Académique Documents
Professionnel Documents
Culture Documents
The Chemical Institute of Canada L'Institut de Chimie Du Canada
Transféré par
Popa ElenaDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
The Chemical Institute of Canada L'Institut de Chimie Du Canada
Transféré par
Popa ElenaDroits d'auteur :
Formats disponibles
THE CHEMICAL INSTITUTE OF CANADA
LINSTITUT DE CHIMIE DU CANADA
Chemists, engineers and technologists working together.
Les chimistes, les ingnieurs et les technologistes travaillant ensemble.
THE CANADIAN CHEMISTRY CONTEST 2007
for high school and CEGEP students
(formerly the National High School Chemistry Examination)
PART B EXTENDED RESPONSE SECTION (90 minutes)
In this section you should respond to TWO topics only, writing in the form of scientific essays (or, for Question
4, an experimental description) including any appropriate equations, formulae and diagrams. Some suggestions
are made about the direction(s) you could take, but these are not exclusive. Each essay/experiment is of equal
value, and the quality of both responses will be considered in the final competition: you should therefore
allocate approximately equal time to each of the subjects you choose. The judging of the responses will be
based on both factual accuracy and presentation. A clear, concise and well-organized piece of written work will
be rated more highly than a long rambling one that contains the same information.
1. Period 3
In this essay you should discuss the properties of the Period 3 elements (from sodium to argon) with particular
reference to how these elements and their compounds illustrate trends in properties across the Periodic Table.
Although it is appropriate to consider atomic scale properties such as atomic and ionic radii, ionization energies,
electron affinities and electronegativity, your essay will be more interesting if it shows how these factors are
related to macro-scale physical properties such as molar volume, melting points and boiling points, and also to
chemical reactivity. With regard to compounds of the elements you might like to consider the periodicity of the
formulae of oxides, chlorides and hydrides, together with their chemical properties. You might also wish to
discuss the structure and bonding of the elements and their compounds.
2.
The Greenhouse Effect
This essay should be written in the form of a magazine article. You should explain what is meant by the
greenhouse effect, and how it is important in controlling the climate of the Earth. Some of the gases that you
might like to consider are: water vapour, carbon dioxide, methane, ozone, chlorofluorocarbons (CFCs),
hydrochlorofluorocarbons (HCFCs) and halons (bromine-containing halogenoalkanes). Using some or all of
these examples, or other gases of your choice, you should explain the differences between natural greenhouse
gases and those that are produced by human activity, and indicate the mechanisms by which the gases are
produced by writing chemical equations. You could also discuss the problems that might arise if the
concentration of greenhouse gases becomes significantly elevated, and how these difficulties might be averted
by human intervention.
/continued overleaf
CCC 2007 Part B Page 2
3.
Chemical Equilibrium
In answering this question you should explain what is meant by dynamic equilibrium systems and how these
relate to reversible reactions: in doing this you need to specify the characteristics of dynamic equilibrium. You
should also discuss the effect of changing conditions, such as concentration, pressure and temperature, on the
position of equilibrium. You should consider Le Chteliers principle, and how this relates to the equilibrium
law expression (equilibrium constant). You might also like to compare equilibrium constants for homogeneous
and heterogeneous processes, and to consider special case equilibrium constants, such as those governing acid
dissociation (Ka), solubility (Ksp), and the ionisation of water (Kw). You could also discuss how equilibrium
considerations are used to optimise steady state conditions for industrial processes, with appropriate examples.
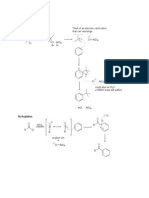
You may use some or all of the following equations as examples, and/or other reaction equations if you so wish.
Br2(l) Br2(g)
+
2 H2O(l) H3O (aq) + OH (aq)
CaCO3(s) CaO(s) + CO2(g)
+
AgCl(s) + aq Ag (aq) + Cl (aq)
2 SO2(g) + O2(g) 2 SO3(g)
CH3COOH(l) + H2O(l) CH3COO (aq) + H3O (aq)
CH3COOH(l) + C2H5OH(l) CH3COOC2H5(l) + H2O(l)
4. Experimental Design
In this question you are required to design an experiment to determine the enthalpy change of solution (heat of
solution) of anhydrous copper sulfate in water, which is a very exothermic reaction.
