Académique Documents
Professionnel Documents
Culture Documents
Periodicity in Atomic Properties:: Period Group
Transféré par
javidfTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Periodicity in Atomic Properties:: Period Group
Transféré par
javidfDroits d'auteur :
Formats disponibles
Periodicity in Atomic Properties:
1.
Atomic Radius:
Within a given period atomic radius decreases from left to right. This is due to the
effect of increase in nuclear charge while the electrons are being added to the same shell.
Within a given group atomic radius increases down the group. This is due to the increase
in number of shells.
Period
1.
2.
3.
Group
IA
IIA
IIIA
IVA
VA
VIA
VIIA
Zero
He
0.37
0.93
Li
Be
Ne
1.34
0.90
0.82
0.77
0.73
0.74
0.72
1.31
Na
Mg
Al
Si
Cl
Ar
1.54
1.30
1.18
1.11
1.06
1.02
0.99
1.74
In the first transition series the atomic size slightly decreases from Sc to Mn because
effect of effective nuclear charge is stronger than the shielding effect. The atomic size from Fe to
Ni remains almost the same because both the effects balance each other.
The atomic size from Cu to Zn slightly increases because shielding effect is more than
effective nuclear charge due to d10 structure of Cu and Zn.
Inner transition elements As we move along the lanthanide series, there is a decrease
in atomic as well as ionic radius. The decrease in size is regular in ions but not so regular in
atoms. This is called lanthanide contraction.
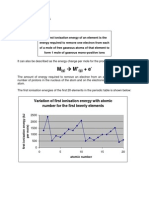
2) Ionisation potential or Ionisation Energy:
Ionization energy increases along the period while decreases down the group.
Factors which influence I.E.
Atomic size: the larger the size of the atom, the smaller the I.E. i.e., I.E.
Effective nuclear charge: The greater the effective charge on the nucleus of an atom, the
more difficult it would be to remove an electron from the atom because electrostatic force of
attraction between the nucleus and the outermost electron increases. So greater energy will be
required to remove the electron.
Penetration effect of orbitals: The order of energy required to remove electron from s,p,d-
and-orbitals of a shell is s>p>d>.
Shielding or screening effect: Screening effect results in decrease of force of attraction
between the nucleus and the outermost electron and lesser energy is required to separate the
electron. Thus the value of I.P. decreases.
Stability of half-filled and fully-filled orbitals: According to Hund's rule the stability of half
filled or completely filled degenerate orbitals is comparatively high. So comparatively more
energy is required to separate the electron from such atoms.
Successive Ionisation Energies
Element
IE1
IE2
IE3
IE4
Li
520.1
7297
11813
1086.2
2352
4620
6221
1402.1
2856
4577
7474
3) Electron Affinity:
Electron affinity increases along the period while decreases down the group.
Factors affecting the magnitude of electron affinity
Atomic size In general electron affinity value decreases with the increasing atomic
radius because electrostatic force of attraction decreases between the electron being added and
the atomic nucleus due to increase of distance between them.
Effective nuclear charge Electron affinity value of the element increase as the effective
nuclear charge on the atomic nucleus increases because electrostatic force of attraction between
the electron being added and the nucleus increases. As the electrostatic force of attraction
increases, amount of energy released is more.
Screening or Shielding effect Electron affinity value of the elements decreases with the
increasing shielding or screening effect. The shielding effect between the outer electrons and the
nucleus increases as the number of electrons increases in the inner shells.
Stability of half filled and completely filled orbitals The stability of half filled and completely filled
degenerate orbitals of a sub shell is comparatively more, so it is difficult to add electron in such
orbitals and lesser energy is released on addition of electron hence the electron affinity value will
decrease.
