Académique Documents
Professionnel Documents
Culture Documents
Review On Pulse Therapy A Novel Approach in The Treatment of Pemphigus Vulgaris PDF
Transféré par
taniaDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Review On Pulse Therapy A Novel Approach in The Treatment of Pemphigus Vulgaris PDF
Transféré par
taniaDroits d'auteur :
Formats disponibles
JIAOMR
REVIEW ARTICLE
Review on Pulse Therapy: A Novel Approach in the Treatment of Pemphigus Vulgaris
Review on Pulse Therapy: A Novel Approach in
the Treatment of Pemphigus Vulgaris
1
Rachna Kaul 2Sanjay CJ
1
Senior Lecturer, Department of Oral Medicine and Radiology, Syamala Reddy Dental College Hospital and Research Center
Bengaluru, Karnataka, India
2
Senior Lecturer, Department of Oral Medicine and Radiology, Dayananda Sagar College of Dental Sciences, Bangaluru
Karnataka, India
Correspondence: Rachna Kaul, Senior Lecturer, Department of Oral Medicine and Radiology, Syamala Reddy Dental College
Hospital and Research Center, No 111/1, SGR Main Road, Munnekolala, Marathahalli, Bengaluru-560037, Karnataka, India
e-mail: rachnakaul2000@yahoo.com
ABSTRACT
Objective: Pemphigus vulgaris, a fatal autoimmune mucocutaneous disorder commonly seen involving the oral cavity, has since a long time remained
a topic of concern regarding its treatment modalities. Pulse therapy, introduced in 1984, employs high-dose corticosteroids along with certain
immunosuppressive agents and has gained wide popularity since the last three decades due to its advantage of minimizing the adverse effects of
conventional corticosteroid therapy. This article provides a detailed review about various studies conducted utilizing different regimens in pulse
therapy and the outcome of these studies.
Materials and methods: Information from various studies conducted over the last three decades was collected and a thorough analysis of the results
of these studies has been provided in this article.
Results: Extensive review of the existing data revealed that pulse therapy minimizes the adverse effects of long-term corticosteroid therapy, controls
the disease process faster and has quicker and long-lasting remission rates.
Conclusion: Pulse therapy appears to be successful in the treatment of pemphigus vulgaris. Various studies have proven the efficacy of pulse therapy
along with reduced side effects of conventional corticosteroid therapy. However, long-term follow-up is necessary to compare the incidence of malignancy
in patients receiving pulse doses of immunosuppressive agents with that in patients receiving continuous oral treatment.
Keywords: Pemphigus vulgaris, Corticosteroids, Pulse therapy, Cyclophosphamide, Azathioprine.
INTRODUCTION
Pemphigus vulgaris (PV) is a chronic autoimmune blistering
disease of skin and mucous membrane.1 The demonstration by
Beutner and Jordan of serum antibodies directed against the
intercellular substance of stratified squamous epithelium was the
first in a series of findings that suggested that PV may be an
autoimmune disease.2 It is a potentially fatal disease with a
mortality rate of 50% at 2 years, 100% at 5 years, if untreated.3
keratinocyte membrane antigen in a receptor-ligand fashion.6
Recently, nondesmoglein autoantibodies to cholinergic receptors
(human alpha 9 acetylcholine receptor) have also been found
capable of inducing clinical features of PV. These receptors are
thought to regulate adhesion and motility of keratinocytes. Their
activation, hence brings about disassembly of desmosomes and
acnatholysis. Also, there is evidence regarding the role of TNF-
and interleukin-6 (IL-6) as mediators in the blistering process of
PV.6
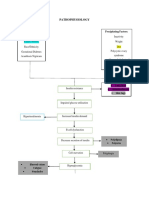
PATHOPHYSIOLOGY
PV is precipitated by IgG autoantibodies binding to desmoglein
3 (Dsg3) and desmoglein 1 (Dsg1).4,5 Antibodies to Dsg3 are
associated with mucosal dominant pemphigus, whereas the
presence of both Dsg3 and Dsg1 antibodies feature mucocutaneous
pemphigus with lesions both in oral cavity and on the skin. The
antigen-antibody reaction activates the complement system
resulting in acantholysis and fluid accumulation forming the
characteristic vesiculobullous lesions.6 The autoantibodies can be
detected in the epithelium using direct immunofluorescent staining
techniques, which reveal a characteristic spider-web distribution
of reaction products. Circulating antibodies are detectable in 80%90% of patients with PV, and the titer is generally corelated with
the level of clinical disease.2,7
The current body of research argues that acantholysis in PV
occurs as an active process resulting from intracellular signaling
triggered as a result of immunoglobulin (IgG) binding to the
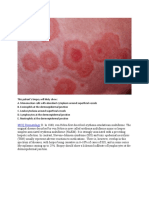
CLINICAL FEATURES
While there are various types of pemphigus such as vulgaris,
foliaceous, vegetans and paraneoplastic, 80% of all patients with
pemphigus have PV.7 It affects both sexes equally and is more
common among Ashkenazic jews. It is commonly seen in fifth to
seventh decade of life though some pediatric cases have also been
reported. Oral mucosa is affected in nearly all the cases and is the
site of first lesion in majority of cases.7
DIAGNOSIS
The formation of trauma-induced bullae is one of the diagnostic
tools: A positive Nikolskys sign or Asboe-Hansen sign
(Nikolskys sign II) are indicative of mucocutaneous bullous
disease, such as PV.3,7 However, the definitive diagnosis is not
solely dependent on positive examination since many other oral
vesiculo-bullous and ulcerative lesions like erosive lichen planus,
