Académique Documents
Professionnel Documents
Culture Documents
Uses and Abuses of Sodium Bicarbonate in The Nicu
Transféré par
Trejito XDTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Uses and Abuses of Sodium Bicarbonate in The Nicu
Transféré par
Trejito XDDroits d'auteur :
Formats disponibles
Uses and abuses of sodium bicarbonate in the neonatal
intensive care unit
Amer N. Ammari, MD, and Karl F. Schulze, MD
Despite the lack of evidence for its effectiveness in the
treatment of acid-base disturbances in critically ill patients of
all ages, and despite several lines of evidence that indicate it
might be dangerous, bicarbonate therapy is used routinely in
many neonatal intensive care units. The justification for the
persistent use of this controversial therapy comes from a
variety of sources, many based more in philosophy than in
science. Clinicians contemplating the use of bicarbonate
therapy should consider what they expect the intervention to
accomplish and what evidence exists that their therapeutic
objective will be met. Without rigorous scientific support for
this therapy, it should be considered of unproven value and,
therefore, experimental. Curr Opin Pediatr 2002, 14:151156 2002
Lippincott Williams & Wilkins, Inc.
Division of Neonatal-Perinatal Medicine, Department of Pediatrics, College of
Physicians and Surgeons, Columbia University, New York, New York, USA.
Correspondence to Amer N. Ammari, MD, Division of Neonatal-Perinatal Medicine,
Department of Pediatrics, College of Physicians and Surgeons, Columbia
University, 3959 Broadway, BHN 1201, New York, NY 10032, USA; e-mail:
ana2003@columbia.edu
Current Opinion in Pediatrics 2002, 14:151156
ISSN 10408703 2002 Lippincott Williams & Wilkins, Inc.
One of the few principles of acid-base regulation not
surrounded by controversy is that treatment of the primary cause of the acid-base disturbance should be the
first step [1,2,3,4]. Adequate oxygen delivery and carbon dioxide elimination should be securely established
[5], and excess intakes or losses of acids or bases should
be identified and controlled [6]. Once these issues have
been addressed, however, clinicians are often left with
refractory disturbances in acid-base physiology that seem
to call for prompt and aggressive intervention. The disturbance is typically excess H+ activity, and it is usually
accompanied by reduced concentrations of whole blood
buffer bases, ie, an increased base deficit. The therapy
usually considered is intravenous sodium bicarbonate.
Apart from those few situations where buffer base is lost
via renal or gastrointestinal routes, the efficacy of treatment with buffer base is notoriously controversial and
has been for decades, yet its general use continues as if
it were a proven standard of care in todays neonatal
intensive care units.
In the care of critically ill patients, clinicians often must
use indirect estimates of physiologic or biochemical variables to track important processes that cannot be measured directly. Nowhere is this limitation more central
than in the diagnosis and treatment of excess H+ activity
in the critically ill newborn infant. Although excessive
H+ is the fundamental disturbance in pathologic acidosis
[6], therapy is typically initiated and guided by changes
in other, indirectly related variables, such as the arterial
concentration of HCO3- and the base deficit. These variables are not only calculated rather than measured but
also derived from measurements made in the intravascular fluid compartment, a part of the body remote from
and, perhaps, temporally out of phase with the conditions at the site of the most serious disorder, the intracellular fluid.
In the typical clinical situation, easily obtainable, direct
(but not necessarily accurate [7,8]) measurements of arterial acid-base profile are used to infer the status of an
unmeasured but presumably highly correlated variable,
the buffering capacity of the whole blood buffer system.
Using this same arterial measurement, further assumptions are made about buffer systems (and H+ activity) in
the interstitial and intracellular compartments, independent systems that interact in complicated and poorly understood ways with the whole blood. Based on this indirect information, decisions are made about attempting to
151
152 Neonatology and perinatology
manipulate the whole, interrelated buffering system
with infusions of buffer base into its smallest compartment, the intravascular fluid. The most important limitation in the formulation of treatment of acid-base disturbances in newborns is that it is not certain what
changes in these compartments are desirable for specific
clinical syndromes or whether these changes can be
brought about reliably by intravascular infusion of bicarbonate. Given these conceptual limitations, the paucity
of systematic studies of this intervention, and the fact
that solid training in acid-base chemistry is no longer
universal among clinicians, it is hardly surprising that the
use of bicarbonate in the management of neonatal acidosis is controversial. Indeed, most commentaries on this
topic include reference to the bicarbonate controversy
[1,2,3,4,911].
This article reviews the bicarbonate controversy as it pertains to some common syndromes encountered in the
care of critically ill neonates. Ideally, the article will motivate the reader to evaluate rigorously (1) the perceived
justifications for bicarbonate therapy, (2) the specific
physiologic changes (not merely a change in buffer base
concentration) that the intervention is expected to induce, and (3) the potential benefits and risks associated
with these physiologic changes. It is hoped that a better
understanding of the classical principles of acid-base
chemistry will persuade the practitioner to view the administration of bicarbonate in its larger context and to
recognize that bicarbonate therapy does considerably
more than alter the parameters of the whole blood bicarbonate buffer system. Such awareness may lead to
more judicious use of this poorly understood therapeutic
modality.
After a brief review of general acid-base physiology, several specific clinical syndromes are discussed in terms of
their acid-base physiology and their physiologic response
to bicarbonate therapy.
ing H+, hydrating CO2, and so forth) and physiologic
(pulmonary ventilation, renal acidification) processes.
Neonatologists track the success or failure of intrinsic
regulatory systems by measuring the difference between
this normal value and the prevailing levels of buffer base
in the patients whole blood. This difference is called the
base excess, with units of mEq/L, and may be positive,
indicating a relative excess of buffer base, or negative,
indicating a reduction in the whole blood buffer base
pool. Blood buffering is accomplished by both bicarbonate and nonbicarbonate buffers [12]. Nonbicarbonate
buffers include hemoglobin, oxyhemoglobin, phosphates, and proteins [6,12]. Total buffering capacity is
divided approximately equally between the two buffer
systems.
