaceae) Endocarp ExtractsSumi Mariya BabuPas encore d'évaluation
- Asian Pacific Journal of Tropical DiseaseDocument7 pagesAsian Pacific Journal of Tropical DiseaseSaya AfieraPas encore d'évaluation
- Gynura Procumbens - Research ArticleDocument8 pagesGynura Procumbens - Research ArticleAndreline Ansula100% (1)
- Phytochemical Standardization, Antioxidant, and Antibacterial Evaluations of Leea Macrophylla: A Wild Edible PlantDocument8 pagesPhytochemical Standardization, Antioxidant, and Antibacterial Evaluations of Leea Macrophylla: A Wild Edible Plantalem010Pas encore d'évaluation
- 4 RNP 0907 125Document11 pages4 RNP 0907 125Soumen ChoudhuryPas encore d'évaluation
- Analysis and Identification of Phenolic Compounds in Dioscorea Hispida DennstDocument14 pagesAnalysis and Identification of Phenolic Compounds in Dioscorea Hispida DennstRainolPas encore d'évaluation
- Dandelion Phenolic Compounds..Document22 pagesDandelion Phenolic Compounds..gail CieloPas encore d'évaluation
- Phytochemical, TLC Profile, and Antioxidant Activity of Malinau Endemic Plant of Tabar Kedayan (Aristolochia Papilifolia Ding Hou) Root FractionsDocument7 pagesPhytochemical, TLC Profile, and Antioxidant Activity of Malinau Endemic Plant of Tabar Kedayan (Aristolochia Papilifolia Ding Hou) Root FractionsAriePas encore d'évaluation
- in Vitro Antioxidant Activity of The Methanolic Extracts of Leaf and Fruit of Calamus Rotang Linn.Document5 pagesin Vitro Antioxidant Activity of The Methanolic Extracts of Leaf and Fruit of Calamus Rotang Linn.Ganesh PrasaiPas encore d'évaluation
- Antioxidant Potency and Gcms Composition of Leaves of Artocarpus Altilis Park FosbDocument5 pagesAntioxidant Potency and Gcms Composition of Leaves of Artocarpus Altilis Park Fosbfitriani fajriPas encore d'évaluation
- Food Chemistry: Shu-Yao Tsai, Shih-Jeng Huang, Sheng-Hua Lo, Tsai-Ping Wu, Pei-Ying Lian, Jeng-Leun MauDocument7 pagesFood Chemistry: Shu-Yao Tsai, Shih-Jeng Huang, Sheng-Hua Lo, Tsai-Ping Wu, Pei-Ying Lian, Jeng-Leun MauAnonymous LqOnAwPas encore d'évaluation
- In Vitro Analysis of Anti Diabetic and ADocument5 pagesIn Vitro Analysis of Anti Diabetic and ASri LakshmiPas encore d'évaluation
- Antioxidant Flavonoid Glycosides From The Leaves of Ficus Pumila L.Document6 pagesAntioxidant Flavonoid Glycosides From The Leaves of Ficus Pumila L.Hajrah SuhardiPas encore d'évaluation
- Nutritional Composition of Tumeric Curcuma LongaDocument6 pagesNutritional Composition of Tumeric Curcuma LongaagungsoPas encore d'évaluation
- Qualitative and Quantitative Analysis of Micropropagated: Centella Asiatica L.UrbDocument5 pagesQualitative and Quantitative Analysis of Micropropagated: Centella Asiatica L.UrbNanda Trisna OliviaPas encore d'évaluation
- Paniculata Nees.) and Patikan Kebo (Euphorbia Hirta L.) EthanolicDocument6 pagesPaniculata Nees.) and Patikan Kebo (Euphorbia Hirta L.) EthanolicAlifPas encore d'évaluation
- Comparison of Different Solvents and Extraction Methods For Isolation of Phenolic Compounds From Horseradish Roots (Armoracia Rusticana)Document6 pagesComparison of Different Solvents and Extraction Methods For Isolation of Phenolic Compounds From Horseradish Roots (Armoracia Rusticana)Apple-oren ZatieylshahieydaPas encore d'évaluation
