Académique Documents
Professionnel Documents
Culture Documents
Kinet HW
Transféré par
BobDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Kinet HW
Transféré par
BobDroits d'auteur :
Formats disponibles
Errors?
Please direct to Jay Joshi
<jjoshi7@gatech.edu>
2. Vinyl chloride can be synthesized by reaction of acetylene with hydrochloric acid over a
mercuric chloride catalyst at 500 K and 5.0 atm total pressure. An undesirable side reaction is
the subsequent reaction of vinyl chloride with HCl. These reactions are illustrated below.
The equilibrium constants at 500 K are 6.6 x 103 and 0.88 for reaction 1 and 2, respectively.
Assume ideal behavior.
Solution
a) Find the equilibrium composition at 5.0 atm and 500 K for the case when acetylene
and HCl are present initially as an equimolar mixture. What is the equilibrium
conversion of acetylene?
First, write out the ICE table for the two reactions. Keep in mind, the feed is equimolar:
The respective equilibrium constants can also be broken up into Ky and KP, using the ideal
gas assumption to ignore K:
1 = 1 1 = (
2 = 2 2 = (
) (1 )
) (1 )
You can then substitute in 1 and 2 for the vapor mole fractions in both equations. Plugging
in the given equilibrium constants at reaction conditions as well transforms the equations:
(1 2 )(1 1 2 )
1
5 (0.5 1 )(0.5 1 2 )
1
2 (1 1 2 )
0.88 =
5 (1 2 )(0.5 1 2 )
6.6 103 =
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
Now, we can construct our ICE table to relate the equilibrium constants to composition:
Initial
Change
Final
Toluene
1 -21-22-23 1-21-22-23
Para
0 1
Ortho
0 2
Meta
0 3
1+2+3
0 1+2+3
TOTAL
1
So we can relate our individual equilibrium constants to composition directly, since we made the
ideal gas assumption, and are operating at an assumed standard state pressure of 1 atm:
Benzene
( )( )
1 (1 + 2 + 3 )
=
= 0.075
2
(1 21 22 23 )2
( )( )
2 (1 + 2 + 3 )
2 =
=
= 0.078
(1 21 22 23 )2
2
( )( )
3 (1 + 2 + 3 )
1 =
=
= 0.167
(1 21 22 23 )2
2
This is a system of three equations and three unknowns. The s can be solved to find:
1 =
1 = 0.062
2 = 0.065
3 = 0.138
Now, the composition can be evaluated:
Toluene:
Benzene:
Ortho-xylene:
Meta-xylene:
Para-xylene:
= 1 21 22 23 = 1 2 0.062 2 0.065 2 0.138 = .
= 1 + 2 + 3 = 0.062 + 0.065 + 0.138 = .
= 2 = .
= 3 = .
= 1 = .
A shape-selective catalyst can be utilized to increase para-xylene selectivity, since paraxylene has a smaller kinetic diameter than the other isomers. The catalyst could allow mostly
para-xylene to diffuse out of it to shift the product distribution.
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
Solving the system of equations gives 1 and 2, which are can then be directly plugged in to
the equations found in the ICE table above to find the outlet composition:
To find equilibrium conversion of acetylene, we use the equation:
= 1
0.012
=1
= .
0.5
b) Redo part (a) with a large excess of inert gas. Assume the inert gas constitute 90 vol.
% of the initial gas mixture.
We now have to rewrite the ICE table. To account for the addition of inerts, add 9 moles to
the previous 1 mole basis to obtain 90 vol% of inerts:
Relating the information on the table above to the equilibrium constants, our system of
equations now becomes:
1 (1 2 )(10 1 2 )
5 (0.5 1 )(0.5 1 2 )
1
2 (10 1 2 )
0.88 =
5 (1 2 )(0.5 1 2 )
6.6 103 =
Solving for 1 and 2, we can evaluate the compositions of the final mixture:
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
Analogously to part (a), the equilibrium conversion is found using the new final acetylene
amount:
, = 1
0.0014
= .
0.5
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
3. A first-order homogenous reaction of A going to 3B is carried out in a constant pressure
batch reactor. It is found that starting with pure A, the volume after 12 min is increased by 70
percent at a pressure of 1.8 atm. If the same reaction is to be carried out in a constant volume
reactor and the initial pressure is 1.8 atm, calculate the time required to bring the pressure to
2.5 atm.