Changes in total blood buffer base cannot be estimated
accurately from bicarbonate levels alone, because the
Henderson-Hasselbalch equation computes bicarbonate
levels with reasonable accuracy despite the variation in
the dissociation constant (pK) of carbonic acid caused by
the nonaqueous physical and chemical properties of
whole blood and the methodologic limitations in the primary measurements. When the pK was calculated from
separate measurements of pH, partial pressure of carbon
dioxide (pCO2), and HCO3, it varied from 5.87 to 6.43
[13], and the variation was greatest in the sicker patients.
Even if HCO3 is estimated accurately, the base deficit
will be erroneous unless adjustments are made for nonbicarbonate buffering. Without correction for the hemoglobin concentration (which is seldom performed) or,
preferably, multipoint carbon dioxide titration data, the
base deficit measurement and the HCO3 measurement
contain the same information. Most estimates of acidbase balance in the neonatal intensive care unit do not
take these covariates into account. Finally, it must be
remembered that, although all buffers will equilibrate
according to the isohydric principle, the time to equilibrium within the body is variable, and transient differences will necessarily exist among body compartments.
Physiologic buffer systems
Acid-base physiology, once a leading area of scientific
inquiry, is currently one of the quieter areas of clinical
physiology. Trainees are now instructed in the practical
interpretation of blood gas analyses rather than the principles of acid-base chemistry. For an in-depth review of
the area, no better treatment of the field is available than
the classic textbook by Dr. Robert Winters [6].
Blood buffers
The whole blood buffer base is the sum of all conjugate
bases in whole blood. The normal value is 48 mEq/L
when the hemoglobin is 15 g/dL. In healthy humans and
in many disease states, regulation of this value appears to
be the major goal of acid-base control mechanisms. Control of acid-base balance involves both chemical (buffer-
The intravascular fluid compartment communicates
freely with the interstitium, which is roughly three times
the size of the intravascular fluid and is buffered primarily by bicarbonate. The intracellular fluid compartment
houses the metabolic machinery; ultimately, it is the mitochondrial pH that therapeutic manipulations of the
blood buffer base are used to protect [14,15]. The intracellular fluid is buffered by a mixture of phosphates,
protein, and bicarbonate. Experimental evidence suggests that 15 to 20% of an infusion of strong acid is
buffered by the blood, 30% by the interstitium, and 55%
by intracellular buffers [16], which means that clinicians
must monitor the quantitatively least important body
buffer system and infer indirectly what is happening in
the intracellular metabolic engine room.
Bicarbonate in the neonatal intensive care unit Ammari and Schulze
Clinical syndromes with acidosis:
respiratory distress syndrome
By far the most common disease leading to bicarbonate
therapy is respiratory distress syndrome. In the typical
case, a small infant is on substantial mechanical ventilatory assistance but remains persistently hypercapnic,
with a pH hovering at approximately 7.25 and a whole
blood buffer base deficit of 8 to 15 mEq/L. This condition is deceptively complex. Assume that the primary
disorder begins as a pure respiratory acidosis caused by
diminished alveolar ventilation. Metabolic carbon dioxide accumulates in the blood, is hydrated to H2CO3, and
dissociates to H+ and HCO3. Hydrogen ions are buffered by the nonbicarbonate buffers, and HCO3 is liberated into the solution. Were the intravascular compartment a closed system, as is the case in vitro, total buffer
base would remain unchanged, and there would be no
change in total body buffering because the HCO3
would replace the nonbicarbonate buffer base (mostly
hemoglobin) consumed in neutralizing the free H+.
However, in vivo, the HCO3 liberated when H+ is buffered by hemoglobin is not contained in a closed system
but diffuses out into the interstitium [12]. This process results in a reduced concentration of bicarbonate in
the total buffers of the blood and, all other things being
equal, an increase in the base deficit. Plasma bicarbonate
concentration at any given elevation of plasma pCO2
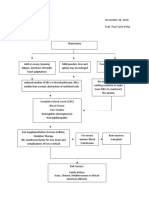
rises higher in vitro than in vivo. The in vivo curve represents steady-state measurements on blood from normal
subjects exposed to various known concentrations of carbon dioxide in the ambient atmosphere (Fig. 1).
Because the interstitial space is larger in the infant than
in the adult and larger still in the premature, sick infant,
the measured base deficit is significant even though the
carbon dioxide has been adequately buffered and no
metabolic acid has been produced. Although the limits of
the decrease in bicarbonate have not been defined in
premature infants, it is simplistic to assume that reduction in whole blood buffer base during transient changes
153
in pCO2 represents waxing and waning metabolic acidosis. Usually in this scenario, there is little or no gain in
strong acid, ketones, or lactic acid. The diffusion of
HCO3 into the enlarging extracellular fluid compartment of the premature, sick infant might be viewed as
dilution of HCO3, reminiscent of expansion acidosis.
Thus, a better way to think of this syndrome is as incompletely compensated respiratory acidosis. Ultimately, renal mechanisms will compensate for this
diluted buffering and replenish HCO3 in the extracellular fluid, but it is not clear how quickly this process is
accomplished.
Bicarbonate therapy for the acidosis of the
respiratory distress syndrome
It is tempting to say, No role, next question, when
asked for the indications for bicarbonate therapy during
management of respiratory distress syndrome; in the
practice of evidence-based medicine, this is the only
valid response. There are no systematic studies that testify to its benefits, either acutely or in terms of survival
[10,1725], and several that suggest significant, potentially negative accompaniments, such as enhanced hypercapnia with acute worsening of intracellular acidosis,
acute hyperosmolarity and longer-term salt loading, decreased cerebral blood flow, increased lactate production,
and increased venous carbon dioxide loading [24,2629].
Furthermore, the timing and direction of clinical changes
after administration of bicarbonate are variable [30]. But
clinicians have been reluctant to close the door completely on this venerable therapy. There are several
popular arguments in favor of treatment. First, bicarbonate therapy will move abnormal parameters of acid-base
status in the blood toward the normal range. What can be
wrong with that? A second, related argument is that myocardial, diaphragmatic, and neuronal function will be improved, response to circulating catecholamines will be
enhanced [31], and the infant will be better able to tolerate or self-correct the primary physiologic disturbance,
ie, diminished alveolar ventilation. The most common
Figure 1. Carbon dioxide titration curve
Carbon dioxide equilibration curves in response to addition
of carbon dioxide to plasma in vitro and in vivo. Reprinted
with permission [6].
