Académique Documents
Professionnel Documents
Culture Documents
YaleNews Sugar Rush Shrinks Brain Cell Powerhouse
Transféré par
Jose Luis Pino MatosCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
YaleNews Sugar Rush Shrinks Brain Cell Powerhouse
Transféré par
Jose Luis Pino MatosDroits d'auteur :
Formats disponibles
Sugar rush shrinks brain cell powerhouse
By Karen N. Peart
February 25, 2016
The spike in blood sugar levels that can come after a meal is controlled by the brains neuronal mitochondria, which are
considered the powerhouse of cells, Yale School of Medicine researchers found in a new study.
Published in the Feb. 25 issue of the journal Cell, the ndings could provide a better understanding of how type 2 diabetes
develops.
Blood glucose levels are thought to be primarily controlled by the pancreatic hormone insulin, the liver, and the muscles.
This new study, however, highlights a crucial role for mitochondria in a small subset of neurons of the brain in systemic
glucose control.
The study was designed to explore how neurons in the brain adapt to the glucose rush. The researchers were surprised to
nd that not only do mitochondria of neurons feel the change in circulating glucose levels, but that adaptive changes in
these same mitochondria are at the core of the bodys ability to handle sugar in the blood. To test this point, the research
team generated several mouse models in which a specic mitochondrial protein called uncoupling protein 2 (UCP2) was
either missing or present in varying amounts in the subset of brain cells that sense circulating sugar levels.
We found that when sugar increases in the body, mitochondria in subsets of brain neurons rapidly change their shape and
their function is altered, said senior author Sabrina Diano, professor in the Departments of Obstetrics, Gynecology and
Reproductive Sciences, Neuroscience, and Comparative Medicine.Diano said what surprised the research team is not that
these changes occur in response to glucose, but that these seemingly subtle adjustments in a housekeeping cellular event
in a handful of brain cells has such a powerful impact in circulating glucose levels by affecting many peripheral tissue
functions.
The ndings imply that alterations in this mechanism may be crucial for the development of metabolic diseases such as
type 2 diabetes, in which the body is not able to clear the blood from high levels of sugar that occur after meals, said Diano,
who is also director of the Reproductive Neurosciences Group and a member of the Program in Integrative Cell Signaling
and Neurobiology of Metabolism at Yale School of Medicine.
Diano and her team will focus their future research on assessing whether alteration of this mitochondrial mechanism in the
brain is involved in the development and propagation of type 2 diabetes.
Other authors on the study include Chitoku Toda, Jung Dae Kim, Daniela Impellizzeri, Salvatore Cuzzocrea, and Zhong-Wu
Liu.
The study was funded by the National Institutes of Health, the Manpei Suzuki Diabetes Foundation, and JSPS Postdoctoral
Fellowships for Research Abroad.
(Photo courtesy of Shutterstock)
Copyright 2016, Yale University. All rights reserved. Privacy policy.
Browse our archives | Contact us | Ofce of Public Affairs & Communications
Vous aimerez peut-être aussi
- The Last Great Nomadic Challenges: From Chinggis Khan To TimurDocument10 pagesThe Last Great Nomadic Challenges: From Chinggis Khan To TimurJose Luis Pino MatosPas encore d'évaluation
- Los Nomades Dela HistoriaDocument22 pagesLos Nomades Dela HistoriaJose Luis Pino MatosPas encore d'évaluation
- The Last Great Nomadic Challenges: From Chinggis Khan To TimurDocument10 pagesThe Last Great Nomadic Challenges: From Chinggis Khan To TimurJose Luis Pino MatosPas encore d'évaluation
- Etnicidad y Grupos Etnicos en Los Estados Tempranos KHAZANOVDocument11 pagesEtnicidad y Grupos Etnicos en Los Estados Tempranos KHAZANOVJose Luis Pino MatosPas encore d'évaluation
- JWSR v9n1 TurchinhallDocument15 pagesJWSR v9n1 TurchinhallJose Luis Pino MatosPas encore d'évaluation
- Bastien 1985 Agricultura AntiguaDocument17 pagesBastien 1985 Agricultura AntiguaJose Luis Pino MatosPas encore d'évaluation
- Accion Social ConceptosDocument15 pagesAccion Social ConceptosJose Luis Pino MatosPas encore d'évaluation
- Critical Archaeology LEONEDocument21 pagesCritical Archaeology LEONEJose Luis Pino MatosPas encore d'évaluation
- Architecture and Legacy of the Inca Royal Estate at ChincheroDocument1 pageArchitecture and Legacy of the Inca Royal Estate at ChincheroJose Luis Pino MatosPas encore d'évaluation
- Identidad UrbanaDocument1 pageIdentidad UrbanaJose Luis Pino MatosPas encore d'évaluation
- La Urbanizacion y La Comunicacion en La Arqueologia AndinaDocument1 pageLa Urbanizacion y La Comunicacion en La Arqueologia AndinaJose Luis Pino MatosPas encore d'évaluation
- Camino Inca Resumen de La Convencion DohaDocument253 pagesCamino Inca Resumen de La Convencion DohaJose Luis Pino MatosPas encore d'évaluation
- Decisiones Sobre El Camino Inca Patrimonio MundialDocument274 pagesDecisiones Sobre El Camino Inca Patrimonio MundialJose Luis Pino MatosPas encore d'évaluation