- 65 IFRJ 20 (01) 2013 ChanthaiDocument5 pages65 IFRJ 20 (01) 2013 ChanthaiArfaa SajidPas encore d'évaluation
- JournalDocument13 pagesJournalNur FitriPas encore d'évaluation
- Phytochemical Analysis and Cytotoxicity Studies of Curcuma Amada Rhizomes in BHK-21 CellsDocument7 pagesPhytochemical Analysis and Cytotoxicity Studies of Curcuma Amada Rhizomes in BHK-21 CellsJessica ClarkPas encore d'évaluation
- Variation in Phenolic Content and Antioxidant Activity of Different Plant Parts of Primula VerisDocument5 pagesVariation in Phenolic Content and Antioxidant Activity of Different Plant Parts of Primula Verisalexia24_andronachePas encore d'évaluation
- Qualitative and Quantitative Phytochemical Evaluations of Strophanthus Hispidus Stem BarkDocument5 pagesQualitative and Quantitative Phytochemical Evaluations of Strophanthus Hispidus Stem BarkInternational Organization of Scientific Research (IOSR)Pas encore d'évaluation
- 30 HPLC Analysis of 6gingerolDocument7 pages30 HPLC Analysis of 6gingerolTanesh SelvarajuPas encore d'évaluation
- Antioxidant Properties of Various Solvent Extracts From Lychee FlowersDocument5 pagesAntioxidant Properties of Various Solvent Extracts From Lychee FlowersArinaAdilaPas encore d'évaluation
- Maity 2013Document8 pagesMaity 2013Sri LakshmiPas encore d'évaluation
- DR Ololade 007Document5 pagesDR Ololade 007suntolPas encore d'évaluation
- DR Ololade 007Document5 pagesDR Ololade 007suntolPas encore d'évaluation
- Chiet Qua Mycobala Bang PG Va EADocument6 pagesChiet Qua Mycobala Bang PG Va EANgô HưngPas encore d'évaluation
- 3 Ijbrddec20183Document8 pages3 Ijbrddec20183TJPRC PublicationsPas encore d'évaluation
- Determination of Total Phenolics and Ascorbic Acid Related To An Antioxidant Activity and Thermal Stability of The Mao Fruit JuiceDocument7 pagesDetermination of Total Phenolics and Ascorbic Acid Related To An Antioxidant Activity and Thermal Stability of The Mao Fruit Juicemaulidara riksaPas encore d'évaluation
- Phytochemicals and Nutritional Characteristics of Ethanol Extract of The Leaf and BarkDocument6 pagesPhytochemicals and Nutritional Characteristics of Ethanol Extract of The Leaf and BarkAdedayo A J AdewumiPas encore d'évaluation
- Flavonoids in Juglans Regia L. Leaves and Evaluation of in Vitro Antioxidant Activity Via Intracellular and Chemical MethodsDocument10 pagesFlavonoids in Juglans Regia L. Leaves and Evaluation of in Vitro Antioxidant Activity Via Intracellular and Chemical MethodsDyanne NegruPas encore d'évaluation
- Food Chemistry: Pronprapa Wongfhun, Michael H. Gordon, Arunee ApichartsrangkoonDocument6 pagesFood Chemistry: Pronprapa Wongfhun, Michael H. Gordon, Arunee ApichartsrangkoonSaif EvonyPas encore d'évaluation
- Apea-Bah, Et Al. 2009Document22 pagesApea-Bah, Et Al. 2009khairul anharPas encore d'évaluation
- Antioxidant Activity of Selected Phenols and Herbs Used in Diets For Medical ConditionsDocument9 pagesAntioxidant Activity of Selected Phenols and Herbs Used in Diets For Medical ConditionsJimmy C K ChoiPas encore d'évaluation