30.0
+2.5
Blood [BE], mEq/l
Plasma [HCO3], mEq/l
32.0
In Vitro
28.0
In Vivo
26.0
50
60
70
80
Plasma PCO2, mm Hg
90
0
2.5
In Vitro
In Vivo
5.0
50
60
70
80
Plasma PCO2, mm Hg
90
154 Neonatology and perinatology
rebuttal to the first assertion is that numerous effective
therapies (most recently, permissive hypercapnia
[32,33]) and, indeed, numerous day-to-day, physiologic
adaptive responses of the body lead to out-of-range biochemical and physiologic measurements. Rebuttal to the
second argument is similar, ie, inherent physiologic adjustments represent signs of a larger wisdom at work;
variables such as myocardial contractile force, myocardial
oxygen consumption, and peripheral vascular tone are
downregulated to bring metabolic activity into line with
metabolic gas transport capabilities. Some have called
this a hibernation response, a period of reduced work
while the cells wait for better times [5]. There is little
question that cell functions that require energy are
downregulated by intracellular acidosis. The important
question, and the central issue in the bicarbonate controversy, is whether this intracellular acidosis can and
should be manipulated by intravascular infusions of bicarbonate buffer [14,20].
True metabolic acidosis caused by
diminished oxygen delivery
Despite intense and continuing study, the impact of reduced oxygen delivery to the peripheral tissues on the
energetics of the human tissues remains poorly understood. We tend to think of pO2 as an indicator of tissue
oxygenation, but, of course, pO2 is a measure of oxygen
concentration and not of oxygen delivery. To assess accurately the status of the patient, the clinician would like
to know whether oxygen consumption is limited by oxygen delivery and, if so, whether the change in cellular
energetics induced by hypoxia/ischemia can be predicted from changes in blood buffer base. Reduced oxygen availability ultimately leads to acidosis in all body
compartments, although the time to equilibrium differs
among the compartments. How does this metabolic acidosis develop? One prevailing model has oxygendeprived cells convert to anaerobic metabolism and liberate lactic acid which, in turn, consumes blood buffer
bases, most notably HCO3 in the extracellular fluid.
Actually, evidence suggests that lactic acid is not the
major source of H+ and that most of the metabolic H+
load derives from the H+ ion liberated in the hydrolysis
of adenosine triphosphate to adenosine diphosphate and
inorganic phosphate. Thus, the metabolic acidosis associated with tissue hypoxia parallels the reduction in the
energy stores or energy charge, ie, the adenosine
triphosphate/(adenosine diphosphate + inorganic phosphate ratio of the cell. When energy stores of the cells are
reduced, it can be assumed that compensatory mechanisms are exhausted and the cells are seriously compromised. Metabolic acidosis caused by tissue hypoxia is,
therefore, an ominous sign. The presence of metabolic
acidosis as estimated by reduced blood buffer base or pH
does not correlate well with measured levels of lactate.
Although lactate accompanies metabolic acidosis in the
premature infant with respiratory distress syndrome, its
presence in the blood serves only to identify a group of
critically ill patients who are at very high risk [34]. An
association between elevated lactate levels and high
mortality rates has been observed in adults [35], in children [36,37], and in both term [38] and preterm [34]
infants.
The cellular response to oxygen deprivation is to activate
negative feedback controllers of adenosine triphosphate
hydrolysis that interact with the calcium ion and slow the
metabolic engine. This adjustment underlies the myocardial hibernation and, presumably, the changes in
mental status, diaphragmatic contractility, and renal
function as well [39]. Another adaptive response is the
activation of the Na+/H+ pump, resulting in the extrusion
of H+ into the extracellular fluid, where it is buffered.
Finally, H+ is buffered by intracellular buffers, which
consist mostly of proteins; intracellular HCO3 is thought
to be approximately 10 mEq/L. When these adjustments
are overwhelmed by the acid load, intracellular pH falls
progressively.
Bicarbonate therapy for the acidosis of the
hypoxia and ischemia
Given the scheme outlined, is it reasonable to give
HCO3? Again, if we are guided by the principles of
evidence-based medical practice, bicarbonate therapy is
not indicated. Because hypoxic/lactic acidosis is much
more common in adults than in children, most of the
systematic experience with HCO3 therapy for this condition comes from adults. Everyone agrees that successful treatment of whatever process underlies the excess
intracellular production of H+ will lead to resolution of
the acidosis. Does buffering the blood, extracellular
fluid, and intracellular fluid either slow the production of
metabolic acid or facilitate its removal? Herein lies the
heart of the bicarbonate controversy. No observer believes that the evidence is conclusive [40,41,42,
43,44], so the argument revolves around how each
practitioner weighs the relevance of the model and the
physiologic data, keeping in mind that exact models of
human clinical syndromes are rare. Most reports favor
avoidance of bicarbonate therapy for lactic acidosis, particularly in association with septic shock. It is worth noting that, should HCO3 be introduced today as a new
drug for the treatment of acidosis, its approval could not
be supported on the basis of existing experimental data.
Resuscitation in the delivery room and
after a cardiac arrest
Insufficient oxygen delivery to the fetus before delivery
or as a consequence of cardiac arrest or severe bradycardia leads quickly to exhaustion of energy stores and global metabolic acidosis. Without systematic observations
concerning HCO3 treatment of neonates after cardiac
arrest, the clinician must again turn to studies of animals
Bicarbonate in the neonatal intensive care unit Ammari and Schulze
and adults for guidance. Early experience with bicarbonate therapy in lambs showed that the level of blood buffers could be raised by glucose and bicarbonate infusions
and that alkali therapy was accompanied by increased
survival and less brain injury [45]. These studies failed to
separate the effects of alkalinization from the change in
intravascular fluid volume. However, it was soon observed in adults that this improved acid-base profile in
blood was accompanied by increased intracellular acidosis, increased venous carbon dioxide loading, and no improvement in resuscitability [9]. It was also noted that
the important correlate of successful resuscitation was
improved coronary perfusion pressure and not pH [46
48]. Bicarbonate alone may not change and may worsen
myocardial perfusion [5,49]; thus, it is not recommended
as front line therapy for resuscitation of adults who experience cardiac arrest [46,50], although its use in certain
circumstances (after protracted periods of resuscitation,
preexisting metabolic acidosis, hyperkalemia) is thought
to be beneficial [51].