Journal of Indian Academy of Oral Medicine and Radiology, October-December 2010;22(4):211-214
211
Rachna Kaul, Sanjay CJ
pemphigoid and erythema multiforme have a similar clinical
appearance. Histological examination of PV on hematoxylin and
eosin staining reveals intraepithelial clefts or bullae, acantholysis
and a dense mononuclear lymphocytic infiltration. A smear taken
from new lesion can also be used to identify acantholytic cells
called Tzanck cells, which are indicative of PV. However, the
identification of Tzanck cells is not diagnostic since these can
also be found in herpes simplex, carcinoma and transient
acantholytic dermatosis.7
TREATMENT
The initial aim of the treatment of PV is to control the disease and
induce disease remission.6,8 In a study conducted among various
Asian physicians treating cases of PV, the expert opinion regarding
the definition of disease control was the development of no new
lesions and healing most of the previous lesions.9 This should be
followed by a period of maintenance treatment using the minimum
drug doses required for disease control in order to minimize their
side effects.8
Systemic corticosteroids are the most useful drugs for the
treatment of PV, first used for this purpose by Thorn et al and
popularized by Costello et al and Lever and White.10 Since their
introduction, the mortality associated with PV has declined, and
is now estimated to be 5 to 15%.5,11
Due to severe side effects like infection, thromboembolic
phenomenon, gastrointestinal complications, osteoporosis,
diabetes, psychological disorders, cardiovascular disorders,
myopathy, etc. associated with their prolonged administration and
high doses, there has been a considerable effort to find alternate
methods of treatment.10,12 While serious and fatal complications
of corticosteroid therapy may occur at any dosage, a marked
increase in mortality and morbidity was noted with dosages greater
than 120 mg of prednisolone daily.12
Various adjuvant therapies employed include
immunesuppressive agents (cyclophosphamide, azathioprine),
dapsone, cyclosporine, antimalarials, gold, monoclonal antibodies
(rituximab, etanercept), intravenous immunoglobulins, cholinergic
drugs, tetracyclines and their derivatives, immunomodulators
(mycophenolate mofetil, tacrolimus), etc. Among various types
of therapies used pulse therapy (PT) deserves a mention along
with plasmapheresis, extracorporeal photochemotherapy and
immunoadsorption.1, 6,10-12
PULSE THERAPY
Pulse therapy (PT) was first described by Pasricha and Ramji in
1984.4,6,8,13 It has been defined as discontinuous or intermittent
intravenous infusion of very high doses (megadoses) of drugs over
a short time.3,6,14,15 The theoretical aims of pulsing are to achieve
more rapid and effective disease control compared with
conventional oral dosing, thus allowing a reduction in long-term
maintenance corticosteroid doses and their side effects.8
In PT, doses of each pulse are not standardized but are usually
10 to 20 mg per kilogram of body weight for methylprednisolone
(250-1000 mg) and 2 to 5 mg per kilogram of body weight
(50-200 mg) for dexamethasone. Single doses of 500 mg of
methylprednisolone and 100 mg of dexamethasone are both
considered equivalent to 625 mg of prednisone. These very high
212
doses, sometimes termed megadoses, are usually given as
intravenous infusions over 30 minutes to 1 hour daily or every
other day for a total of 1 to 5 administrations. In most indications,
pulse glucocorticoid therapy is accompanied and/or followed by
the continuous administration of lower intermediate-dose
glucocorticoids and/or immunosuppressive agents like cyclophosphamide and azathioprine.15
In 1982, Pasricha et al treated five patients suffering from PV
with an arbitrarily designed regimen. The regimen consisted of
giving 100 mg dexamethasone in 500 mL of 5% glucose by a
slow intravenous infusion on three consecutive days, along with
500 mg cyclophosphamide on one day only. Such dexamethasonecyclophosphamide pulses (DCP) were given once a month and in
between these pulses the patients received only 50 mg
cyclophosphamide orally per day. After having received a total
of 14 to 48 DCPs, further treatment for PV was withdrawn
(Table 1). All the patients were in continuous clinical remission
for 4 to 9 years.16
In another study, 79 patients with PV were treated with an
arbitrarily designed regimen of 100 mg dexamethasone dissolved
in 5% glucose given by an intravenous infusion over 1 hour, daily
on three consecutive days and in addition, 500 mg
cyclophosphamide on day 1 only. The intermittent high doses
(IHD) of dexamethasone were repeated every 2 to 4 weeks, and
the patient continued to take 50 mg/day oral cyclophosphamide.
This treatment was divided into four phases. During Phase I, the
patient continued to develop relapses of PV a variable number of
days after IHD, but the lesions healed up quickly after IHD. These
relapses became progressively milder and stopped after a few
months, but the IHD were continued once a month for 6-9 months
(Phase II). In the next phase (Phase III), the monthly IHD were
stopped, and the patient continued to take 50 mg/day
cyclophosphamide orally (Table 1). After approximately 1 year,
this maintenance treatment was withdrawn and the patient was
observed for any relapses (Phase IV). It was found that only seven
patient had active disease at the end of the study while majority
had either attained remission or were just on maintenance doses.