In your response you should state what you understand by enthalpy change of solution, and outline your
experimental procedure, giving details of the apparatus and materials you would need for performing the
experiment in a high school laboratory. It is important to specify any safety precautions that might be required.
You should indicate what readings you would take and how you would use these readings to calculate a result.
You should consider any problems that you might encounter when performing your experiment (including what
might happen if the copper sulfate is partly hydrated), and how these factors might give rise to errors in the final
value obtained. You might also like to discuss how your experiment could be improved in order to overcome
these problems.
Vous aimerez peut-être aussi
- The Chemical Institute of Canada L'Institut de Chimie Du CanadaDocument2 pagesThe Chemical Institute of Canada L'Institut de Chimie Du CanadaPopa ElenaPas encore d'évaluation
- Canada Chemistry OlympiadDocument1 pageCanada Chemistry OlympiadCorneliaPas encore d'évaluation
- November 2013 ChE Board Exam QuestionsDocument3 pagesNovember 2013 ChE Board Exam QuestionsJayson Ordinaria100% (1)
- Gasification PHD ThesisDocument8 pagesGasification PHD Thesistfwysnikd100% (2)
- Formal Lab Report FormatDocument10 pagesFormal Lab Report FormatrebbiegPas encore d'évaluation
- Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarD'EverandCarbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarPas encore d'évaluation
- Arshad 2016Document24 pagesArshad 2016Maythee SaisriyootPas encore d'évaluation
- Name - : (Excludes Brønsted-Lowry)Document22 pagesName - : (Excludes Brønsted-Lowry)John23Pas encore d'évaluation
- Explo 5 Support PDFDocument18 pagesExplo 5 Support PDFMuthu KumarPas encore d'évaluation
- Xperiments With Arbon Ioxide: HapterDocument12 pagesXperiments With Arbon Ioxide: HapterBashar Al ZoobaidiPas encore d'évaluation
- PV NOx and SOxDocument10 pagesPV NOx and SOxQuang Lã ViệtPas encore d'évaluation
- Unit 1 Cape Chemistry Lab Manual2013-14Document18 pagesUnit 1 Cape Chemistry Lab Manual2013-14Jaz10080% (5)
- Project Descriptions 2012-13. Supplementary List 9.10.12Document7 pagesProject Descriptions 2012-13. Supplementary List 9.10.12Moao AomoPas encore d'évaluation
- Dehydration of 2 Methylcyclohexanol S18 ReportDocument4 pagesDehydration of 2 Methylcyclohexanol S18 ReportprakharPas encore d'évaluation
- A Computational Model of Catalyzed Carbon Sequestration: Back To Exploration - 2008 CSPG CSEG CWLS ConventionDocument4 pagesA Computational Model of Catalyzed Carbon Sequestration: Back To Exploration - 2008 CSPG CSEG CWLS ConventionsahanchemPas encore d'évaluation
- 2012 Usnco National Exam Part IIDocument9 pages2012 Usnco National Exam Part IILizAndrei Jaja MarquezPas encore d'évaluation
- Module in (Ge-Ad: General CHEMISTRY (Organic) ) : Palawan State University Roxas Campus (Department Name) DepartmentDocument50 pagesModule in (Ge-Ad: General CHEMISTRY (Organic) ) : Palawan State University Roxas Campus (Department Name) DepartmentJohn Mark JuarezPas encore d'évaluation
- SPE 167184 Fully Compositional and Thermal Reservoir Simulations Efficiently Compare EOR TechniquesDocument17 pagesSPE 167184 Fully Compositional and Thermal Reservoir Simulations Efficiently Compare EOR TechniquesSifat TanveerPas encore d'évaluation