A review of the use of bicarbonate in neonatal resuscitation [11] concluded that the treatment is more likely to
lead to problems such as intracellular acidosis
[52,53,54] and hyperosmolality [28], than to facilitate
resuscitation. The guidelines from the American Heart
Association manual on Neonatal Advanced Life Support
[55] no longer recommend bicarbonate as front line
therapy, noting that there is no systematic evidence for
its efficacy.
Conclusions
Faced with an infant with reduced stores of blood buffer
base in the vascular space, the clinician should not attempt immediate replenishment. First, the limitations of
the actual measurement should be considered. Are the
numbers in the acid-base profile internally consistent
and consistent with the clinical condition and clinical
course of the patient? Are the samples representative?
Are there ways to address immediately the primary cause
of the acid-base disturbanceie, can alveolar ventilation
or oxygen transport be improved? Is there an unintended
addition of acid or loss of base? Once these issues have
been addressed, the neonatologist should articulate specific therapeutic objectives. One goal might be to reduce
the acidosis in the microenvironment surrounding essential energy-generating organelles and assist the cell in
restoring normal bioenergetics. Then the clinician
should ask why this change might be effected by bicarbonate infusion and whether the change would be certain to benefit the patient. If satisfied that available evidence suggests that meeting the desired objective is
possible and safe, action should be taken. If not, bicarbonate therapy should be avoided. Following this line of
reasoning is unlikely to stem the flow of this popular
treatment through todays ICUs but might force the
practitioner to confront the fact that treatment with bicarbonate is seldom consistent with principles of evi-
155
dence-based medical practice. Its use is better viewed as
an unplanned human experiment tinged with desperation. The resulting discomfiture among neonatologists
would be welcome.
References and recommended reading
Papers of particular interest, published within the annual period of review,
have been highlighted as:
Of special interest
Of outstanding interest
1
Ginsberg HG, Goldsmith JP: Controversies in neonatal resuscitation. Clin
Perinatol 1998, 25:115.
2
Cuhaci B, Lee J, Ahmed Z: Sodium bicarbonate controversy in lactic acidosis
[letter]. Chest 2000, 118:882884.
In this letter to the editor in response to [43], the authors argue that clinicians
should be cautious in discarding treatment options that are not harmful if appropriately used, especially if there are no alternatives and if their use is based on logical
explanation.
3
Bar-Joseph G: Is sodium bicarbonate therapy during cardiopulmonary resuscitation really detrimental? Crit Care Med 2000, 28:16931694.
4
Rosival V: Evaluating sodium bicarbonate controversy [letter]. Chest 2001,
119:16221623.
This letter to the editor was followed by a response from the authors of [2]. Here,
the author argued that the killer is the hydrogen cation, not the lactate anion. The
negative cardiovascular effect of decreased pH in human patients is most important because of its influence on the level of consciousness. The decreased pH level
seen in humans inhibits the activity of the pH-dependent glycolytic enzyme phosphofructokinase, resulting in the impaired use of glucose and resultant decreased
activity of the brain cells. In response, the authors of [2] argued that the patients
with severe lactic acidosis have a poor prognosis and generally do not live long. It
is hard to claim that they die of acidosis per se, and it is very difficult to isolate the
role of severe acidosis. They argue in favor of a better correlation between spinal
fluid pH and encephalopathy rather than arterial pH.
5
Kamel KS, Mazer CD: Effect of NaHCO3 on cardiac energy metabolism and
contractile function during hypoxemia. Crit Care Med 2001, 29:344350.
Winters RW: The Body Fluids in Pediatrics. Boston: Little Brown & Co.;
1973.
Hood I, Campbell EJM: Sounding Boards: is pK OK? N Engl J Med 1982,
306:864866.
Story DA, Poustie S: Agreement between two plasma bicarbonate assays in
critically ill patients. Anaesth Intensive Care 2000, 28:399402.
Weil MH, Trevino RP, Rackow EC: Sodium bicarbonate during CPR: does it
help or hinder? Chest 1985, 88:487.
10
Sinclair JC: Neonatal acidosis and respiratory distress syndrome in the preterm infant: role of early pH correction with bicarbonate. J Pediatr 1972,
81:11881189.
11
Hein HA: The use of sodium bicarbonate in neonatal resuscitation: help or
harm? Pediatrics 1993, 91:496497.
12 Adrogue HE, Adrogue HJ: Acid-base physiology. Respir Care 2001,
46:328341.
An excellent review of acid-base physiology with discussion of buffer systems,
hydrogen ion generation, consumption and excretion, intracellular regulation, and
the interaction of the pulmonary and renal systems in excretion of acids. There are
two components to the space of distribution of bicarbonate: an anatomic portion
and a nonanatomic one. The anatomic portion corresponds to the extracellular fluid
volume, and the nonanatomic portion is a theoretical space large enough to accommodate all the administered HCO3 unaccounted for by the anatomic boundaries. Approximately 30 minutes must elapse after bicarbonate infusion before the
clinical effect is judged.
13
Trenchard D, Noble MI, Guz A: Serum carbonic acid pK1 abnormalities in
patients with acid-base disturbances. Clin Sci 1967, 32:189200.
14
Siegel SR, Phelps DL, Leake RD, et al.: The effects of rapid infusion of hypertonic sodium bicarbonate in infants with respiratory distress. Pediatrics
1973, 51:651654.
15
Bonventre JV, Cheung JY: Effects of metabolic acidosis on viability of cells
exposed to anoxia. Am J Physiol 1985, 249:C149C159.