- Encuentro de Estudios Andinos 2016Document7 pagesEncuentro de Estudios Andinos 2016Jose Luis Pino MatosPas encore d'évaluation
- Jardines Tropicales de La MemoriaDocument23 pagesJardines Tropicales de La MemoriaJose Luis Pino MatosPas encore d'évaluation
- Fire England Recursos FuegoDocument28 pagesFire England Recursos FuegoJose Luis Pino MatosPas encore d'évaluation
- Tiwanaku OriginsDocument33 pagesTiwanaku OriginsJose Luis Pino MatosPas encore d'évaluation
- Quality and Creativity for Cultural Tourism DevelopmentDocument7 pagesQuality and Creativity for Cultural Tourism DevelopmentJose Luis Pino MatosPas encore d'évaluation
- Inka BibliografiaDocument4 pagesInka BibliografiaJose Luis Pino MatosPas encore d'évaluation
- Introduction: Hierarchy, Hegemony, and Resist Ance in A "Syncretic" Ritual System 1.1. The Research ProblemDocument2 pagesIntroduction: Hierarchy, Hegemony, and Resist Ance in A "Syncretic" Ritual System 1.1. The Research ProblemJose Luis Pino MatosPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5784)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (72)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- University of Phoenix Anatomy and Physiology Week 2 QuizDocument2 pagesUniversity of Phoenix Anatomy and Physiology Week 2 QuizSophia FHSPas encore d'évaluation
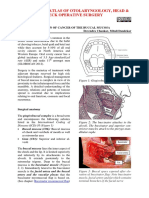
- Surgical Resection of Cancer of The Buccal MucosaDocument21 pagesSurgical Resection of Cancer of The Buccal MucosapradeepPas encore d'évaluation
- Principle of The Method Quality Control: Alkaline PicrateDocument1 pagePrinciple of The Method Quality Control: Alkaline PicrateRisqon Anjahiranda Adiputra100% (1)
- Autonomic Nervous System For MBBSDocument20 pagesAutonomic Nervous System For MBBSjacobsPas encore d'évaluation
- How Sleep Deprivation Affects Students' Academic PerformanceDocument50 pagesHow Sleep Deprivation Affects Students' Academic PerformanceAstrophile0% (2)
- Manifest From The HeartDocument25 pagesManifest From The HeartTuta Velesije Bg100% (2)
- Teachers' Health Examination Card (CS Form 86)Document4 pagesTeachers' Health Examination Card (CS Form 86)Maria Angellie S. Bellido - EramisPas encore d'évaluation
- Uncontrolled Cell DivisionDocument51 pagesUncontrolled Cell Divisionm43Pas encore d'évaluation
- BIO01 CO1 PPT - An Overview of The CellDocument60 pagesBIO01 CO1 PPT - An Overview of The CellCHRISTIAN MATTHEW DELA CRUZPas encore d'évaluation
- Chronic UrticariaDocument40 pagesChronic UrticariaeeeeewwwwwPas encore d'évaluation
- RECONSTRUCTION MAndibula 2Document62 pagesRECONSTRUCTION MAndibula 2RadianNasution100% (1)
- Dental Appliances For Snoring and Obstructive Sleep Apnoea - Construction Aspects For General Dental PractitionersDocument9 pagesDental Appliances For Snoring and Obstructive Sleep Apnoea - Construction Aspects For General Dental Practitionersdocx1975100% (1)
- Limbic System (Behaviour and Emotion)Document19 pagesLimbic System (Behaviour and Emotion)Patterson MachariaPas encore d'évaluation
- Drug StudyDocument14 pagesDrug StudyTin BernardezPas encore d'évaluation
- Gastric Funcn TestDocument18 pagesGastric Funcn TestArshdeep SinghPas encore d'évaluation
- Ch. 13 EyeDocument46 pagesCh. 13 Eyefatucha87Pas encore d'évaluation
- Structure of BacteriaDocument13 pagesStructure of BacteriaRirin UtariPas encore d'évaluation
- NCM107 - Midterm - Newborn CareDocument6 pagesNCM107 - Midterm - Newborn CareLjc JaslinPas encore d'évaluation
- Hillside School: Biology For Grade 12 Note 2 Topic: The Structure and Function of Bacterial CellDocument8 pagesHillside School: Biology For Grade 12 Note 2 Topic: The Structure and Function of Bacterial Celloli JrPas encore d'évaluation
- Learning Theories of PersonalityDocument13 pagesLearning Theories of Personalitypavitra_madhusudanPas encore d'évaluation
- Receptors IntroductionDocument53 pagesReceptors IntroductionSunilPas encore d'évaluation
- Assessment of HearingDocument51 pagesAssessment of HearingSwetha PasupuletiPas encore d'évaluation
- Skripsi Tanpa Pembahasan PDFDocument64 pagesSkripsi Tanpa Pembahasan PDFBillyDwiSaputraPas encore d'évaluation
- Nursing Care PlanDocument5 pagesNursing Care Planruggero07100% (2)
- 100 Benefits of ExerciseDocument3 pages100 Benefits of ExercisePaul RavenWolf JuniorPas encore d'évaluation
- A Presentation On Pantothenic Acid or b5Document28 pagesA Presentation On Pantothenic Acid or b5jainsaketPas encore d'évaluation
- Test Bank Chapter 11: The Pediatric Patient in The Adult Critical Care UnitDocument8 pagesTest Bank Chapter 11: The Pediatric Patient in The Adult Critical Care UnitAnonymous ZzjzIcmPas encore d'évaluation
- Inventarisasi Rs Type DDocument63 pagesInventarisasi Rs Type DAnonymous 5wBxtyIPas encore d'évaluation
- FCA (SA) - Part - II - Past - Papers 10Document30 pagesFCA (SA) - Part - II - Past - Papers 10matentenPas encore d'évaluation
- Figure 1 Human Nervous System Source:courses - Lumenlearning.c Om/microbiology/chapter/anato My-Of-The-Nervous-SystemDocument14 pagesFigure 1 Human Nervous System Source:courses - Lumenlearning.c Om/microbiology/chapter/anato My-Of-The-Nervous-SystemShekaina Faith Cuizon LozadaPas encore d'évaluation