Solution
First, we need to solve for the reaction rate constant. Start with the batch reactor design
equation. Remember, we are told the reaction is first-order, so we can write:
= =
Integrate the differential equation to obtain the solution form for a first order reaction:
= []
We do not know . However, we can find it through the molar expansion factor. To do this,
start by using the ideal gas law to define the ratio of initial and final total moles:
The vessel is constant pressure, so P does not change. We are also given that there is a 70%
increase in volume after 12 minutes. This information let us simplify our ratio:
0.7 +
=
=
= 1.7
We relate this ratio of total moles to conversion using the following equation:
= 1 +
The expansion factor can be found using the given information
31
= (1) (
)=2
|1|
Write conversion in terms of . Then, plug in the appropriate numbers and solve:
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
1.7 = 1 + 2 (1
= 0.65
Now, we can plug this into our solution of the batch reactor balance to solve for the reaction
rate constant:
0.65 = [ 12]
= 0.0359 1
Now, we can start to consider the actual variable pressure case in the problem statement.
However, from the design equation will not be the same as before. We can still find it
using the same method though. So, we write out the ideal gas law again:
2.5
= =
= 1.389
1.8
The expansion factor is unchanged. Solving for
1.389 = 1 + 2 (1
= 0.8055
Now, plug this into the batch reactor design equation we derived before to solve for time:
0.8055 = [(0.03591 )]
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
4. At room temperature, sucrose is hydrolyzed by the catalytic action of the enzyme sucrase
as follows:
sucrose products
Starting with a sucrose concentration CA0 = 1.0 millimol/liter and an enzyme concentration
CE0 = 0.01 millimol/liter, the following kinetic data are obtained in a batch reactor
(concentrations calculated from optical rotation measurements):
CA, millimol/liter
t, hr
0.84 0.68 0.53
1
0.38 0.27
4
0.16
0.09 0.04
7
0.018 0.006 0.0025
9
10
Determine whether these data can be reasonably fitted by a kinetic equation of the MichaelisMenten type, or
where CM = Michaelis constant. If the fit is reasonable, evaluate the constants k3 and CM. Solve
by the integral method.
Solution
This problem is an example of using linear regression to fit data. The Michaelis-Menten equation
can be put into a linear form, where it can then be compared to the collected data to see if the
data fits the model.
With this in mind, we can attempt to linearize the given rate equation. To simplify this, we make
some substitutions:
4 =
5 =
Substituting:
=
3
4
=
=
+ 1 + 5
11
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
Now, we have an ODE, which we can separate and integrate. This is the integral method of
fitting rate equations:
HINT: This integral
appears difficult, but try
separating the fraction,
and then integrating it in
two, simpler parts
1 + 5
=
4
5 ( 0 ) + | |
0
5 ( 0 ) |0 |
+
=
4
4
Transforming to put into a more useful form (linear equation):
5 +
| |
0
(0 )
| |
0
(0 )
Dependent variable
4
(0 )
4
5
(0 )
Slope
y-intercept
Independent
Variable
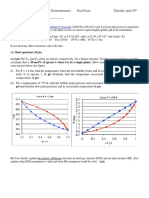
We can now plot our data, and see if it fits our derived model. A sketch of the graph is shown
below:
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
As written on the figure, fitting the data to our model reveals our unknown parameters:
So our final rate equation becomes:
3 = .
= .
.
+ .
To verify if our fit is appropriate, we can compare the given data to that generated from our
linearized Michaelis-Menten equation. The data that was used to generate the plot above is
shown. Because we successfully fit our data to the Michaelis-Menten model, we have shown
that it can be reasonably fitted:
=
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
5. An ampoule of radioactive Kr-89 (half life = 76 minutes) is set aside for a day. What does
this do to the activity of the ampoule? Note that radioactive decay is a first-order process
Solution
To begin, activity is a thermodynamic quantity, which is defined from the chemical
potential of a species relative to a selected standard state. Iin this context, it can be assumed
to be the effective concentration of Kr-89.