16
Winters RW, Dell RB: Regulation of acid-base equilibrium. In Physiological
Controls and Regulations. Edited by Yamamoto WS, Brobeck JR. Philadelphia: Saunders; 1965:3960.
17
Howell JH: Sodium bicarbonate in the perinatal setting-revisited. Clin Perinatol 1987, 14:807816.
156 Neonatology and perinatology
18
Steichen JJ, Kleinman LI: Studies in acid-base balance, I: effect of alkali
therapy in newborn dogs with mechanically fixed ventilation. J Pediatr 1977,
91:287291.
19
Prodhom LS: Rules of evidence applied to treatments of neonatal hypoxemia
and acidosis. Pediatrics 1968, 42:563564.
20
Sinclair JC, Engel K, Silverman WA: Early correction of hypoxemia and acidemia in infants of low birth weight: a controlled trial of oxygen breathing,
rapid alkali infusion, and assisted ventilation. Pediatrics 1968, 42:565589.
facet of medicine, care should be taken. The authors reviewed [52] on human
hepatocytes in both bicarbonate and nonbicarbonate buffers. There is a transient
initial decrease in intracellular pH after NaHCO3 administration that tends to correlate with the rise in pCO2, depending on the dose and rate of bicarbonate administration, the initial intracellular pH, cardiac output/ventilation and total CO2
clearance, and, most importantly, the presence of greater amounts of nonbicarbonate buffers (such as high hemoglobin concentration).
43 Forsythe SM, Schmidt GA: Sodium bicarbonate for the treatment of lactic
acidosis. Chest 2000, 117:260267.
This review of the adult literature answers the following questions: is a low pH bad?
Can sodium bicarbonate raise the pH in vivo? Does increasing the blood pH with
sodium bicarbonate have any salutary effects? Does sodium bicarbonate have
negative side effects? The authors do not give or advise bicarbonate infusion regardless of the pH in adults with lactic acidosis.
21
Van Vliet PK, Gupta JM: THAM v. sodium bicarbonate in idiopathic respiratory
distress syndrome. Arch Dis Child 1973, 48:249255.
22
Hobel CJ, Oh W, Hyvarinen MA, et al.: Early versus late treatment of neonatal
acidosis in low-birth-weight infants: relation to respiratory distress syndrome.
J Pediatr 1972, 81:11781187.
23
Baum JD, Robertson NR: Immediate effects of alkaline infusion in infants with
respiratory distress syndrome. J Pediatr 1975, 87:255261.
44
Arieff AI: Efficacy of buffers in the management of cardiac arrest. Crit Care
Med 1998, 26:13111313.
24
Rhodes PG, Hall RT, Hellerstein S: The effects of single infusion of hypertonic
sodium bicarbonate on body composition in neonates with acidosis. J Pediatr
1977, 90:789795.
45
Dawes GS, Jakobsen HN, Mott JC, et al.: The treatment of asphyxiated mature fetal lambs and rhesus monkeys with intravenous glucose and sodium
carbonate. J Physiol 1963, 169:167184.
25
Corbet AJ, Adams JM, Kenny JD, et al.: Controlled trial of bicarbonate therapy
in high-risk premature newborn infants. J Pediatr 1977, 91:771776.
46
Weisfeldt ML, Guerci AD: Sodium bicarbonate in CPR. JAMA 1991,
266:21292130.
26
Kravath RE, Aharon AS, Abal G, et al.: Clinically significant physiologic
changes from rapidly administered hypertonic solutions: acute osmol poisoning. Pediatrics 1970, 46:266275.
47
Bishop RL, Weisfeldt ML: Sodium bicarbonate administration during cardiac
arrest: effect on arterial pH, PCO2, and osmolality. JAMA 1976, 235:506
509.
27
Dell RB, Winters RW: Acid-base effects of hypertonic sodium bicarbonate
solution: a commentary. J Pediatr 1972, 80:681682.
48
Weil MH, Tang W: Cardiac arrest and sodium bicarbonate. Crit Care Med
1996, 24:547548.
28
Lou HC, Lassen NA, Fris-Hansen B: Decreased cerebral blood flow after
administration of sodium bicarbonate in the distressed newborn infant. Acta
Neurol Scand 1978, 57:239247.
49
Kette F, Weil MH, Gazmuri RJ: Buffer solutions may compromise cardiac
resuscitation by reducing coronary perfusion pressure. JAMA 1991,
266:21212126.
29
Kamel KS, Mazer CD: Effect of NaHCO3 on cardiac energy metabolism and
contractile function during hypoxemia. Crit Care Med 2001, 29:344350.
50
30
Adrogue HJ, Brensilver J, Cohen JJ, et al.: Influence of steady-state alterations
in acid-base equilibrium on the fate of administered bicarbonate in the dog. J
Clin Invest 1983, 71:867883.
Gambassi G, Carbonin P: Cardiopulmonary resuscitation: bicarbonate is of
no value. BMJ 1993, 307:627.
51
Bar-Joseph G, Weinberger T, Castel T, et al.: Response to repeated equal
doses of epinephrine during cardiopulmonary resuscitation in dogs. Ann
Emerg Med 2000, 35:310.
31
Campbell GS, Houle DB, Crisp NW, et al.: Depressed response to intravenous sympathomimetic agents in humans during acidosis. Dis Chest 1958,
33:1822.
32
Hickling KG, Joyce C: Permissive hypercapnia in ARDS and its effect on
tissue oxygenation. Acta Anesthesiol Scand Suppl 1995, 107:201208.
33
Tuxen DV: Permissive hypercapnic ventilation. Am J Respir Crit Care Med
1994, 150:870874.
34
Deshpande SA, Platt MP: Association between blood lactate and acid-base
status and mortality in ventilated babies. Arch Dis Child Fetal Neonatal Ed
1997, 76:F15F20.
35
Weil MH, Afifi AA: Experimental and clinical studies on lactate and pyruvate
as indicators of the severity of acute circulatory failure (shock). Circulation
1970, 41:9891001.