- Antioxidant and Memory Enhancing Effects of Purple Sweet Potato Anthocyanin and Cordyceps Mushroom ExtractDocument5 pagesAntioxidant and Memory Enhancing Effects of Purple Sweet Potato Anthocyanin and Cordyceps Mushroom Extracthoabk1510Pas encore d'évaluation
- 11 Vol.1 12 Ijpsr Paper 4Document7 pages11 Vol.1 12 Ijpsr Paper 4kerta100% (1)
- Investigation On The Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera Indica L.)Document4 pagesInvestigation On The Antioxidant Activity of Leaves, Peels, Stems Bark, and Kernel of Mango (Mangifera Indica L.)eliezer999Pas encore d'évaluation
- Determination of Primary and Functional Metabolites of Salvia Argentea and Evaluation of Its Leaves and Roots AntioxidanDocument6 pagesDetermination of Primary and Functional Metabolites of Salvia Argentea and Evaluation of Its Leaves and Roots Antioxidanام محمدPas encore d'évaluation
- HPTLC R PDocument8 pagesHPTLC R PDrSantosh TarkePas encore d'évaluation
- Non Specific and Specific Parameter Standardization of Banana Peel (Musa Paradisciata Sapientum) and Andrographis PaniculataDocument7 pagesNon Specific and Specific Parameter Standardization of Banana Peel (Musa Paradisciata Sapientum) and Andrographis PaniculataDinata KusumaPas encore d'évaluation
- 2387 4622 1 SM PDFDocument8 pages2387 4622 1 SM PDFMd. Hasanur RahmanPas encore d'évaluation
- Antibacterial and Antioxidant Activity of Parmotrema Reticulatum Obtained F PDFDocument5 pagesAntibacterial and Antioxidant Activity of Parmotrema Reticulatum Obtained F PDFIrin TandelPas encore d'évaluation
- Tea and Herbal InfusionsDocument10 pagesTea and Herbal InfusionsNada PetrovićPas encore d'évaluation
- Characteristics of Cemcem (Spondiaz Pinnata L.F. Kurz) Instant PowderDocument14 pagesCharacteristics of Cemcem (Spondiaz Pinnata L.F. Kurz) Instant Powdersgd 2Pas encore d'évaluation
- Anti Microbial 9Document5 pagesAnti Microbial 9AyoeYoeRamndaniPas encore d'évaluation
- Food Chemistry: Geetha Samak, Revathi P. Shenoy, S.M. Manjunatha, K.S. VinayakDocument4 pagesFood Chemistry: Geetha Samak, Revathi P. Shenoy, S.M. Manjunatha, K.S. VinayakdzenitaPas encore d'évaluation
- Vermaetal 2019Document11 pagesVermaetal 2019Rosa RosmawatiPas encore d'évaluation
- Determination of Chloride Ion Concentration by GravimetryDocument2 pagesDetermination of Chloride Ion Concentration by GravimetryMaam Jesecca JeckPas encore d'évaluation
- Rev MSDS Arjuna Floor Klein LemonDocument1 pageRev MSDS Arjuna Floor Klein Lemonhanks ardianPas encore d'évaluation
- Carbon and Its Compounds Class 10 Notes Science Chapter 4 - Learn CBSEDocument1 pageCarbon and Its Compounds Class 10 Notes Science Chapter 4 - Learn CBSEArnav KumarPas encore d'évaluation
- C) 2-Methyl-2-Propanol and Isobutyl AlcoholDocument4 pagesC) 2-Methyl-2-Propanol and Isobutyl AlcoholZia RathorePas encore d'évaluation
- CHEMICAL INVESTIGATION AND EVALUATION OF ANTIMICROBIAL ACTIVITY OF TRICHODESMA EHRENBERGII SCHWEINF. EX BOISS.GROWING WIDELY IN GEBEL ELBA, EGYPT Amani M. D. El-Mesallamy, Fatma A. Ahmed, Mohammed H. Elhaw , Taha A. Ibrahim*Document6 pagesCHEMICAL INVESTIGATION AND EVALUATION OF ANTIMICROBIAL ACTIVITY OF TRICHODESMA EHRENBERGII SCHWEINF. EX BOISS.GROWING WIDELY IN GEBEL ELBA, EGYPT Amani M. D. El-Mesallamy, Fatma A. Ahmed, Mohammed H. Elhaw , Taha A. Ibrahim*iajpsPas encore d'évaluation