We are given that this is a first order process. From the design equation, we know that the
general equation for this reaction becomes:
0
| =
We are also given information about the half-life of the ampoule. At t = 76 min, we know that
CA = 0.5CA0. This allows us to solve the reaction rate constant:
|
0
|
| = |2| = (76)
0.50
= 0.00912 1
So to gauge the relative change in activity, we can pick another time, and see how the
concentration changes. For the half-life, we found = 0.5. Lets find what this ratio
becomes after one day has elapsed:
24 60
= [(0.00912 1 ) (1
)]
0
= 1.98 106
0
So, the activity has dropped to about 2 106 of its original value
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
6. Find the first-order rate constant for the disappearance of A in the gas reaction 2A R if,
on holding the pressure constant, the volume of the reaction mixture, starting with 80% A,
decreases by 20% in 3 min.
Solution
Assume: Ideal gas
This problem is very similar to #3 on this assignment. To begin, we write the batch reactor
design equation. We are told implicitly through the information regarding the reaction rate
constant that the reaction must be first order:
= =
Which yields the solution:
= []
To find k, we must first find
. We can do this by first evaluating what the change in the
total number of moles are. This can be done by through the ideal gas assumption:
Pressure and temperature are held constant. We are also told that the volume decreases by
20% at a given time, so:
0.2
=
=
= 0.8
Additionally, we write the relationship relating the change in total moles to the conversion of
our reactant and the molar expansion factor:
= 1 +
Conversion of A can be written in terms of moles of A:
= 1
Plugging in:
Errors? Please direct to Jay Joshi
<jjoshi7@gatech.edu>
=
1
+
(1
Now, rearrange this equation so that it can be used to plug in for :
+ 1
=
Plug this into our derived solution to the design equation:
+ 1
= []
+ 1
1
= |
|
We can almost solve for the reaction rate constant through the design equation, but we still
need to solve for the molar expansion factor. Refer to the equation from Fogler:
= 0
The problem statement gives us that the initial mole fraction of A is 80%. So, we can
evaluate A:
= (0.8) ( 1)
--Where "r" and "a" are the stoichiometric coefficients of species R and A, respectively
1
= (0.8) ( 1) = 0.4
2
Now, we solve for the reaction rate constant directly through our modified design equation:
=
(0.4) + 1 0.8
1
|
|
(0.4)
3
= .
Vous aimerez peut-être aussi
- Part 1 Dynamic Modeling - 2022Document19 pagesPart 1 Dynamic Modeling - 2022MUHAMMAD LUQMAN HAKIMI MOHD ZAMRIPas encore d'évaluation
- MATLAB Ordinary Differential Equation (ODE) Solver For A Simple ExampleDocument8 pagesMATLAB Ordinary Differential Equation (ODE) Solver For A Simple ExampleVENKI CHPas encore d'évaluation
- P11 5BDocument7 pagesP11 5BFachri MunadiPas encore d'évaluation
- CBE3508 Sp21 FinalDocument6 pagesCBE3508 Sp21 Finalsasuke uchihaPas encore d'évaluation
- Chemical Equibria - 2021 - 2023Document27 pagesChemical Equibria - 2021 - 2023Vincent AnzoPas encore d'évaluation
- CH 301 Practice Questions 2023Document20 pagesCH 301 Practice Questions 2023Fortune VushePas encore d'évaluation
- Problem 2: First and Second Law of Thermodynamics Thermodynamics Coursechemical Engineering DepartmentDocument17 pagesProblem 2: First and Second Law of Thermodynamics Thermodynamics Coursechemical Engineering DepartmentTita Ari UtamiPas encore d'évaluation
- Themo Tutorials Part 1Document59 pagesThemo Tutorials Part 1Jenae CarlsonPas encore d'évaluation