36
Charpie JR, Dekeon MK, Goldberg CS, et al.: Serial blood lactate measurements predict early outcome after neonatal repair or palliation for complex
congenital heart disease. J Thorac Cardiovasc Surg 2000, 120:7380.
37
Hatherill M, McIntyre AG, Wattie M, et al.: Early hyperlactataemia in critically
ill children. Intensive Care Med 2000, 26:314318.
38
Cheung P, Finer N: Plasma lactate concentration as a predictor of death in
neonates with severe hypoxemia requiring extracorporeal membrane oxygenation. J Pediatr 1994, 125:763768.
39
Yanos J, Wood LD, Davis K, et al.: The effect of respiratory and lactic acidosis
on diaphragm function. Am Rev Respir Dis 1993, 147:616619.
40
Adrogue HJ, Madias NE: Management of life-threatening acid-base disorders: first of two parts. N Engl J Med 1998, 338:2634.
41
Epstein SK, Singh N: Respiratory acidosis. Respir Care 2001, 46: 366383.
42 Cuhaci B, Lee J, Ahmed Z: Sodium bicarbonate and intracellular acidosis:
myth or reality?. Crit Care Med 2001, 29:10881090.
This article reviews adult studies of the use of sodium bicarbonate in treatment of
lactic acidosis and stresses the fact that this issue is not resolved and, as with any
Levraut J, Ginuti C, Ciebiera J-P, et al.: Initial effect of sodium bicarbonate on
intracellular pH depends on the extracellular nonbicarbonate buffering capacity. Crit Care Med 2001, 29:10331039.
Human hepatocytes were first perfused with pH 7 bicarbonate-buffered medium (5
mmol/L HCO3, 20 torr pCO2) containing no nonbicarbonate buffer, small
amounts of nonbicarbonate buffer, or large amounts of nonbicarbonate buffer. In
the second experiment, hepatocytes were placed in acidotic human blood (pH 7) at
three hematocrits (40%, 20%, and 5%), and the intracellular pH was measured
with a pH-sensitive fluorescent dye. The authors concluded that the effect of sodium bicarbonate on intracellular pH depends on changes in pCO2 in the medium
bathing the cells. Sodium bicarbonate may exacerbate cell acidosis under buffering
conditions close to those in vivo. Initial changes in the cell pH depend on the
extracellular nonbicarbonate buffering capacity.
52
53
Goldsmith DJ, Forni LG, Hilton PJ: Bicarbonate therapy and intracellular acidosis. Clin Sci (Colch) 1997, 93:593598.
54 Wyckoff MH, Perlman J, Niermeyer S: Medications during resuscitation: what
is the evidence? Semin Neonatol 2001, 6:251259.
This article discusses the use of sodium bicarbonate, naloxone hydrochloride, epinephrine, and volume expanders during delivery room resuscitation. Bicarbonate
functions as a physiologic buffer only in an open system in which carbon dioxide
can be transported to the lungs and eliminated. The article stressed the idea that
the investigations to determine the effects of sodium bicarbonate administration on
resuscitation have largely been uncontrolled, descriptive, adult human studies. Decreased cerebral blood flow, metabolic alkalosis, paradoxical tissue and intracellular hypercarbic acidosis, hyperosmolality, and intracellular shifts of potassium and
calcium were side effects noted.
55
Niermeyer S, Kattwinkel J, Van Reempts P, et al.: International guidelines for
neonatal resuscitation: an excerpt from the Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International
Consensus on Science. Contributors and Reviewers for the Neonatal
Resuscitation Guidelines. Pediatrics 2000, 106: http://www.pediatrics.
org/cgi/reprint/106/3/e29.
Vous aimerez peut-être aussi
- 2019 Article 1596Document9 pages2019 Article 1596Arina FitrianiPas encore d'évaluation
- Gases Arteriales - BE NEJMDocument10 pagesGases Arteriales - BE NEJMalexsr36Pas encore d'évaluation
- 2.1DisparadorMaterialartigoAmyL - Dzierba 20201125101652Document12 pages2.1DisparadorMaterialartigoAmyL - Dzierba 20201125101652João VitorPas encore d'évaluation
- Diagnostic Use of Base Excess in Acid-BaseDocument10 pagesDiagnostic Use of Base Excess in Acid-Basejcarl_20063003Pas encore d'évaluation
- The Use of Bioimpedance Spectroscopy To Guide Fluid Management in Patients Receiving DialysisDocument7 pagesThe Use of Bioimpedance Spectroscopy To Guide Fluid Management in Patients Receiving Dialysisnelbut2Pas encore d'évaluation
- A Balanced View of Balanced SolutionsDocument12 pagesA Balanced View of Balanced SolutionsManish VijayPas encore d'évaluation
- Acid-Base Analysis in The Operating Room - A Bedside Stewart ApproachDocument8 pagesAcid-Base Analysis in The Operating Room - A Bedside Stewart ApproachJulian SanchezPas encore d'évaluation
- An Overview of Hyperinsulinemic-Euglycemic Therapy in Calcium Channel Blocker and β-blocker OverdoseDocument30 pagesAn Overview of Hyperinsulinemic-Euglycemic Therapy in Calcium Channel Blocker and β-blocker OverdoseFitra AlfaniPas encore d'évaluation
- 1 s2.0 S1051227622001881 MainDocument17 pages1 s2.0 S1051227622001881 MainsdaaPas encore d'évaluation
- Analysis of Acid-Base Disorders in An ICU Cohort UDocument13 pagesAnalysis of Acid-Base Disorders in An ICU Cohort UJack FruitPas encore d'évaluation