The remissions were found to be long lasting. Side-effects of
corticosteroids and cyclophosphamide were minimal and not
clinically significant except for increased susceptibility to
infections.17
In their study on 50 patients with PV, Kaur et al used the DCP
PT. The pulse consisted of 136 mg dexamethasone dissolved in
5% dextrose given in a drip over a period of 1 to 2 hours on
3 consecutive days. In addition, 500 mg cyclophosphamide was
added in the drip on the first day. Such pulses were given at monthly
intervals. In between the pulses, patients were given 50 mg
cyclophosphamide orally each day (Table 1). The lesions were
found to heal in 3 to 4 days time in majority of patients and the
chief advantage found was freedom from side-effects of
corticosteroids.18
In another study, it was found that the patients responded well
to monthly doses of intravenous cyclophosphamide with rapid
decrease in the frequency and severity of blistering resulting in
resolution of their disease after 7 to 10 months19 (Table 1).
In their study, Chryssomallis and coworkers treated eight
patients with PV alternate-day, one hour, infusions of 8, 9 or
JAYPEE
Review on Pulse Therapy: A Novel Approach in the Treatment of Pemphigus Vulgaris
10 mg/kg methylprednisolone Na succinate. Oral prednisone and
a second immunosuppressive agent were simultaneously
administered. These were rapidly decreased when control of the
disease was achieved (Table 1). They found that all the patients
initially responded well to therapy but the disease recurred in four
patients after 3, 4, 9, and 16 months of remission, respectively.
Three of these patients were treated again with PT and went into
remission. None of the patients who received cyclophosphamide
had a recurrence.20
In a retrospective, case-controlled study, Wreth et al included
15 patients with PV dividing them into two groupsone group
received very high-dose intravenous pulses of methylprednisolone
sodium succinate and the other did not receive intravenous PT
(Table 1). In this study, patients treated with PT and control patients
treated with conventional oral prednisone had similar initial disease
severity. Six of the nine patients who received PT showed
improvement of their PV. Therapeutic benefit was seen in patients
who received PT early or late after beginning glucocorticoid
therapy. In contrast, none of the six control patients has achieved
long-term remissions without therapy. Hence, they concluded that
patients treated with conventional orally administered doses of
prednisone had protracted courses requiring years of glucocorticoid
therapy with no long-term remissions.21
In a study, DCP was used to treat 20 patients with bullous
dermatoses, including six with PV. On each of days 1 to 3, 100
mg dexamethasone was administered intravenously and on day 1,
500 mg cyclophosphamide IV. In the therapy interval between the
pulses 50 mg cyclophosphamide per day was administered. Initially
the pulses were repeated every two weeks and later at 10-week
intervals (Table 1). After six months of this regimen, 13 patients
were symptom-free, 4 had improved, and 3 showed no change.
They concluded that DCP PT appears to be a good alternative to
the standard continuous corticosteroid treatment.22
In their study on 123 patients with PV, Dadras and coworkers
divided the patients into two groupsone group received pulse
therapy with methylprednisolone within three consecutive monthly
doses (1000 mg intravenous methylprednisolone for four days plus
500 mg intravenous cyclophosphamide for one day) and the second
group where the patients received 1 to 2 mg/kg/day oral
prednisolone for 28 days plus 1.5 mg/kg/day azathioprine. They
found that PT with methylprednisolone-cyclophosphamide was
generally well tolerated, safe and associated with lesser side
effects.23
Recently, a variant of pulse therapy has been proposed for
patients with significant corticosteroid related toxicity and
unresponsiveness to multiple immunosuppressive adjunctive
therapies. Here, cyclophosphamide is given intravenously at the
dosage of 50 mg/kg/day for four consecutive days and followed
by the administration of granulocyte-colony stimulating factor (GCSF), 5 g/kg/day, beginning six days after last day of
cyclophosphamide until the absolute neutrophil count exceeds
1000/ mm3. Febrile neutropenia and sepsis are common side effects
of this immunoablative therapy.3
SIDE-EFFECTS OF PULSE THERAPY
Cyclophosphamide, an alkylating agent is one of the most potent
chemical immunosuppressants available and used as an adjuvant
in PT. It induces a decrease in cellularity of lymphoid organs in
Table 1: Summary of studies conducted in the field of PT
Sl. no
Researchers
Year in
Number of Regimen used
which
patients
reported treated
1
2
Pandya AG, Sontheimer RD19
Appelhans M, Bonsmann G, Orge C, Brocker EB22
1992
1993
2
20
Chryssomallis F, Dimitriades A, Chaidemenos GC,
Panagiotides D, Karakatsanis G20
1995
4
5
Wreth VP21
Pasricha JS, Thanzama J, Khan UK17
1996
2006
15
79
Kaur S, Kanwar AJ18
2007
50
Pasricha JS, Das SS16
2007
IV cyclophosphamide
100 mg dexamethasone IV on days 1 to 3 with 500 mg
cyclophosphamide IV on day 1 along with 50 mg/day oral
cyclophosphamide. PT repeated initially every 2 weeks and
then at 10 weeks interval. Total duration of treatment was
6 months.
Alternate day 1 hour infusion of 8, 9 or 10 mg/kg
methylprednisolone sodium succinate along with oral
prednisolone and cyclophosphamide.
High dose IV methylprednisoone sodium succinate.
100 mg dexamethasone in 5% glucose by IV infusion over
1 hour daily on 3 consecutive days along with 500 mg
cyclophosphamide IV on day 1 only plus 50 mg oral
cyclophosphamide. Doses of dexamethasone repeated at
2 to 4 weeks
136 mg dexamethasone in 5% dextrose drip 1 to 2 hours on
three consecutive days along with 500 mg cyclophosphamide
added to the drip on day 1 given at monthly intervals plus
50 mg oral cyclophosphamide.
100 mg dexamethasone in 500 ml of glucose slow IV infusion
on three consecutive days along with 500 mg cyclophosphamide on day 1 only. DCP given once a month only.