- Chemical Equations & ReactionsDocument78 pagesChemical Equations & ReactionsDelsie FalculanPas encore d'évaluation
- Hydrogen Sulfide Combustion ChemistryDocument24 pagesHydrogen Sulfide Combustion Chemistryivan a gargurevichPas encore d'évaluation
- Airbag LabDocument5 pagesAirbag LabTamara HamiltonPas encore d'évaluation
- Absorber Intercooling Configurations Using Aqueous Piperazine For Capture From Sources With 4 To 27% CODocument20 pagesAbsorber Intercooling Configurations Using Aqueous Piperazine For Capture From Sources With 4 To 27% COasmaa aliPas encore d'évaluation
- Aces 2013080817241354Document9 pagesAces 2013080817241354Muhammad Abdul RaufPas encore d'évaluation
- Experimental Study Co2 CaptureDocument20 pagesExperimental Study Co2 CaptureserviciosdecomisionamientoPas encore d'évaluation
- 2020 UKChO ASDAN FinalDocument16 pages2020 UKChO ASDAN FinalXuPas encore d'évaluation
- Chemistry Revision POINTERSDocument10 pagesChemistry Revision POINTERSJohn TanPas encore d'évaluation
- Co Gasification of Coal and TyreDocument8 pagesCo Gasification of Coal and TyreJeff Ong Soon HuatPas encore d'évaluation
- 9701 Nos Ps 20Document5 pages9701 Nos Ps 20lianchen251110Pas encore d'évaluation
- Chem1031 Tutorial Set 1Document22 pagesChem1031 Tutorial Set 1Adithya RajendranPas encore d'évaluation
- Rate Based Simulation of DEA Promoted PoDocument6 pagesRate Based Simulation of DEA Promoted PoMuhammad JunaidPas encore d'évaluation
- 120 Formal Chemistry Lab Report FormatDocument10 pages120 Formal Chemistry Lab Report FormatRangothri Sreenivasa SubramanyamPas encore d'évaluation
- 4.3 Chemical EquationsDocument23 pages4.3 Chemical EquationsJagitkanthan RajPas encore d'évaluation
- Understanding Carbonate Equilibria by MeasuringDocument16 pagesUnderstanding Carbonate Equilibria by MeasuringJohn Jairo RamosPas encore d'évaluation
- Carbon Dioxide Capture ThesisDocument6 pagesCarbon Dioxide Capture ThesisProfessionalPaperWritingServiceCanada100% (2)
- Achapter7 8TextbookSolutionDocument91 pagesAchapter7 8TextbookSolutionjimPas encore d'évaluation
- PChem EA Sept 2021 - PostingDocument3 pagesPChem EA Sept 2021 - Postingmicheal ellenggaPas encore d'évaluation
- Chemistry OlympiadsDocument10 pagesChemistry OlympiadsLouiseflemingPas encore d'évaluation
- Bogaerts Et Al-2016-Plasma Processes and PolymDocument21 pagesBogaerts Et Al-2016-Plasma Processes and Polymhemedi kitilaPas encore d'évaluation
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationD'EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationPas encore d'évaluation
- Summer Chemistry Olympiad 21: Chemistry Olympiads Discord ServerDocument12 pagesSummer Chemistry Olympiad 21: Chemistry Olympiads Discord ServerChampion ReaderPas encore d'évaluation
- Chemical and Materials Engineering Department: Course SyllabiDocument35 pagesChemical and Materials Engineering Department: Course SyllabiAbdullah SalemPas encore d'évaluation
- CO2 and SO2 RemovalDocument12 pagesCO2 and SO2 RemovalAnumFarooqPas encore d'évaluation
- A Case of Negative Apparent Activation Energy Due To Pore Diffusion EffectsDocument3 pagesA Case of Negative Apparent Activation Energy Due To Pore Diffusion EffectsBamrung SungnoenPas encore d'évaluation
- Tokumura 2007 Dynamic Modeling andDocument7 pagesTokumura 2007 Dynamic Modeling andAle EcoPas encore d'évaluation
- Thermal Behaviour of RDX and HMXDocument23 pagesThermal Behaviour of RDX and HMXDaniel CaririPas encore d'évaluation