Vous aimerez peut-être aussi
- Periodicity in Elements NotesDocument7 pagesPeriodicity in Elements NotesjqgjwgnnwkPas encore d'évaluation
- Unit Objectives: Periodic TrendsDocument9 pagesUnit Objectives: Periodic Trendsmartin mulengaPas encore d'évaluation
- Classification of Elements & Periodic Table: Iind PartDocument31 pagesClassification of Elements & Periodic Table: Iind PartKeshan PaudelPas encore d'évaluation
- Chapter 3 Periodic Table (SUEC) AnswerDocument12 pagesChapter 3 Periodic Table (SUEC) AnswerLee AustinPas encore d'évaluation
- Chem 111 Week Three Notes BDocument7 pagesChem 111 Week Three Notes BnyachiosirPas encore d'évaluation
- Electron Configuration and Periodic PropertiesDocument48 pagesElectron Configuration and Periodic Propertiesahmad batataPas encore d'évaluation
- Engineering Chemistry Notes UNIT 4Document9 pagesEngineering Chemistry Notes UNIT 4Nivetha EPas encore d'évaluation
- Self HelpDocument33 pagesSelf HelpHimanshu KumarPas encore d'évaluation
- Document From Michi?Document55 pagesDocument From Michi?audrey abaasaPas encore d'évaluation
- A - Level Inorganic Chemistry 2-1Document171 pagesA - Level Inorganic Chemistry 2-1kitderoger_391648570Pas encore d'évaluation
- Inorganic Chemistry NotesDocument105 pagesInorganic Chemistry NotesOdongo TonnyPas encore d'évaluation
- Atomic RadiusDocument2 pagesAtomic RadiusnapilgabethPas encore d'évaluation
- 2 chapter 2 原子半径以及电离能Document33 pages2 chapter 2 原子半径以及电离能Pingping chenPas encore d'évaluation
- Periodic Properties Copy 2Document30 pagesPeriodic Properties Copy 2Rodayna HosniPas encore d'évaluation
- Ionization EnergyDocument8 pagesIonization EnergyHafiza Sikder AnishaPas encore d'évaluation
- UntitledDocument27 pagesUntitledFatimah AgboolaPas encore d'évaluation
- Periodic Properties of ElementsDocument53 pagesPeriodic Properties of Elementschandro57Pas encore d'évaluation
- Btech Chem NIFFT Part 1 PDFDocument53 pagesBtech Chem NIFFT Part 1 PDFকৃ ষ্ণাPas encore d'évaluation
- Classification of ElementsDocument74 pagesClassification of ElementsJimit Patel BankPas encore d'évaluation
- Periodic Trends: ObjectivesDocument41 pagesPeriodic Trends: Objectivessabhari_ramPas encore d'évaluation
- Chapter 3 NotesDocument7 pagesChapter 3 NotesNickPas encore d'évaluation
- Periodic Properties and Their Graduation in Group andDocument14 pagesPeriodic Properties and Their Graduation in Group andbhogalsahib1408Pas encore d'évaluation
- Chem HL PeriodicityDocument5 pagesChem HL PeriodicityCsya Raynold NgPas encore d'évaluation
- Ion EnergiesDocument4 pagesIon EnergiesNamratha NairPas encore d'évaluation
- Nuclearchemistrybsci 170708171032Document42 pagesNuclearchemistrybsci 170708171032Victor OkosunPas encore d'évaluation
- Periodic Properties: Chemistry For (I.I.T. J.E.E.)Document11 pagesPeriodic Properties: Chemistry For (I.I.T. J.E.E.)Gagan Raj JaiswalPas encore d'évaluation
- Periodic Table and PeriodicityDocument11 pagesPeriodic Table and Periodicityakeemoluwadamilare623Pas encore d'évaluation
- Chapter 7 Jan13Document98 pagesChapter 7 Jan13kumuthaPas encore d'évaluation
- S5 Inorganic Chemistry NotesDocument30 pagesS5 Inorganic Chemistry Notesssekandie79Pas encore d'évaluation
- Ionisation Energy: Chong Zhi Ling & Chang Jie YingDocument7 pagesIonisation Energy: Chong Zhi Ling & Chang Jie YingJun Hong TeePas encore d'évaluation
- CHE222 - Periodicity - 2023Document45 pagesCHE222 - Periodicity - 2023Student 365Pas encore d'évaluation
- Periodic Table (4,5,6)Document37 pagesPeriodic Table (4,5,6)rehanfazal9669Pas encore d'évaluation
