Académique Documents
Professionnel Documents
Culture Documents
Unit 1 Target Sheets
Transféré par
xraylembsy0 évaluation0% ont trouvé ce document utile (0 vote)
118 vues7 pagesunit 1 chemistry edexcel sylabus
Copyright
© Attribution Non-Commercial (BY-NC)
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentunit 1 chemistry edexcel sylabus
Droits d'auteur :
Attribution Non-Commercial (BY-NC)
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
118 vues7 pagesUnit 1 Target Sheets
Transféré par
xraylembsyunit 1 chemistry edexcel sylabus
Droits d'auteur :
Attribution Non-Commercial (BY-NC)
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 7
Edexcel AS Chemistry
Target sheets
pages Chapter 1.1 Formulae, equations and amount of At the I have I have
substance start… studied.. revised…
10-11 I understand the terms atom, element, ion,
molecule, compound, empirical & molecular 1 2 3 1 2 3 1 2 3
formula (1.3a)
12-13 I can write balanced equations (full & ionic) for
simple reactions, including the use of state symbols 1 2 3 1 2 3 1 2 3
(1.3b)
14-15 I understand the terms relative atomic mass,
16-17 amount of substance, molar mass & parts per 1 2 3 1 2 3 1 2 3
million (ppm) (1.3c)
24-25 I can calculate the amount of substance in a
1 2 3 1 2 3 1 2 3
solution of known concentration (1.3d)
18-19 I can use chemical equations to calculate reacting
masses & vice versa using the concepts of amount 1 2 3 1 2 3 1 2 3
of substance and molar mass(1.3e)
20-21 I can use chemical equations to calculate volumes
of gases & vice versa using the concepts of
1 2 3 1 2 3 1 2 3
amount of substance and molar volume of gases
(1.3f)
30-31 I can use chemical equations & experimental
results to deduce percentage yields & atom
1 2 3 1 2 3 1 2 3
economies in laboratory and industrial processes
and understand why they are important(1.3g)
16-17 I understand, and can carry out, calculations using
1 2 3 1 2 3 1 2 3
the Avogadro constant (1.3h)
22-23 I can analyse & evaluate results obtained from
28-29 finding a formula or confirming an equation by
experiment, e.g. the reaction of lithium with water 1 2 3 1 2 3 1 2 3
and deducing the equation from the amounts in
moles of lithium and hydrogen (1.3i)
30-31 I can make a salt & calculate the percentage yield
of product, e.g. preparation of a double salt
1 2 3 1 2 3 1 2 3
(ammonium iron (II) sulphate from iron, ammonia
and sulphuric acid) (1.3j)
28-29 I can carry out & interpret results of simple test tube
reactions, such as replacements, reactions of
acids, precipitations, to relate the observations to 1 2 3 1 2 3 1 2 3
the state symbols used in equations and to practise
writing full and ionic equations (1.3k)
pages Chapter 1.2 Energetics At the I have I have
start… studied.. revised…
32-33 I understand the term enthalpy change, ΔH (1.4a)
1 2 3 1 2 3 1 2 3
34-35
34-35 I can construct simple enthalpy level diagrams
1 2 3 1 2 3 1 2 3
showing the enthalpy change (1.4b)
34-35 I can recall the signs for ΔH exothermic and
endothermic reactions, eg illustrated by the use of
1 2 3 1 2 3 1 2 3
exo- and endothermic reactions in hot and cold
packs (1.4c)
36-39 I know the definitions of standard enthalpy changes
of reaction, formation, combustion, neutralization &
atomization and can use experimental data to 1 2 3 1 2 3 1 2 3
calculate energy transferred in a reaction and
hence the enthalpy change of the reaction (1.4d)
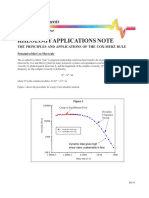
42-45 I know Hess's Law & can apply this to calculating
enthalpy changes of reaction from data provided,