- Clase 7 Articulo Ejemplo 11-03-15Document8 pagesClase 7 Articulo Ejemplo 11-03-15Betzi HannlyPas encore d'évaluation
- Hipertensión IntraabdominalDocument24 pagesHipertensión IntraabdominalAnonymous QNRRaQUPas encore d'évaluation
- Glicerofosfato de Sodio Vs InogarnicoDocument8 pagesGlicerofosfato de Sodio Vs InogarnicojhonPas encore d'évaluation
- New Acid BaeDocument6 pagesNew Acid BaeEnrique MartinezPas encore d'évaluation
- Intensive Care 1Document6 pagesIntensive Care 1raitchuPas encore d'évaluation
- Nacl Kad 2Document12 pagesNacl Kad 2Ida LemonPas encore d'évaluation
- Acid-Base Disorders - The American College of Chest PhysiciansDocument9 pagesAcid-Base Disorders - The American College of Chest PhysiciansGustavo MartinezPas encore d'évaluation
- Journal Reading: Resuscitation FluidsDocument26 pagesJournal Reading: Resuscitation FluidsKikyPas encore d'évaluation
- Acid-Base and Electrolyte Teaching Case Assessing Acid-Base Status: Physiologic Versus Physicochemical ApproachDocument10 pagesAcid-Base and Electrolyte Teaching Case Assessing Acid-Base Status: Physiologic Versus Physicochemical Approachgasman2003Pas encore d'évaluation
- Blood Conservation in Pediatric Cardiac SurgeryDocument5 pagesBlood Conservation in Pediatric Cardiac Surgerymohanakrishna007Pas encore d'évaluation
- An Innovative Approach to Understanding and Treating Cancer: Targeting pH: From Etiopathogenesis to New Therapeutic AvenuesD'EverandAn Innovative Approach to Understanding and Treating Cancer: Targeting pH: From Etiopathogenesis to New Therapeutic AvenuesPas encore d'évaluation
- Bicarbonate in Diabetic Ketoacidosis - A SystematiDocument13 pagesBicarbonate in Diabetic Ketoacidosis - A SystematiNurul Kamilah SadliPas encore d'évaluation
- 16 PDF PDFDocument5 pages16 PDF PDFAlfan KurniantoPas encore d'évaluation
- Gluud 2016Document15 pagesGluud 2016FitriaPas encore d'évaluation
- Albumin DissertationDocument6 pagesAlbumin DissertationPaperWriterServicesCanada100% (1)
- Fluid and Dietary Restriction's Effi Cacy On Chronic Kidney Disease Patients in HemodialysisDocument9 pagesFluid and Dietary Restriction's Effi Cacy On Chronic Kidney Disease Patients in HemodialysisfitriPas encore d'évaluation
- Zhang 2018Document8 pagesZhang 2018luisdespinozahPas encore d'évaluation
- ArtifDocument10 pagesArtifIstván PortörőPas encore d'évaluation
- CRRT Acid Base BalanceDocument10 pagesCRRT Acid Base BalanceJAMUNAPas encore d'évaluation
- Fluid Overload in Peritoneal Dialysis PatientsDocument11 pagesFluid Overload in Peritoneal Dialysis PatientsHaryonoPas encore d'évaluation
- Avoiding Common Errors in The Emergency Department-571-800Document230 pagesAvoiding Common Errors in The Emergency Department-571-800Hernando CastrillónPas encore d'évaluation
- GiftasupDocument50 pagesGiftasupFizza SiddiquiPas encore d'évaluation
- Transfusión en Pacientes PediatricosDocument14 pagesTransfusión en Pacientes PediatricosAndrea Abigail ChacónPas encore d'évaluation
- Common Sense Approach To Managing SepsisDocument12 pagesCommon Sense Approach To Managing SepsiszepedrocorreiaPas encore d'évaluation
- Hipoxemia PermisivaDocument10 pagesHipoxemia Permisivafrancia perezPas encore d'évaluation
- Updates On Medical Management of HyperkalemiaDocument8 pagesUpdates On Medical Management of Hyperkalemiapaulina naranjoPas encore d'évaluation
- Pathophysiology of Hypertrophic PyloricDocument9 pagesPathophysiology of Hypertrophic PyloricVașadi Razvan CristianPas encore d'évaluation
- Interpreting Abgs: The Basics: 2.0 Contact HoursDocument23 pagesInterpreting Abgs: The Basics: 2.0 Contact HoursThorgan HazardPas encore d'évaluation
- Management of Lifestyle Factors in Individuals With Cirrhosis: A Pragmatic ReviewDocument9 pagesManagement of Lifestyle Factors in Individuals With Cirrhosis: A Pragmatic ReviewsavitageraPas encore d'évaluation
- Review Article Use of Albumin: An UpdateDocument9 pagesReview Article Use of Albumin: An UpdateLicea Bco JosePas encore d'évaluation
- Relative Potency of Proton-Pump Inhibitors-ComparisonDocument13 pagesRelative Potency of Proton-Pump Inhibitors-ComparisonTonii SoberanisPas encore d'évaluation
- Ruth 2013Document17 pagesRuth 2013Paul SimononPas encore d'évaluation
- Hba1c StandarizaciónDocument13 pagesHba1c StandarizaciónAsesoria TecnicaPas encore d'évaluation
- Metabolic AcidosisDocument14 pagesMetabolic Acidosispara todoPas encore d'évaluation
- 述评 Human Albumin Infusion Strategy in Liver Cirrhosis Liberal or RestrictiveDocument3 pages述评 Human Albumin Infusion Strategy in Liver Cirrhosis Liberal or Restrictive倪沁赟Pas encore d'évaluation
- 2017-Water During FastingDocument8 pages2017-Water During Fastingpedro vargasPas encore d'évaluation
- Mergency Medicine, Second Edition: 60 Acid-Base DisordersDocument21 pagesMergency Medicine, Second Edition: 60 Acid-Base DisordersLukman NurfauziPas encore d'évaluation
- The Search For Disease-Modifying Agents in Decompensated Cirrhosis: From Drug Repurposing To Drug DiscoveryDocument17 pagesThe Search For Disease-Modifying Agents in Decompensated Cirrhosis: From Drug Repurposing To Drug Discoverymuhamad deniansyahPas encore d'évaluation