- Chapter 2 - Electrons in AtomsDocument32 pagesChapter 2 - Electrons in AtomsFandyPas encore d'évaluation
- AS Level Summary-Unit 1Document11 pagesAS Level Summary-Unit 1Filiz Kocayazgan ShanablehPas encore d'évaluation
- Ionisation EnergyDocument12 pagesIonisation Energyhahajing1980Pas encore d'évaluation
- CMOS Fundamentals ?Document45 pagesCMOS Fundamentals ?Sushma ShivaniPas encore d'évaluation
- Atomic Radii: R Is A Radius of AtomDocument7 pagesAtomic Radii: R Is A Radius of AtomGHS Chak JhumraPas encore d'évaluation
- Lattice Energy CIE Chemistry A2 Chemical EnergeticsDocument2 pagesLattice Energy CIE Chemistry A2 Chemical EnergeticsdanielmahsaPas encore d'évaluation
- Periodicity: Ionisation EnergyDocument3 pagesPeriodicity: Ionisation EnergyFouziaPas encore d'évaluation
- A039level Chemistry Inorganic NotesDocument108 pagesA039level Chemistry Inorganic NotesNasser SsennogaPas encore d'évaluation
- Nuclei in One Shot - Class Notes - LEC-6 - PPT-6 - Physics - Nuclei - Vijeta Series - Kshitiz Kanik Sir - RAM NIWASDocument61 pagesNuclei in One Shot - Class Notes - LEC-6 - PPT-6 - Physics - Nuclei - Vijeta Series - Kshitiz Kanik Sir - RAM NIWASprajwal jhaPas encore d'évaluation
- Atomic Radii: Atomic Structure: Periodic TrendsDocument5 pagesAtomic Radii: Atomic Structure: Periodic TrendsRajat SharmaPas encore d'évaluation
- Factors Affecting The Ionisation EnergyDocument3 pagesFactors Affecting The Ionisation EnergyTi Yee SanPas encore d'évaluation
- Tutorial 38, Ionisation Energy, Electron Gain Enthalpy and ElecronegativityDocument16 pagesTutorial 38, Ionisation Energy, Electron Gain Enthalpy and ElecronegativityDYES Motion GraphicsPas encore d'évaluation
- 05 - CHEM 111 - Lecture 5 - Periodicity of The ElementsDocument50 pages05 - CHEM 111 - Lecture 5 - Periodicity of The ElementsBrian NkhomaPas encore d'évaluation
- Factors Affecting Atomic RadiiDocument1 pageFactors Affecting Atomic RadiiChun Hui Lee50% (2)
- Ionisation EnergyDocument6 pagesIonisation EnergyAathifa ThowfeekPas encore d'évaluation
- 4.2: Variation of Physical and Chemical PropertiesDocument68 pages4.2: Variation of Physical and Chemical PropertiesAnisha Syazwana Binti RoslyPas encore d'évaluation
- Electronegativity Is The Ability of An Atom To Attract A Pair of Electrons Towards Itself in ADocument5 pagesElectronegativity Is The Ability of An Atom To Attract A Pair of Electrons Towards Itself in AREZA FARDGHASSEMIPas encore d'évaluation
- 5033 375 2 51613PeriodicpropertiesofElementsDocument31 pages5033 375 2 51613PeriodicpropertiesofElementsvader1534Pas encore d'évaluation
- Periodic Properties - Part 3Document38 pagesPeriodic Properties - Part 3Bhavesh GargPas encore d'évaluation
- Group Trends of Chemical PropertiesDocument12 pagesGroup Trends of Chemical PropertiesZeeshan ul-haqPas encore d'évaluation
- Chapter 3 ing - - نسخةDocument11 pagesChapter 3 ing - - نسخةThë Nãüght RayenëPas encore d'évaluation
- The Electron Density in An Atom Extends Far Beyond The Nucleus But We Define The Size of An Atom in Terms of Its Atomic Radius - One-Half TheDocument31 pagesThe Electron Density in An Atom Extends Far Beyond The Nucleus But We Define The Size of An Atom in Terms of Its Atomic Radius - One-Half TheAhRun5171Pas encore d'évaluation
- Electrons in Atoms.Document62 pagesElectrons in Atoms.Md.Tanjim reza TurjoPas encore d'évaluation
- 3.2 Periodic TrendsDocument45 pages3.2 Periodic TrendsAli Mohamed ShiplPas encore d'évaluation
- Periodic Trends Resource 1Document8 pagesPeriodic Trends Resource 1anitPas encore d'évaluation