selected from a table of data or obtained from
1 2 3 1 2 3 1 2 3
experiments. I understand why standard data is
necessary to carry out calculations of this type
(1.4e)
36-39 I can evaluate the results obtained from
46-47 experiments using the expression: energy
transferred in joules = mass x specific heat
1 2 3 1 2 3 1 2 3
capacity x temp change. I can comment on sources
of error and assumptions made in the experiments
(1.4f)
48-49 I understand the terms bond enthalpy and mean
bond enthalpy, and can use bond enthalpies in
1 2 3 1 2 3 1 2 3
Hess cycle calculations and recognise their
limitations (1.4g)
48-49 I understand that bond enthalpy data gives some
indication about which bond will break first in a
reaction, how easy or difficult it is and therefore 1 2 3 1 2 3 1 2 3
how rapidly a reaction will take place at room
temperature (1.4g)
pages Chapter 1.3 Atomic structure and the periodic table At the I have I have
start… studied.. revised…
52-53 I know the definitions of relative atomic mass,
relative isotopic mass & relative molecular mass
1 2 3 1 2 3 1 2 3
and understand that they are measured relative to
1/12 mass 12C atom (1.5a)
52-53 I understand the basic principles of a mass
spectrometer & can interpret data to i) determine
isotopic comp of a sample of an element, e.g.
polonium 1 2 3 1 2 3 1 2 3
ii) deduce relative atomic mass of an element
iii) measure relative molecular mass of a
compound(1.5b)
56-59 I can describe some uses of mass spectrometers,
e.g. in radioactive dating, in space research, in
sport to detect the use of anabolic steroids, in the
1 2 3 1 2 3 1 2 3
pharmaceutical industry to provide and identifier for
compounds synthesised for possible identification
as drugs(1.5c)
62-63 I understand the definition of ionization energies of
gaseous atoms –and that they are endothermic 1 2 3 1 2 3 1 2 3
processes(1.5d)
62-63 I can recall ideas about electronic structure
developed from i) an understanding that
successive ionization energies provide evidence
for the existence of quantum shells and the group
1 2 3 1 2 3 1 2 3
to which the elements belong,
ii) an understanding that the first ionization energy
of successive elements provides evidence for
electron sub shells(1.5e)
66-67 I can describe the shapes of electron density plots
1 2 3 1 2 3 1 2 3
(or maps) for s and p orbitals(1.5f)
64-65 I can predict electronic structure and configuration
of atoms of the elements from hydrogen to krypton
inclusive using 1s…notation and electron in-boxes 1 2 3 1 2 3 1 2 3
notation (recall electrons populate orbits singly
before pairing up) (1.5g)
68-73 I understand that electronic structure determines
1 2 3 1 2 3 1 2 3
the chemical properties of an element(1.5h)
68-73 I know that the periodic table is divided into blocks
1 2 3 1 2 3 1 2 3
such as s, p and d(1.5i)
74-77 I can represent data for the elements in graphical
form for elements 1 to 36 and use this to explain 1 2 3 1 2 3 1 2 3
the meaning of the term ‘periodic property’(1.5j)
74-77 I can explain trends from periods 2 & 3 i) melting
temp of elements based on given data using the
structure and the bonding between the atoms or
molecules of the element ii) ionization energy
1 2 3 1 2 3 1 2 3
based on given data or recall of the shapes of the
plots of ionisation energy versus atomic number
using ideas of electronic structure and the way that
electron energy levels vary across the period(1.5k)
pages Chapter 1.4 Bonding At the I have I have
start… studied.. revised…
1 ionic bonding