- A+ Blog - Class-9-First Bell 2.0-Chemistry-Chapter-2-Science Diary-Class-13 - (Em)Document3 pagesA+ Blog - Class-9-First Bell 2.0-Chemistry-Chapter-2-Science Diary-Class-13 - (Em)Shefeena muneerPas encore d'évaluation
- 18章 PDFDocument41 pages18章 PDF台師大蔡銘揚Pas encore d'évaluation
- Beatty Secondary Prelim 2021 ChemistryDocument45 pagesBeatty Secondary Prelim 2021 ChemistryAlexisPas encore d'évaluation
- Brand, N. W., Butt, C. R. M., Elias, M. 1998. Nickel Laterites: Classification and Features. AGSO Journal of Australian Geology & Geophysics, 17 (4), 81-88Document8 pagesBrand, N. W., Butt, C. R. M., Elias, M. 1998. Nickel Laterites: Classification and Features. AGSO Journal of Australian Geology & Geophysics, 17 (4), 81-88Nabila SalehPas encore d'évaluation
- The Ideal Soil Chart (Agricola's Best Guess V 2.0 January 2014)Document1 pageThe Ideal Soil Chart (Agricola's Best Guess V 2.0 January 2014)Sultan Ahmad KhanPas encore d'évaluation
- Restoration and Conservation of Works of Art Made of StoneDocument68 pagesRestoration and Conservation of Works of Art Made of StoneCrisencio M. PanerPas encore d'évaluation
- Titration QuestionsDocument4 pagesTitration QuestionsZeeshan AhmadPas encore d'évaluation
- SQP 20 Sets ChemistryDocument144 pagesSQP 20 Sets Chemistrypoornima9739100% (1)
- Activity 1 - Formula Writing and Naming Inorganic CompoundsDocument5 pagesActivity 1 - Formula Writing and Naming Inorganic CompoundsPrecious Mae Cuerquis Barbosa0% (1)
- Chapter 7Document30 pagesChapter 7Apichat Junsod100% (4)
- ChemistryDocument13 pagesChemistrymayaPas encore d'évaluation
- 415 Chapter 1 - Final PDFDocument25 pages415 Chapter 1 - Final PDFFelipe FonsecaPas encore d'évaluation
- Physico-Chemical and Ecotoxicological Characterization of Slaughterhouse Wastewater Resulting From Green Line SlaughterDocument12 pagesPhysico-Chemical and Ecotoxicological Characterization of Slaughterhouse Wastewater Resulting From Green Line SlaughterPetronilo SuarezPas encore d'évaluation
- Indian Institute of Technology Jammu: Department of Chemical EngineeringDocument9 pagesIndian Institute of Technology Jammu: Department of Chemical EngineeringPiyush VermaPas encore d'évaluation
- Chemistry 30AP Electrochemistry Workbook: Net Ionic EquationsDocument27 pagesChemistry 30AP Electrochemistry Workbook: Net Ionic EquationsDayanul AlamPas encore d'évaluation
- Carbohydrates TestsDocument3 pagesCarbohydrates TestsKings PridePas encore d'évaluation
- 8 - Citric-Acid-Monohydrate EP 10Document2 pages8 - Citric-Acid-Monohydrate EP 10asmae.labindusPas encore d'évaluation
- Total Synthesis of ErmophilaneDocument4 pagesTotal Synthesis of ErmophilanePrabodh SatyalPas encore d'évaluation
- Mineral Fuels, Mineral Oils and Products of Their Distillation Bituminous Substances Mineral WaxesDocument3 pagesMineral Fuels, Mineral Oils and Products of Their Distillation Bituminous Substances Mineral WaxesSakib Ex-rccPas encore d'évaluation