- Periodicity Part 1Document1 pagePeriodicity Part 1Green SlimePas encore d'évaluation
- Chem Inorganic A'levelDocument95 pagesChem Inorganic A'levelAlvin HavinzPas encore d'évaluation
- Gss Ug en PDFDocument165 pagesGss Ug en PDFk_gh22Pas encore d'évaluation
- Conserved Vector CurrentDocument11 pagesConserved Vector CurrentAbhass KumarPas encore d'évaluation
- All MAR17Document1 571 pagesAll MAR17mkromelPas encore d'évaluation
- Cam B3lypDocument7 pagesCam B3lypAnonymous QfKMOvREJ1Pas encore d'évaluation
- Elektronika Daya Dan PenerapannyaDocument30 pagesElektronika Daya Dan PenerapannyaMuhammad AfdalPas encore d'évaluation
- Grade 10 IB Bridging Course - Chemistry: Topic 1 Atomic Structure Part 3 Electron ArrangementDocument9 pagesGrade 10 IB Bridging Course - Chemistry: Topic 1 Atomic Structure Part 3 Electron ArrangementMarc LoPas encore d'évaluation
- Quantum Mechanics NotesDocument285 pagesQuantum Mechanics NotesJoshPas encore d'évaluation
- Revised Lecture 10 - GaN SemiconductorDocument42 pagesRevised Lecture 10 - GaN SemiconductorHabib ShinwariPas encore d'évaluation
- D & F Block Short NotesDocument4 pagesD & F Block Short NotesAlokPas encore d'évaluation
- Electronic Devices and Circuits ECE 001 (TIP Reviewer)Document33 pagesElectronic Devices and Circuits ECE 001 (TIP Reviewer)James Lindo100% (2)
- Notes On Semiconductor Physics For Electronic DevicesDocument27 pagesNotes On Semiconductor Physics For Electronic DevicesspyseetunaPas encore d'évaluation
- PS1Document2 pagesPS1Saswata RoyPas encore d'évaluation
- Niels BohrDocument2 pagesNiels Bohrsonuisbeast123456Pas encore d'évaluation
- Physics and Technology - Vishay SemiconductorsDocument8 pagesPhysics and Technology - Vishay Semiconductorsdanny_nikhilPas encore d'évaluation
- HW 4 Solution PDFDocument5 pagesHW 4 Solution PDFyesPas encore d'évaluation
- Structural ProblemsDocument217 pagesStructural ProblemsAbdelqader ShurrabPas encore d'évaluation
- Metallic Bonding Explained:-: NotesDocument2 pagesMetallic Bonding Explained:-: NotesAlex noslenPas encore d'évaluation
- Niels Hansen. Metallurgical Transactions 16A (1985) - P. 2167-2190.Document24 pagesNiels Hansen. Metallurgical Transactions 16A (1985) - P. 2167-2190.Bruna OliveiraPas encore d'évaluation
- Darmstadt Vlsi Design CourseDocument533 pagesDarmstadt Vlsi Design Courseapi-3722592100% (1)
- Chemical Bonding I: Lewis TheoryDocument33 pagesChemical Bonding I: Lewis TheoryBiruk BtPas encore d'évaluation
- Inhomogeneous Electron GasDocument8 pagesInhomogeneous Electron GasJosé Luis Rosas HuertaPas encore d'évaluation
- Ceramics Tech #ElectroceramicsDocument51 pagesCeramics Tech #Electroceramicsnawa100% (2)
- Las Science 9 Melc 3 q2 Week 3Document6 pagesLas Science 9 Melc 3 q2 Week 3LINDSY MAE SULA-SULAPas encore d'évaluation
- Anderson Review Magnetic Exchange 1963Document116 pagesAnderson Review Magnetic Exchange 1963GustavoDinizPas encore d'évaluation
- hw2 (NEW)Document3 pageshw2 (NEW)Willie ChangPas encore d'évaluation
- Vlsi Design Unit 1Document51 pagesVlsi Design Unit 1reneeshczPas encore d'évaluation
- Physics of Semiconductor Devices: 12.1.1 Direct Band Gap SemiconductorsDocument4 pagesPhysics of Semiconductor Devices: 12.1.1 Direct Band Gap SemiconductorsNinad GawhalePas encore d'évaluation
- Bloch's Theorem - Definition From AnswersDocument1 pageBloch's Theorem - Definition From AnswersJustice SompoPas encore d'évaluation
- Ch. 7 Dislocation and StrengtheningDocument6 pagesCh. 7 Dislocation and Strengtheningravi hargassnerPas encore d'évaluation