78-79 I can recall & interpret evidence for the existence of
82-83 ions by reference to the physical properties of ionic
compounds, electron density maps & migration of 1 2 3 1 2 3 1 2 3
ions, e.g. electrolysis of aqueous copper chromate
(VI) (1.6.1a)
78-79 I can describe the formation of ions in terms of
1 2 3 1 2 3 1 2 3
electron loss or gain (1.6.1b)
78-79 I can draw electron configuration diagrams of
cations and anions using dots or crosses to 1 2 3 1 2 3 1 2 3
represent electrons (1.6.1c)
80-81 I can describe ionic crystals as giant lattices of ions
1 2 3 1 2 3 1 2 3
(1.6.1d)
78-79 I can describe ionic bonding as the result of strong
1 2 3 1 2 3 1 2 3
net electronic attraction between ions (1.6.1e)
80-81 I can recall trends in ionic radii down the group and
for a set of isoelectronic ions , e.g. N3- to Al3+ 1 2 3 1 2 3 1 2 3
(1.6.1f)
? I can recall the stages in the formation of a solid
ionic crystal from its elements & know that this
1 2 3 1 2 3 1 2 3
leads to a measure value for the lattice energy
(1.6.1g)
84-85 I can test the ionic model for ionic bonding of a
particular compound by a comparison of lattice
energies obtained from the experimental values in 1 2 3 1 2 3 1 2 3
Born-Haber cycles, with provided values calculated
from electrostatic theory (1.6.1h)
86-87 I can explain the meaning of the term polarization
1 2 3 1 2 3 1 2 3
as applied to ions (1.6.1i)
86-87 I understand that the polarizing power of a cation
depends on its radius and charge, and
1 2 3 1 2 3 1 2 3
polarizaribility of an anion depends on its size
(1.6.1j)
86-87 I understand that the polarization of anions by
cations leads to some covalency in an ionic bond,
1 2 3 1 2 3 1 2 3
based on evidence from Born-Haber cycles
(1.6.1k)
84-85 I can use values calculated for standard heats of
formation based on Born-Haber cycles to explain
why particular ionic compounds exist, eg the 1 2 3 1 2 3 1 2 3
relative stability of MgCl2 over MgCl or MgCl3 and
NaCl over NaCl2 (1.6.1l)
2 covalent bonding
90-91 I can demonstrate an understanding that covalent
bonding is strong & arises from the electrostatic
attraction between the nucleus & electrons which
are between nuclei, based on the evidence: 1 2 3 1 2 3 1 2 3
i ) the physical properties of giant atomic structures
ii) electron density maps for simple molecules
(1.6.2a)
88-91 I can draw electron configuration diagrams for
simple covalently bonded molecules including
those with multiple bonds and dative covalent 1 2 3 1 2 3 1 2 3
bonds, using dots or crosses to represent electrons
(1.6.2b)
3 metallic bonding
92-93 I understand that metals consist of giant lattices of
1 2 3 1 2 3 1 2 3
metal ions in a sea of delocalised electrons (1.6.3a)
92-93 I can describe metallic bonding as the strong
attraction between metal ions and the sea of 1 2 3 1 2 3 1 2 3
delocalised electrons (1.6.3b)
92-93 I can use the models in 1.6.3a and 1.6.3b to
interpret simple properties of metals e.g. 1 2 3 1 2 3 1 2 3
conductivity & melting temperatures (1.6.3c)
pages Chapter 1.5 Introductory organic chemistry At the I have I have
start… studied.. revised…
100-103 I understand that a series of organic compounds is
characterised by a general formula with one or 1 2 3 1 2 3 1 2 3
more functional groups (1.7.1a)
100-103 I can apply the rules of IUPAC nomenclature to
organic compounds and draw these compounds,
1 2 3 1 2 3 1 2 3
as I encounter them, using structural, displayed &
skeletal formulae (1.7.1b)
94-99 I appreciate the difference between hazard and risk
1 2 3 1 2 3 1 2 3