50 mg oral cyclophosphamide is continued. Total of 14 to
18 DCP pulses are given.
Journal of Indian Academy of Oral Medicine and Radiology, October-December 2010;22(4):211-214
213
Rachna Kaul, Sanjay CJ
low doses, while not affecting hematopoietic cells. At high doses,
it is selectively toxic for B-lymphocytes with regenerating
B-lymphocytes being much more sensitive than resting
B-lymphocytes.19 As an adjuvant in PT, it may prevent the disease
from recurring. However, some studies have found a risk of cardiac
arrest with its prolonged use. The risk of cardiac arrest exists even
with less aggressive form of PT. A medical history of
supraventricular arrhythmias may be considered a risk factor.20
Also, myelosuppression, gonadal damage, teratogenecity,
hemorrhagic cystitis, leukopenia and reversible alopecia are other
side-effects associated with the use of cyclophosphamide.19,24
Cummins et al found adverse effects like hematuria, nonlifethreatening infections and transitional cell carcinoma of urinary
bladder as the adverse effects associated with its use. Though,
there were no deaths reported with its use, they recommended
close monitoring of the patients on cyclophoshamide.25
Pandya et al have recommended a pulse cyclophosphamide
protocol to be followed in using this drug as a constituent of PT.
They have suggested that the intermittent high-dose protocol with
cyclophosphamide impairs immune surveillance for relatively short
periods of time compared with daily oral therapy. Therefore, the
malignant potential using this protocol may be less than those using
standard doses. Good hydration is extremely important in
protecting the bladder when giving cyclophosphamide both orally
and intravenously.19 It has been reported that concomitant pulsecyclophosphamide and corticosteroid lead to complete remission
in 37% and mortality in 3% of cases.18,22
Azathioprine, another commonly used immunosuppressant and
adjuvant in PT has been associated with side effects like
myelosuppression, nausea, hepatotoxicity, hypersensitivity
reactions and increased susceptibility to infections.8 Skin cancer
and lymphoma after long-term use of azathioprine have been
reported in renal transplant patients.19 Reports suggest slow steroid
sparing action as compared to cyclophosphamide, complete
remission rates of 28 to 45% and mortality rates of 1.4 to 7%
associated with the use of azahioprine.8
CONCLUSION
PT appears to be successful in the treatment of PV. High-dose
steroid therapy can be tapered with the use of this treatment.
Various studies have proven the efficacy of pulse therapy along
with reduced side-effects of conventional corticosteroid therapy.
However, long-term follow-up is necessary to compare the
incidence of malignancy in patients receiving pulse doses of
immunosuppressive agents with that in patients receiving
continuous oral treatment.
REFERENCES
1. Sacchidanand S, Hiremath NC, Natraj HV, Revathi TN, Rani SRH,
Pradeep G, Tenneti V. Dexamethasone-cyclophosphamide pulse therapy
for autoimmune-vesiculobullous disorders at Victoria hospital,
Bangalore. Dermatol Online J 2003;9(5):2.
2. Fitzpatrick RE, Newcomer VD. The correlation of disease activity and
antibody titers in pemphigus. Arch Dermatol 1980;116:285-90.
3. Rucocco E, Baroni A, Wolf R, Ruocco V. Life-threatening bullous
dermatoses: Pemphigus vulgaris. Clin Dermatol 2005;23:223-26.
214
4. Mentink LF, Mackenzie MW, Tth GG, Laseur M, Lambert FPG,
Veeger NJ, Cianchin G, et al. Randomized controlled trial of adjuvant
oral dexamethasone pulse therapy in pemphigus vulgaris. Arch Dermatol
2006;142:570-76.
5. Rose E, Wever S, Zillikens D, Linse R, Haustein UF, Brocker EB.
Intravenous dexamethasone-cyclophosphamide pulse therapy in
comparison with oral methylprednisolone-azathioprine therapy in
patients with pemphigus: Result of a multicenter prospectively
randomized study. JDDG 2005;3:200-06.
6. Raviraj J, Nayak AG. Current pathophysiological aspects and
therapeutic modalities for pemphigus vulgaris: A review. JIAOMR
2007;19(4):503-11.
7. Sirois D, Leigh JE, Sollecito TP. Oral pemphigus vulgaris preceding
cutaneous lesions: Recognition and diagnosis. J Am Dent Assoc
2000;131:1156-60.
8. Harman KE, Albert S, Black MM. Guidelines for the management of
pemphigus vulgris. Br J Dematol 2003;149:926-37.
9. Samadi Z, Gorouhi F, Davari P, Firooz A. Think globally, act locally:
Expert opinions from Asia on the diagnosis and treatment of pemphigus
vulgaris. Indian J Med Sci 2007;61(3):144-51.
10. Bystryn JC. Adjuvant therapy of pemphigus. Arch Dermatol
1984;120:941-51.
11. Fleischli ME, Valek RH, Pandya AG. Pulse intravenous cyclophosphamide therapy in pemphigus. Arch Dermatol 1999;135:57-61.
12. Rosenberg FR, Sanders S, Nelson CT. Pemphigus. Arch Dermatol
1976;112:962-70.
13. Pasricha SJ, Gupta R. Pulse therapy with dexamethasonecyclophosphamide in pemphigus. Indian J Dermatol Venerol Leprol
1984;50(4):199-203.
14. Toth GG, van de Meer JB, Jonkman MF. Dexamethasone pulse therapy
in pemphigus. JEADV 2002;142:570-76.
15. Roujeau JC. Pulse glucocorticoid therapy: The Big Shot revisited.
Arch Dermatol 1996;132(12):1499-1502.