- Chemical Engineering ThermodynamicsDocument10 pagesChemical Engineering ThermodynamicsP P DPas encore d'évaluation
- Centeno, CRE PDFDocument10 pagesCenteno, CRE PDFrj centenoPas encore d'évaluation
- First LawDocument10 pagesFirst LawAhmed Al-ayatPas encore d'évaluation
- Example CH 9Document4 pagesExample CH 9Uday Prakash SahuPas encore d'évaluation
- HW1 SolutionsDocument2 pagesHW1 SolutionsAbdul Aziz KhanPas encore d'évaluation
- Kathmandu University: School of EngineeringDocument19 pagesKathmandu University: School of EngineeringBishal LamichhanePas encore d'évaluation
- MP 2 Finite Element MethodDocument12 pagesMP 2 Finite Element MethodDon Nelson CaserPas encore d'évaluation
- CET I 3.PVT Relationship 2021 Part 2Document47 pagesCET I 3.PVT Relationship 2021 Part 2Dhruv AgarwalPas encore d'évaluation
- Problem Set 1 SolutionsDocument15 pagesProblem Set 1 SolutionsNathan HalePas encore d'évaluation
- Tugas Termo DameDocument13 pagesTugas Termo DamedesisitompulPas encore d'évaluation
- Reactors Design - 1603888, 1599618 I 1603509Document9 pagesReactors Design - 1603888, 1599618 I 1603509saramartori.2002Pas encore d'évaluation
- The ClausiusDocument12 pagesThe ClausiusjokishPas encore d'évaluation
- Gas Technology-4th Stage Lecture 2 12 November 2017Document7 pagesGas Technology-4th Stage Lecture 2 12 November 2017muhammadPas encore d'évaluation
- Week 10 Tutorial Chem R Eng 08-02-2023Document13 pagesWeek 10 Tutorial Chem R Eng 08-02-2023Zain Ul AbedinPas encore d'évaluation
- 2016년봄 열전기Exam1Document7 pages2016년봄 열전기Exam1윤성욱Pas encore d'évaluation
- Preinforme 3Document8 pagesPreinforme 3Andres Felipe MozoPas encore d'évaluation
- TDY1 - Les 3 EngDocument28 pagesTDY1 - Les 3 EngBrandon GesterkampPas encore d'évaluation
- Virial Equation of State2Document46 pagesVirial Equation of State2ShainaBagonPas encore d'évaluation
- Graphite Oxide (GO) Decomposition Kinetics: Electronic Supplementary InformationDocument7 pagesGraphite Oxide (GO) Decomposition Kinetics: Electronic Supplementary Informationbai tap hoa vo coPas encore d'évaluation
- Tugas 3 - TRK 02 - Kelompok 8Document61 pagesTugas 3 - TRK 02 - Kelompok 8Sulthan DaffaPas encore d'évaluation
- Control System LabDocument15 pagesControl System Labhassan ullah khanPas encore d'évaluation
- MCEE5210 2023F L6 Rate-Based MethodDocument74 pagesMCEE5210 2023F L6 Rate-Based Methodtc1992423Pas encore d'évaluation
- Taller Ingenieria de Las Reacciones - Determinacion Del Orden de ReaccionDocument15 pagesTaller Ingenieria de Las Reacciones - Determinacion Del Orden de ReaccionJesus JulioPas encore d'évaluation
- HW 01 SolutionDocument12 pagesHW 01 Solutionmaulida rahmiPas encore d'évaluation
- Reversible Work: Constant and Constant Closed System ProcessesDocument4 pagesReversible Work: Constant and Constant Closed System ProcessesnnbPas encore d'évaluation
- ProblemsDocument4 pagesProblemsmarslanjameelmalikPas encore d'évaluation
- Chemistry 26: Analytical Chemistry Long Exam 1: Equation Bank + GuideDocument4 pagesChemistry 26: Analytical Chemistry Long Exam 1: Equation Bank + GuideJustin GonzagaPas encore d'évaluation
- Week 4Document28 pagesWeek 4Mohamed nasserPas encore d'évaluation
- Stoichiometric Numbers Continue...Document11 pagesStoichiometric Numbers Continue...Hambeleleleni NgotipeniPas encore d'évaluation
- 3 - 1 Laplace TransformDocument23 pages3 - 1 Laplace TransformShilpya Kurniasih100% (1)
- RICE Table WKST KEYDocument11 pagesRICE Table WKST KEYJonathan ZhangPas encore d'évaluation
- SLab 2022 Internal QPDocument4 pagesSLab 2022 Internal QPChemical StudentsPas encore d'évaluation