(1.7.1c)
94-99 I understand the hazards associated with organic
compounds and why it is necessary to carry out
1 2 3 1 2 3 1 2 3
risk assessments when dealing with potentially
hazardous materials (1.7.1d)
94-99 I can suggest ways that risk can be reduced and
reactions can be carried out safely by:
i) working on a smaller scale
ii) taking specific precautions or using
1 2 3 1 2 3 1 2 3
alternative techniques depending on
properties of substances involved
carrying out reaction using alternative method that
involves less hazardous substances (1.7.1d)
pages Chapter 1.6 The Alkanes At the I have I have
start… studied.. revised…
104-105 I can state the general formula of alkanes and
understand that they are saturated hydrocarbons 1 2 3 1 2 3 1 2 3
which contain single bonds only (1.7.2a)
106-107 I can explain the existence of structural isomers
1 2 3 1 2 3 1 2 3
using alkanes (up to C5) as examples (1.7.2b)
108-113 I know that alkanes are used as fuels and obtained
from the fractional distillation, cracking and 1 2 3 1 2 3 1 2 3
reformation of crude oil (1.7.2c)
120-123 I can discuss the reasons for developing alternative
fuels in terms of sustainability & reducing emission 1 2 3 1 2 3 1 2 3
of CO2 & its relationship to climate change (1.7.2d)
114-117 I can describe the reactions of alkanes in terms of
118-119 combustion, substitution by chlorine showing the
mechanism of free radical substitution in terms of
initiation, propagation and termination, and using
1 2 3 1 2 3 1 2 3
curly half-arrows in the mechanism to show the
formation of free radicals in the initiation step using
a single dot to represent the unpaired electron
(1.7.2e)
Pages Chapter 1.7 The alkenes At the I have I have
start… studied.. revised…
124-128 I can state the general formula of alkenes and
understand that they are unsaturated hydrocarbons
1 2 3 1 2 3 1 2 3
with a carbon-carbon double bond which consists
of a σ and a π bond (1.7.3a)
124-128 I can explain E-Z isomerism (geometric /cis/trans
isomerism) in terms of restricted rotation around a
1 2 3 1 2 3 1 2 3
C=C double bond and the nature of substituents
on the carbon atoms (1.7.3b)
124-128 I can show an understanding of the E- Z- naming
system and why it is necessary to use this when 1 2 3 1 2 3 1 2 3
the cis- trans- naming system breaks down (1.7.3c)
129-131 I can describe the addition reactions of alkenes,
limited to:
i) addition of hydrogen with nickel catalyst
to form alkane
ii) addition of halogens to produce di-
substituted halogenoalkanes 1 2 3 1 2 3 1 2 3
iii) addition of hydrogen halides to produce
mono-substituted halogenoalkanes
iv) oxidation of the double bond by
potassium manganate (VII) to produce a
diol (1.7.3d)
132-133 I can describe the mechanism (including
diagrams), giving evidence where possible of:
i) electrophillic addition of bromine and
1 2 3 1 2 3 1 2 3
hydrogen bromide to ethane
ii) the electrophillic addition of hydrogen
bromide to propene (1.7.3e)
129-131 I can describe the test for presence of C=C using
bromine water and understand that the product is 1 2 3 1 2 3 1 2 3
the addition of OH and Br (1.7.3f)
134-135 I can describe addition polymerization of alkenes
and ID the repeat unit given the monomer and vice 1 2 3 1 2 3 1 2 3
versa (1.7.3g)
136-141 I can interpret given information about the uses of
energy and resources over the life-cycle of polymer
products to show how the use of renewable
1 2 3 1 2 3 1 2 3
resources, recycling and energy recovery can
contribute to more sustainable use of materials