- Management of Chronic Hepatitis B - An Overview of Practice Guidelines For Primary Care ProvidersDocument16 pagesManagement of Chronic Hepatitis B - An Overview of Practice Guidelines For Primary Care Providersdanny17phPas encore d'évaluation
- Conditioning Chemotherapy Dose Adjustmentin Obesepatients Bubalo BBMT2014Document18 pagesConditioning Chemotherapy Dose Adjustmentin Obesepatients Bubalo BBMT2014Giang Dinh NguyenPas encore d'évaluation
- Mclain JCDocument7 pagesMclain JCkenjiPas encore d'évaluation
- Bic e Acidose LacticaDocument8 pagesBic e Acidose Lacticapriscilla duartePas encore d'évaluation
- Peptide and Protein DeliveryD'EverandPeptide and Protein DeliveryChris Van Der WalleÉvaluation : 2 sur 5 étoiles2/5 (1)
- Perioperativefluid Therapy: Denise Fantoni,, Andre C. ShihDocument12 pagesPerioperativefluid Therapy: Denise Fantoni,, Andre C. ShihDaniela BenavidesPas encore d'évaluation
- Oxygen Saturation and Outcomes in Preterm InfantsDocument17 pagesOxygen Saturation and Outcomes in Preterm InfantsSav GaPas encore d'évaluation
- Trastorno Acido BaseDocument15 pagesTrastorno Acido BaseCami Vergara Caro0% (1)
- Avoiding Common Problems Associated With Intravenous Fluid TherapyDocument13 pagesAvoiding Common Problems Associated With Intravenous Fluid TherapyDeepak AnPas encore d'évaluation
- Complementary and Alternative Medical Lab Testing Part 8: UrologyD'EverandComplementary and Alternative Medical Lab Testing Part 8: UrologyÉvaluation : 3 sur 5 étoiles3/5 (1)
- Antihypertensives in HDDocument6 pagesAntihypertensives in HDamanyPas encore d'évaluation
- Chapter 23Document171 pagesChapter 23Arpit PradhanPas encore d'évaluation
- Final Practical Research 2Document29 pagesFinal Practical Research 2Irene Javier75% (4)
- ADVIA 2120i Technology2012-160208 - 127086901 - 6Document61 pagesADVIA 2120i Technology2012-160208 - 127086901 - 6Олександр100% (3)
- Latihan Soal Eritrosit, Plasma Dan HBDocument20 pagesLatihan Soal Eritrosit, Plasma Dan HBHayckal Hasanal MPas encore d'évaluation
- USMLE 1 Hematology BookDocument368 pagesUSMLE 1 Hematology BookPRINCEPas encore d'évaluation
- Human Performance and Limitations PDFDocument163 pagesHuman Performance and Limitations PDFJustinPas encore d'évaluation
- BloodDocument27 pagesBloodRagda AhmedPas encore d'évaluation
- Part 2 Blood 1.Pptx Biology 22Document5 pagesPart 2 Blood 1.Pptx Biology 22RekaPas encore d'évaluation
- Concept MapDocument21 pagesConcept MapMaila Lariosa100% (1)
- Model Answers Quiz 2Document5 pagesModel Answers Quiz 2Raju RajaPas encore d'évaluation
- Proteins SchematicDocument9 pagesProteins SchematicRuchie Ann Pono BaraquilPas encore d'évaluation
- Concept MapDocument2 pagesConcept MapcheskaotreraPas encore d'évaluation
- HypoxiaDocument15 pagesHypoxiaاحمد حيدر يونس مهديPas encore d'évaluation
- Test Bank For Phlebotomy Worktext and Procedures Manual 3rd Edition WarekoisDocument9 pagesTest Bank For Phlebotomy Worktext and Procedures Manual 3rd Edition Warekoisanthonyelliscnjswramob100% (20)
- Physiology, Oxygen Transport: April 2019Document5 pagesPhysiology, Oxygen Transport: April 2019Soha SonaPas encore d'évaluation
- Nursing Care Management of Patients With Anemia - 01Document38 pagesNursing Care Management of Patients With Anemia - 01Sionur 00Pas encore d'évaluation
- Jus Tomat Untuk Menaikkan Kadar Hemoglobin Ibu HamilDocument8 pagesJus Tomat Untuk Menaikkan Kadar Hemoglobin Ibu HamilDiden Bima Fiya SirfefaPas encore d'évaluation
- MyoglobinDocument6 pagesMyoglobinChandra ReddyPas encore d'évaluation
- AARC Clinical Practice Guideline Blood Gas Analysis and Hemoximetry: 2013Document10 pagesAARC Clinical Practice Guideline Blood Gas Analysis and Hemoximetry: 2013jvalenciagPas encore d'évaluation
- Human Heredity Principles and Issues 10th Edition Michael Cummings Solutions ManualDocument38 pagesHuman Heredity Principles and Issues 10th Edition Michael Cummings Solutions Manualbufochauswu2w100% (16)
- Reviewer Legal MedDocument35 pagesReviewer Legal MedAndrea AmorPas encore d'évaluation
- 10th BIOLOGY PPT CH. NO. 8Document13 pages10th BIOLOGY PPT CH. NO. 8Aastha BorhadePas encore d'évaluation
- Abim Lab ValuesDocument4 pagesAbim Lab ValuesBell GatesPas encore d'évaluation
- 10.1515 - CCLM 2023 7044Document112 pages10.1515 - CCLM 2023 7044sfendri17Pas encore d'évaluation
- One Liner Biology EnglishDocument17 pagesOne Liner Biology Englishkrishna100% (1)
- 2014 Course BookDocument285 pages2014 Course BookWafaa AdamPas encore d'évaluation
- Role Play Bhs Inggris Kep 3Document2 pagesRole Play Bhs Inggris Kep 3widyaayukusumaPas encore d'évaluation
- Lab Workup: Practical Nursing Mohawk College 2014Document2 pagesLab Workup: Practical Nursing Mohawk College 2014Pawel PietruszczakPas encore d'évaluation
- Biology Notes HSCDocument116 pagesBiology Notes HSCYuki SuzukiPas encore d'évaluation
- Medsci 142: Biology For Biomedical Science: Human Organ SystemsDocument8 pagesMedsci 142: Biology For Biomedical Science: Human Organ SystemsthefrenchnerdPas encore d'évaluation