16. Pasricha JS, Das SS. Curative effect of dexaethasone-cyclophosphamide
pulse therapy for the treatment of pemphigus vulgaris. Int J Dermatol
2007;31(12):875-77.
17. Pasricha JS, Thanzama J, Khan UK. Intermittent high dose
dexamethasone-cyclophosphamide therapy for pemphigus. Br J
Dermatol 2006;119(1):73-77.
18. Kaur S, Kanwar AJ. Dexamethasone-Cyclophosphamide pulse therapy
in pemphigus. Int J Dermatol 2007;29(5):371-74.
19. Pandya AG, Sontheimer RD. Treatment of pemphigus vulgaris with
pulse intravenous cyclophosphamide. Arch Dermatol 1992;128(12):
1626-30.
20. Chryssomallis F, Dimitriads A, Chaidemenos GC, Panagiotides D,
Karakatsanis G. Steroid pulse therapy in pemphigus vulgaris long-term
follow-up. Int J Dermatol 1995;34(6):423-42.
21. Wreth VP. Treatment of pemphigus vulgaris with brief, high dose
intravenous glucocorticoids. Arch Dermatol 1996;132(12):1435-39.
22. Appelhans M, Bonsmann G, Orge C, Brocker EB. Dexamethasonecyclophosphamide pulse therapy in bullous autoimmune dermatoses.
Haurtarzt 1993;44(3):143-47.
23. Dadras MS, Karami A, Toosy P, Shafiyan A. Pulse versus oral
methylprednisolone therapy in pemphigus vulgaris. Arch Iranian Med
2007;10(1):1-6.
24. Krain LS, Landau JW, Newcomer VD. Cyclophosphamide in the
treatment of pemphigus vulgaris and bullous pemphigoid. Arch
Dermatol 1972;106(5):657-61.
25. Cummins DL, Mimouni D, Anhalt GJ. Oral cyclophosphamide for
treatment of pemphigus vulgaris and foliaceus. J Am Acad Dermatol
2005;49(2):276-80.
JAYPEE
Vous aimerez peut-être aussi
- Handbook For Dermatology ResidentsDocument186 pagesHandbook For Dermatology ResidentsJohn100% (6)
- Geriatric Soap NoteDocument6 pagesGeriatric Soap Noteapi-282282363100% (6)
- Lasers in DermatologyDocument558 pagesLasers in Dermatologytania100% (2)
- Dermatology MCQ 1700Document45 pagesDermatology MCQ 1700Dr-Jahanzaib Gondal100% (1)
- Emerging Treatments For Crohn's Disease: Cells, Surgery, and Novel TherapeuticsDocument10 pagesEmerging Treatments For Crohn's Disease: Cells, Surgery, and Novel Therapeuticsellya theresiaPas encore d'évaluation
- CNA Practice TestDocument12 pagesCNA Practice TestJoe Prempeh50% (4)
- PapersDocument164 pagesPaperstaniaPas encore d'évaluation
- Dermatology MCQ FRCPDocument101 pagesDermatology MCQ FRCPhesham89% (19)
- Lippincott S Anesthesia Review 1001 Questions And.20Document1 pageLippincott S Anesthesia Review 1001 Questions And.20Reem Salem0% (1)
- Macrocytic Anemia - Megaloblastic AnemiaDocument42 pagesMacrocytic Anemia - Megaloblastic AnemiaDarien LiewPas encore d'évaluation
- Pathophysiology of Nontoxic Nodular GoiterDocument2 pagesPathophysiology of Nontoxic Nodular GoiterKaila Abeleda100% (2)
- Pgs Guidance Exam Book PDFDocument369 pagesPgs Guidance Exam Book PDFUsman IlyasPas encore d'évaluation
- Lesson Plan On PhysiologicalDocument22 pagesLesson Plan On PhysiologicalruchiPas encore d'évaluation
- IntroductionDocument10 pagesIntroductionNareman Alaa50% (2)
- Rubrics For Preparing Blood TransfusionDocument4 pagesRubrics For Preparing Blood TransfusionMARK JOSHUA ALFAROPas encore d'évaluation
- General Dermatology MCQsDocument197 pagesGeneral Dermatology MCQsDr.Tawheed95% (19)
- MSM Brochure PDFDocument14 pagesMSM Brochure PDFLinda Kayser100% (2)
- Sumber 3Document12 pagesSumber 3Setiari DewiPas encore d'évaluation
- Medical Care: "Microbiology, Epidemiology, Clinical Manifestations, and Diagnosis of Leptospirosis"Document5 pagesMedical Care: "Microbiology, Epidemiology, Clinical Manifestations, and Diagnosis of Leptospirosis"Lyra LorcaPas encore d'évaluation
- 2028 Full PDFDocument8 pages2028 Full PDFdhineyPas encore d'évaluation
- Case ReportDocument6 pagesCase ReportGladishPas encore d'évaluation
- Nejmoa 1814917Document11 pagesNejmoa 1814917yasserPas encore d'évaluation
- Double Blind Randomized Placebo-Controlled Trial To Evaluate The Efficacy and Safety of Short-Course Low Dose Oral Prednisolone in Pityriasis RoseaDocument10 pagesDouble Blind Randomized Placebo-Controlled Trial To Evaluate The Efficacy and Safety of Short-Course Low Dose Oral Prednisolone in Pityriasis RoseaSalsabila Munirah AmirPas encore d'évaluation
- Granulomatosis UDocument12 pagesGranulomatosis UCieliito CvPas encore d'évaluation
- ShimanovichDocument7 pagesShimanovichapi-3836160100% (2)
- Jurnal UveitisDocument6 pagesJurnal UveitisWidodo WidoPas encore d'évaluation
- Cid/cix182 (Traducido)Document35 pagesCid/cix182 (Traducido)Carlos Manuel Pérez GómezPas encore d'évaluation