(1.7.3h)
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Astm A510-20Document7 pagesAstm A510-20Ryan Zhang100% (2)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Causes of Long-Term Deflections of Large-Span PreDocument7 pagesCauses of Long-Term Deflections of Large-Span PreXavier NietoPas encore d'évaluation
- F-16c.1 Ginkgo Ginkgolic AcidDocument2 pagesF-16c.1 Ginkgo Ginkgolic AcidNarongchai PongpanPas encore d'évaluation
- 2 Texts About Petroleum For Reading ComprehensionDocument4 pages2 Texts About Petroleum For Reading ComprehensionEmmanuel Tartagal100% (2)
- Bab 07 - k2 - Struktur Dan EseiDocument30 pagesBab 07 - k2 - Struktur Dan EseiazharsarahPas encore d'évaluation
- A Review On Green Hydrogen Future of Green Hydrogen in IndiaDocument7 pagesA Review On Green Hydrogen Future of Green Hydrogen in IndiaSPE Baghdad sectionPas encore d'évaluation
- Biology Term2 Assessment Y10Document10 pagesBiology Term2 Assessment Y109hgdpyk96jPas encore d'évaluation
- Norton Lubricating OilDocument5 pagesNorton Lubricating OilRajesh BPas encore d'évaluation
- Inter 2 Physics Solved Mcqs Battle of Brains by Ambitious AcademyDocument7 pagesInter 2 Physics Solved Mcqs Battle of Brains by Ambitious AcademySaad WaseemPas encore d'évaluation
- Sülfür DöngüsüDocument15 pagesSülfür DöngüsüK Mehmet ŞensoyPas encore d'évaluation
- GT ThermodynamicsDocument22 pagesGT Thermodynamicsforzama100% (1)
- Design of Distillation Column Control SystemsDocument540 pagesDesign of Distillation Column Control SystemsAlessio Scarabelli100% (2)
- Writing and Balancing Chemical EquationsDocument31 pagesWriting and Balancing Chemical EquationsEthan-Dale BrownPas encore d'évaluation
- Group 14 Elements - C, Si, Ge,..Document58 pagesGroup 14 Elements - C, Si, Ge,..Looi Chui YeanPas encore d'évaluation
- Carbozinc 11 - TdsDocument2 pagesCarbozinc 11 - TdsAchraf BoudayaPas encore d'évaluation
- Syllabus PCB PDFDocument5 pagesSyllabus PCB PDFSujay HvPas encore d'évaluation
- 141 Ch.5.1.FrontEnd NFCDocument34 pages141 Ch.5.1.FrontEnd NFCplyx xyPas encore d'évaluation
- Chapter 5Document32 pagesChapter 5bmwk1200rPas encore d'évaluation
- Mil-B-007883 Brazing - Cancelled - See Cancellation NoteDocument26 pagesMil-B-007883 Brazing - Cancelled - See Cancellation NoteChris NenovPas encore d'évaluation
- Arcelor-Mittal Granite PVDFDocument1 pageArcelor-Mittal Granite PVDFZlatko KrsicPas encore d'évaluation
- 2,3,4,5 TetraphenylcyclopentadienoneDocument6 pages2,3,4,5 TetraphenylcyclopentadienoneDenisse Watt Cuarteros100% (2)
- Stereoisomer QuestionsDocument8 pagesStereoisomer QuestionsEden ChanPas encore d'évaluation
- MPS ITP Bare Pipe - PT Sari Dumai Oleo (Signed SAP)Document30 pagesMPS ITP Bare Pipe - PT Sari Dumai Oleo (Signed SAP)Alfian ImaduddinPas encore d'évaluation
- Antifungal Amides From Piper Tuberculatum, Vasquesdasilva2002Document7 pagesAntifungal Amides From Piper Tuberculatum, Vasquesdasilva2002jlavilaPas encore d'évaluation
- Acids and Bases WebquestDocument2 pagesAcids and Bases WebquestAngus DelaneyPas encore d'évaluation
- Chapter 29 Mechanical Separations 18Document101 pagesChapter 29 Mechanical Separations 18Ali HasSsanPas encore d'évaluation
- Stirling EngineDocument16 pagesStirling EngineSumitPrasadPas encore d'évaluation
- Gas Separator Sizing SpreadsheetDocument19 pagesGas Separator Sizing SpreadsheetSaid FerdjallahPas encore d'évaluation
- Material BalanceDocument78 pagesMaterial Balanceusman0553100% (4)
- Cox MerzDocument4 pagesCox MerzJohnPas encore d'évaluation