- Simulation Study of Radial Heat and MassDocument8 pagesSimulation Study of Radial Heat and Massbasura12345Pas encore d'évaluation
- Co2 Capture PHD ThesisDocument5 pagesCo2 Capture PHD Thesisnibaditapalmerpaterson100% (2)
- 28-3-62-Kinetic Study of Dry Reforming of Methane Over Ni-Ce - Al2O3 Catalyst With DeactivationDocument11 pages28-3-62-Kinetic Study of Dry Reforming of Methane Over Ni-Ce - Al2O3 Catalyst With DeactivationWassachol SumarasinghaPas encore d'évaluation
- Research ArticleDocument10 pagesResearch ArticleAnonymous cspbsMcXuhPas encore d'évaluation
- Chapter 5 Sheets AnswersDocument2 pagesChapter 5 Sheets AnswershelloblargPas encore d'évaluation
- Margeti Vasiliki Lab5Document7 pagesMargeti Vasiliki Lab5vickyPas encore d'évaluation
- StoichiometryDocument10 pagesStoichiometryIjazPas encore d'évaluation
- Lab Report Format 2019Document3 pagesLab Report Format 2019Innundated BurritoPas encore d'évaluation
- SCI - Volume 24 - Issue 3 - Pages 1253-1263 PDFDocument11 pagesSCI - Volume 24 - Issue 3 - Pages 1253-1263 PDFhoangvubui4632Pas encore d'évaluation
- Αποθήκευσης υδρογόνου σε μικροπορώσεις οργανομεταλλικές δομές .Document14 pagesΑποθήκευσης υδρογόνου σε μικροπορώσεις οργανομεταλλικές δομές .dsertyPas encore d'évaluation
- Texas Essential Knowledge and Skills (TEKS) : Lesson Plan Type: Inquiry Based Learning, Discovery Learning and DiscussionDocument13 pagesTexas Essential Knowledge and Skills (TEKS) : Lesson Plan Type: Inquiry Based Learning, Discovery Learning and Discussionapi-322902620Pas encore d'évaluation
- Dissolved Gas Concentration in Water: Computation as Functions of Temperature, Salinity and PressureD'EverandDissolved Gas Concentration in Water: Computation as Functions of Temperature, Salinity and PressurePas encore d'évaluation
- Art. 2 AdelantoDocument14 pagesArt. 2 AdelantoYuliiana HernandezPas encore d'évaluation
- Computational Evaluation of Emissions For Non-Premixed Natural Gas CombustionDocument3 pagesComputational Evaluation of Emissions For Non-Premixed Natural Gas CombustionijsretPas encore d'évaluation
- Cervicalcancerscreening ModuleDocument45 pagesCervicalcancerscreening ModulePopa ElenaPas encore d'évaluation
- The Pap Test and Bethesda 2014: ReviewDocument12 pagesThe Pap Test and Bethesda 2014: ReviewpdianaPas encore d'évaluation
- Austrian National Chemistry Olympiad 1998Document21 pagesAustrian National Chemistry Olympiad 1998Popa ElenaPas encore d'évaluation
- Olympiad Support Booklet - Full Text 2Document60 pagesOlympiad Support Booklet - Full Text 2Popa ElenaPas encore d'évaluation
- Articol DR PopaDocument5 pagesArticol DR PopaPopa ElenaPas encore d'évaluation
- Fibroza 1Document8 pagesFibroza 1Popa ElenaPas encore d'évaluation
- C3L6 Teachers Mark Scheme 2011Document8 pagesC3L6 Teachers Mark Scheme 2011Popa ElenaPas encore d'évaluation
- Practical Problems: Safety RulesDocument9 pagesPractical Problems: Safety RulesPopa ElenaPas encore d'évaluation
- 99prepare SolDocument53 pages99prepare SolPopa ElenaPas encore d'évaluation
- Friedel Crafts NotDocument3 pagesFriedel Crafts NotPopa ElenaPas encore d'évaluation
- Olympiad 2013 R1 Mark SchemeDocument9 pagesOlympiad 2013 R1 Mark SchemePopa ElenaPas encore d'évaluation
- Physical ExaminationDocument2 pagesPhysical ExaminationPopa Elena0% (1)
- SpeedFace M4 DatasheetDocument2 pagesSpeedFace M4 DatasheetRENJITH K NAIRPas encore d'évaluation