- Developments in Therapy and Diagnosis of Yaws and Future ProspectsDocument8 pagesDevelopments in Therapy and Diagnosis of Yaws and Future ProspectsOlivia Halim KumalaPas encore d'évaluation
- Prophylaxis Against Fungal Peritonitis in CAPD A Single Center Experience With Low Dose FluconazoleDocument5 pagesProphylaxis Against Fungal Peritonitis in CAPD A Single Center Experience With Low Dose FluconazoleJorge David Gamez PeñarandaPas encore d'évaluation
- M34 16Document2 pagesM34 16Ana Tomas PetrovicPas encore d'évaluation
- Jurnal Intensified Antituberculosis Therapy in Adult With Tuberculous MeningitisDocument13 pagesJurnal Intensified Antituberculosis Therapy in Adult With Tuberculous Meningitisnaila ghinayaPas encore d'évaluation
- Pemphigus Foliaceous: A Rare Case Report AbstractDocument5 pagesPemphigus Foliaceous: A Rare Case Report AbstractVpatelPas encore d'évaluation
- D-Penicillamine-induced Pemphigus Vulgaris in A Patient With Scleroderma-Rheumatoid Arthritis Overlap SyndromeDocument2 pagesD-Penicillamine-induced Pemphigus Vulgaris in A Patient With Scleroderma-Rheumatoid Arthritis Overlap SyndromeNiken Tri HapsariPas encore d'évaluation
- Jurnal RhinosinusitisDocument26 pagesJurnal RhinosinusitisFarah F SifakPas encore d'évaluation
- Adjuvant Steroid Therapy in Community-Acquired Pneumonia: What 'S New in Infectious DiseasesDocument3 pagesAdjuvant Steroid Therapy in Community-Acquired Pneumonia: What 'S New in Infectious DiseasesDianPas encore d'évaluation
- Antibiotic Myths For The Infectious Diseases ClinicianDocument13 pagesAntibiotic Myths For The Infectious Diseases CliniciandrthanallaPas encore d'évaluation
- Original Article Safety of Dexamethasone in PemphigusDocument5 pagesOriginal Article Safety of Dexamethasone in PemphigusTara WandhitaPas encore d'évaluation
- Jhe2021 6455659Document5 pagesJhe2021 6455659Septi MuninggarPas encore d'évaluation
- Ten Common Mistakes in The Management of Lupus Nephritis. 2014Document10 pagesTen Common Mistakes in The Management of Lupus Nephritis. 2014Alejandro Rivera IbarraPas encore d'évaluation
- Jurnal 4Document7 pagesJurnal 4Lutfi MalefoPas encore d'évaluation
- 1-jahnson2018 (polidocanol 3% 2-6ml x1-2 in 3mo, antibiotic+NSAIDs, efficacy=96, SES= 5)Document14 pages1-jahnson2018 (polidocanol 3% 2-6ml x1-2 in 3mo, antibiotic+NSAIDs, efficacy=96, SES= 5)Vangelis PapachristosPas encore d'évaluation
- A Prospective Observational Study of Prescribing Patterns in Peptic Ulcer DiseaseDocument40 pagesA Prospective Observational Study of Prescribing Patterns in Peptic Ulcer DiseaseGeet MaanPas encore d'évaluation
- dst50234 PDFDocument7 pagesdst50234 PDFtaniaPas encore d'évaluation
- Treatment of Toxic Epidermal Necrolysis With Intravenous ImmunoglobulinDocument5 pagesTreatment of Toxic Epidermal Necrolysis With Intravenous ImmunoglobulinUbaidillah HafidzPas encore d'évaluation
- Laprosy Research Paper (Ahmed Tanjimul Islam)Document7 pagesLaprosy Research Paper (Ahmed Tanjimul Islam)AHMED TANJIMUL ISLAMPas encore d'évaluation
- Jurding RhinosinusistisDocument26 pagesJurding RhinosinusistisIhdina Hanifa Hasanal IbrahimPas encore d'évaluation
- Dupilumab Improves Upper and Lower Airway Disease Control in Chronic Rhinosinusitis With Nasal Polyps and AsthmaDocument10 pagesDupilumab Improves Upper and Lower Airway Disease Control in Chronic Rhinosinusitis With Nasal Polyps and AsthmaMeylinda RizkyPas encore d'évaluation
- Meningitis 3Document12 pagesMeningitis 3Kelly MuñozPas encore d'évaluation
- Nature Reviews Nephrology (Formerly Nature Clinical Practice Nephrology)Document12 pagesNature Reviews Nephrology (Formerly Nature Clinical Practice Nephrology)Đại NgưPas encore d'évaluation
- Rheumatology 05Document6 pagesRheumatology 05C Bala DiwakeshPas encore d'évaluation
- Moxifloxaxin For CAPDocument9 pagesMoxifloxaxin For CAPmateri posPas encore d'évaluation
- Chemotherapy For Tuberculous Meningitis: Peter R. Donald, M.DDocument3 pagesChemotherapy For Tuberculous Meningitis: Peter R. Donald, M.DDirga Rasyidin LPas encore d'évaluation
- High Dose Steroid vs Low Dose Steroid + Azathioprine for Oral PemphigusDocument5 pagesHigh Dose Steroid vs Low Dose Steroid + Azathioprine for Oral PemphigusMario CastroPas encore d'évaluation
- EsomeprazoleDocument7 pagesEsomeprazoleSanjay NavalePas encore d'évaluation
- SplitPDFFile 1001 To 1200Document200 pagesSplitPDFFile 1001 To 1200Shafan ShajahanPas encore d'évaluation
- Practice Guidelines for Candidiasis TreatmentDocument17 pagesPractice Guidelines for Candidiasis TreatmentCristian HaesbaertPas encore d'évaluation
- Artigo 3Document17 pagesArtigo 3Raquel BastosPas encore d'évaluation
- Mepolizumab For Prednisone-Dependent Asthma With Sputum EosinophiliaDocument9 pagesMepolizumab For Prednisone-Dependent Asthma With Sputum EosinophiliaSurya Perdana SiahaanPas encore d'évaluation
- A Painful Red EyeDocument2 pagesA Painful Red EyeCristhian Agustin ParedesPas encore d'évaluation
- Lovastatin For The Treatment of Adult Patients With Dengue: A Randomized, Double-Blind, Placebo-Controlled TrialDocument9 pagesLovastatin For The Treatment of Adult Patients With Dengue: A Randomized, Double-Blind, Placebo-Controlled TrialBpmStfbPas encore d'évaluation
- Nebulized Colistin in The Treatment of Pneumonia Due To Multidrug-Resistant Acinetobacter Baumannii and Pseudomonas AeruginosaDocument4 pagesNebulized Colistin in The Treatment of Pneumonia Due To Multidrug-Resistant Acinetobacter Baumannii and Pseudomonas AeruginosaPhan Tấn TàiPas encore d'évaluation
- Mignogna Et Al-2002-Journal of Oral Pathology & Medicine PDFDocument7 pagesMignogna Et Al-2002-Journal of Oral Pathology & Medicine PDFdrjonduPas encore d'évaluation
- New England Journal Medicine: The ofDocument11 pagesNew England Journal Medicine: The ofprabuPas encore d'évaluation
- Eosinophilic Granulomatosis With Polyangiitis Associated With Subcutaneous Nodules in A 10-Year-Old GirDocument2 pagesEosinophilic Granulomatosis With Polyangiitis Associated With Subcutaneous Nodules in A 10-Year-Old GirShelyAzradPas encore d'évaluation
- Case Report Details Successful Response to Pembrolizumab in NSCLC Patient Despite Significant Renal LossDocument4 pagesCase Report Details Successful Response to Pembrolizumab in NSCLC Patient Despite Significant Renal LossegagusmelaPas encore d'évaluation
- Metheng 2010 Control of Itch in Patients Undergoing DialysisDocument8 pagesMetheng 2010 Control of Itch in Patients Undergoing DialysisoctyvaniPas encore d'évaluation
- Analysis of Anc Levels After Filgrastim Therapy in Acute Leukemia Children With NeutropeniaDocument7 pagesAnalysis of Anc Levels After Filgrastim Therapy in Acute Leukemia Children With NeutropeniaOcta VianiPas encore d'évaluation
- Assessment of The Prevalence and Risk Factors Associated With Glucocorticoid-Induced Diabetes Mellitus in Pemphigus Vulgaris PatientsDocument6 pagesAssessment of The Prevalence and Risk Factors Associated With Glucocorticoid-Induced Diabetes Mellitus in Pemphigus Vulgaris PatientsRahma BachriPas encore d'évaluation
- Dupilumab Treatment in Adults With Moderate-to-Severe Atopic DermatitisDocument10 pagesDupilumab Treatment in Adults With Moderate-to-Severe Atopic DermatitisDwi Putri SimamoraPas encore d'évaluation
- Jurnal AsthmaDocument11 pagesJurnal AsthmaNadira Juanti PratiwiPas encore d'évaluation
- Azithromycin For The Prevention of COPD ExacerbationsDocument6 pagesAzithromycin For The Prevention of COPD ExacerbationsI Made AryanaPas encore d'évaluation
- Lack of Efficacy of Long-Term, Low-Dose Azithromycin in Chronic Rhinosinusitis: A Randomized Controlled TrialDocument12 pagesLack of Efficacy of Long-Term, Low-Dose Azithromycin in Chronic Rhinosinusitis: A Randomized Controlled Trialpaijo09Pas encore d'évaluation
- Mepolizumab COPD NEJMDocument17 pagesMepolizumab COPD NEJM5fqkqkcdhtPas encore d'évaluation
- Enteric Fever 2013Document3 pagesEnteric Fever 2013aminceloPas encore d'évaluation
- J Antimicrob Chemother Doi:10.1093/jac/dkw038Document7 pagesJ Antimicrob Chemother Doi:10.1093/jac/dkw038Andhi Riawan Eko WiikramatunggadewaPas encore d'évaluation
- Infections in Cancer Chemotherapy: A Symposium Held at the Institute Jules Bordet, Brussels, BelgiumD'EverandInfections in Cancer Chemotherapy: A Symposium Held at the Institute Jules Bordet, Brussels, BelgiumPas encore d'évaluation
- Sistemic Drug PDFDocument321 pagesSistemic Drug PDFKensel Escudero100% (1)
- Genital WartsDocument1 pageGenital WartstaniaPas encore d'évaluation
- Sistemic Drug PDFDocument321 pagesSistemic Drug PDFKensel Escudero100% (1)
- MCPS Oct 2016 Question # 7 Rook Page # 29.10Document1 pageMCPS Oct 2016 Question # 7 Rook Page # 29.10taniaPas encore d'évaluation
- A MoleDocument1 pageA MoletaniaPas encore d'évaluation