- The Pole and Zeros PDFDocument24 pagesThe Pole and Zeros PDFKim KeatPas encore d'évaluation
- Power Stations Using Locally Available Energy Sources: Lucien Y. Bronicki EditorDocument524 pagesPower Stations Using Locally Available Energy Sources: Lucien Y. Bronicki EditorAmat sapriPas encore d'évaluation
- R OR K C S V: EG Epair Its For Ylinder and Ervice AlvesDocument5 pagesR OR K C S V: EG Epair Its For Ylinder and Ervice AlvesLeonardoFabioCorredorPas encore d'évaluation
- ARTS 9 Q2 M2 Wk2Document21 pagesARTS 9 Q2 M2 Wk2Matt LimPas encore d'évaluation
- Refrigerant Solutions: Refrigerant Retrofit For Existing R22 EquipmentDocument2 pagesRefrigerant Solutions: Refrigerant Retrofit For Existing R22 EquipmentpriyoPas encore d'évaluation
- Sorsogon State College Engineering & ArchitectureDocument11 pagesSorsogon State College Engineering & ArchitectureArianne Mae De Vera GallonPas encore d'évaluation
- 2nd Quarter - Summative Test in TleDocument2 pages2nd Quarter - Summative Test in TleRachelle Ann Dizon100% (1)
- 1 s2.0 S0956713515002546 Main PDFDocument9 pages1 s2.0 S0956713515002546 Main PDFIfwat ThaqifPas encore d'évaluation
- Toolbox Talks Working at Elevations English 1Document1 pageToolbox Talks Working at Elevations English 1AshpakPas encore d'évaluation
- National Railway Museum Annual Review 04-05Document40 pagesNational Railway Museum Annual Review 04-05sol.loredo1705530Pas encore d'évaluation
- Barilla SpaDocument11 pagesBarilla Spavariapratik100% (1)
- Ar ExportsDocument1 pageAr ExportsRais AlamPas encore d'évaluation
- A-ZKD-13 (ZKD-59 (A) ) : Mechanical ParameterDocument1 pageA-ZKD-13 (ZKD-59 (A) ) : Mechanical Parameterwissam zaatuorPas encore d'évaluation
- Lesson 2 Arts of East AsiaDocument21 pagesLesson 2 Arts of East Asiarenaldo ocampoPas encore d'évaluation
- Action, Desire and Subjectivity in Prabhakara MimamsaDocument28 pagesAction, Desire and Subjectivity in Prabhakara Mimamsasiddy_777Pas encore d'évaluation
- Monitoring:: Steps of Adding New SiteDocument8 pagesMonitoring:: Steps of Adding New SiteMohammad ZakoutPas encore d'évaluation
- Air Cooler With Checking DoorDocument2 pagesAir Cooler With Checking DoorSuraj KumarPas encore d'évaluation
- Industrial Training Report (Kapar Power Plant)Document40 pagesIndustrial Training Report (Kapar Power Plant)Hakeemi Baseri100% (2)
- Eureka Forbes ReportDocument75 pagesEureka Forbes ReportUjjval Jain0% (1)
- Computer From ScratchDocument6 pagesComputer From ScratchPaul NavedaPas encore d'évaluation
- Appetizer Summative TestDocument36 pagesAppetizer Summative TestArgelynPadolinaPedernalPas encore d'évaluation
- Aoc f22sDocument43 pagesAoc f22sJoao Jose Santos NetoPas encore d'évaluation
- 32lh250h Commercial Mode PDFDocument46 pages32lh250h Commercial Mode PDFcordero medusaPas encore d'évaluation
- A Duality Principle For The Entanglement Entropy of Free Fermion SystemsDocument12 pagesA Duality Principle For The Entanglement Entropy of Free Fermion SystemsCroco AliPas encore d'évaluation
- History of DiamondsDocument21 pagesHistory of Diamondssilvernitrate1953Pas encore d'évaluation
- Manual OccultismDocument390 pagesManual OccultismJikker Gigi Phatbeatzz Barrow100% (11)
- Ryan's DilemmaDocument11 pagesRyan's DilemmaAkhi RajPas encore d'évaluation
- Audio Spot LightingDocument20 pagesAudio Spot LightingLavanya Vaishnavi D.A.Pas encore d'évaluation
- Electricity and MagnetismDocument29 pagesElectricity and MagnetismNashrul HaqPas encore d'évaluation