- Keamanan Obat Bagi Ibu Hamil Berdasarkan Evidence BasedDocument17 pagesKeamanan Obat Bagi Ibu Hamil Berdasarkan Evidence BasedJasonJejametPas encore d'évaluation
- Adult-Onset Hydroa Vacciniforme-Like Lymphoma in A Long-Term Resident of The United StatesDocument4 pagesAdult-Onset Hydroa Vacciniforme-Like Lymphoma in A Long-Term Resident of The United StatestaniaPas encore d'évaluation
- Dapsone Therapy Side Effects and MonitoringDocument3 pagesDapsone Therapy Side Effects and MonitoringtaniaPas encore d'évaluation
- Bedside Diagnostic in DermatologyDocument22 pagesBedside Diagnostic in DermatologyagusPas encore d'évaluation
- Pics MCQSDocument22 pagesPics MCQStaniaPas encore d'évaluation
- Listening Sample Task - Form CompletionDocument4 pagesListening Sample Task - Form CompletionTrần Đăng Khoa100% (1)
- Pemphigus VulgarisDocument12 pagesPemphigus Vulgarisamirz_4Pas encore d'évaluation
- Prevention and Management of Glucocorticoid-Induced Side Effects: A Comprehensive ReviewDocument6 pagesPrevention and Management of Glucocorticoid-Induced Side Effects: A Comprehensive ReviewtaniaPas encore d'évaluation
- Monitoring key dermatologic medicationsDocument3 pagesMonitoring key dermatologic medicationsriskhakovPas encore d'évaluation
- dst50234 PDFDocument7 pagesdst50234 PDFtaniaPas encore d'évaluation
- A Study To Assess The Effectiveness of Planned Teaching Programme Regarding Stem Cells Among Eligible Couples in Selected Areas of AhmedabadDocument4 pagesA Study To Assess The Effectiveness of Planned Teaching Programme Regarding Stem Cells Among Eligible Couples in Selected Areas of AhmedabadIJAR JOURNALPas encore d'évaluation
- Adult Nursing 2 Teaching PlanDocument5 pagesAdult Nursing 2 Teaching PlanJerilee SoCute WattsPas encore d'évaluation
- Impact of Self-Management Education on Diabetes PatientsDocument3 pagesImpact of Self-Management Education on Diabetes PatientsArief AndriyantoPas encore d'évaluation
- Generi Life Physician Statement FormDocument1 pageGeneri Life Physician Statement FormVennus RacraquinPas encore d'évaluation
- Success Manual and Cheat Sheet Notes To Pass Your Basic Life Support (BLS) CourseDocument11 pagesSuccess Manual and Cheat Sheet Notes To Pass Your Basic Life Support (BLS) CourseanthonyPas encore d'évaluation
- Recipe FormatDocument1 pageRecipe FormatScribdTranslationsPas encore d'évaluation
- Physical & Multiple Disability ChecklistDocument3 pagesPhysical & Multiple Disability ChecklistGheylhu AmorPas encore d'évaluation
- Initial Assessment and Management of Acute StrokeDocument49 pagesInitial Assessment and Management of Acute StrokeIrina DuceacPas encore d'évaluation
- Eye Care ProcedureDocument9 pagesEye Care ProcedureSharon Lawrence100% (1)
- TETRASODIUM EDTA - National Library of Medicine HSDB DatabaseDocument21 pagesTETRASODIUM EDTA - National Library of Medicine HSDB DatabaseElena TrofinPas encore d'évaluation
- 9164 62159 1 PBDocument12 pages9164 62159 1 PBjenegnePas encore d'évaluation
- COVID-19: Further Evidence That The Virus Originated in The USDocument3 pagesCOVID-19: Further Evidence That The Virus Originated in The USRodolfoAPas encore d'évaluation
- Pathophysiology: Predisposing Factors: Precipitating FactorsDocument2 pagesPathophysiology: Predisposing Factors: Precipitating FactorsJemsMei Comparativo MensuradoPas encore d'évaluation
- Case Sheet for Maternity ServicesDocument22 pagesCase Sheet for Maternity ServicesGulfeshan ArshiPas encore d'évaluation
- Seminar: Annelies Wilder-Smith, Eng-Eong Ooi, Olaf Horstick, Bridget WillsDocument14 pagesSeminar: Annelies Wilder-Smith, Eng-Eong Ooi, Olaf Horstick, Bridget WillsAlexander ArguelloPas encore d'évaluation
- Gulan 2003 Klinicka - I - Radioloska - Prezentacija - Kostanih Medri - 1319 Publishedversion H0k4iq SDocument10 pagesGulan 2003 Klinicka - I - Radioloska - Prezentacija - Kostanih Medri - 1319 Publishedversion H0k4iq SMuxoliniPas encore d'évaluation
- MCQ MedicineDocument4 pagesMCQ MedicineKasun PereraPas encore d'évaluation
- Management of Intraocular Foreign BodiesDocument7 pagesManagement of Intraocular Foreign BodiesdebbyPas encore d'évaluation
- Gynzone TrainingDocument62 pagesGynzone TrainingMariana PinheiroPas encore d'évaluation
- HCC (Kuliah DR Masrul)Document38 pagesHCC (Kuliah DR Masrul)Tengku M Ridho AnharPas encore d'évaluation
- Micro-503 - (T) - Course Outline and ScheduleDocument2 pagesMicro-503 - (T) - Course Outline and ScheduleUmer AliPas encore d'évaluation