- Tut6 SolnDocument10 pagesTut6 SolnJaishuu ChoudharyPas encore d'évaluation
- Cre Lab AssignmentDocument7 pagesCre Lab AssignmentRidaPas encore d'évaluation
- C5Document4 pagesC5conker4Pas encore d'évaluation
- Equations & ConstantsDocument5 pagesEquations & ConstantsJoserinePas encore d'évaluation
- Lab Report 1Document24 pagesLab Report 1Jaymac100% (1)
- Reactive Energy BalanceDocument12 pagesReactive Energy BalanceGrey DavePas encore d'évaluation
- Thermal Stresses: Mechanics of Deformable BodiesDocument15 pagesThermal Stresses: Mechanics of Deformable BodiesJake CanlasPas encore d'évaluation
- F 11 Place 2Document8 pagesF 11 Place 2paimoPas encore d'évaluation
- TOLENTINO. Exam1 PDFDocument5 pagesTOLENTINO. Exam1 PDFJoseph TolentinoPas encore d'évaluation
- Chemistry QuestionsDocument8 pagesChemistry QuestionsGundanPas encore d'évaluation
- Arrhenius and Half Life WorksheetDocument12 pagesArrhenius and Half Life WorksheetHaren Aizhel TenderoPas encore d'évaluation
- Chapter Two Volumetric Properties of Pure FluidsDocument18 pagesChapter Two Volumetric Properties of Pure Fluidsc74zdwfbzhPas encore d'évaluation
- Exercises of Integrals and Integro-Differentials EquationsD'EverandExercises of Integrals and Integro-Differentials EquationsPas encore d'évaluation
- O Level Biology Practice Questions And Answers EnzymesD'EverandO Level Biology Practice Questions And Answers EnzymesÉvaluation : 5 sur 5 étoiles5/5 (1)
- 13 - NucleiDocument17 pages13 - Nucleigauthamdoc321Pas encore d'évaluation
- Chem Kinet Meeting 2Document27 pagesChem Kinet Meeting 2Nuril AzmiPas encore d'évaluation
- Nuril Trisnawati Te2Document3 pagesNuril Trisnawati Te2Nuril Trisnawati83% (6)
- Genmath Long ExamDocument3 pagesGenmath Long ExamJessa BarberoPas encore d'évaluation
- 1967 Fallout Shelter ManagementDocument116 pages1967 Fallout Shelter ManagementLouie_popwhatski100% (1)
- Relative AgeDocument20 pagesRelative AgeBing KadulPas encore d'évaluation
- Ans Tutorial Nuclear 1Document8 pagesAns Tutorial Nuclear 1Bisharah NizamPas encore d'évaluation
- NW6Document10 pagesNW6Mamidala HarithaPas encore d'évaluation
- A First Course in DynamicsDocument435 pagesA First Course in DynamicsElseaLi-dongCuiPas encore d'évaluation
- FIS1234 Physics III Chapter 10 Radioactivity Tutorial QuestionsDocument2 pagesFIS1234 Physics III Chapter 10 Radioactivity Tutorial QuestionsrenPas encore d'évaluation
- UntitledDocument6 pagesUntitledAaron EscovillaPas encore d'évaluation
- Exponentials and Logarithms: MathematicsDocument16 pagesExponentials and Logarithms: MathematicsMahima AgrawalPas encore d'évaluation
- Analytic Expressions For Alpha Decay Half-Lives and Potential Barriers-RoyerDocument13 pagesAnalytic Expressions For Alpha Decay Half-Lives and Potential Barriers-RoyerJhoan PerezPas encore d'évaluation
- 83 Revision Questions For IGCSE Questions Solutions PDFDocument5 pages83 Revision Questions For IGCSE Questions Solutions PDFChui Wai100% (4)
- Transes Fundamentals of PharmacologyDocument4 pagesTranses Fundamentals of PharmacologyCHINGCHONG SLAYERPas encore d'évaluation
- Bo de Thi Thu 2019 Tieng Anh LovebookDocument18 pagesBo de Thi Thu 2019 Tieng Anh Lovebookhoanganh ytPas encore d'évaluation
- Reading ToeflDocument6 pagesReading ToeflViciAlfanani100% (3)
- Atomic & Nuclear Physics Answers PDFDocument8 pagesAtomic & Nuclear Physics Answers PDFtaimoor2Pas encore d'évaluation
- Basic & Clinical PKDocument24 pagesBasic & Clinical PKGopal pokhrelPas encore d'évaluation
- Atomic and Nuclear Physics: Determining The Half-Life of Ba 137 MDocument4 pagesAtomic and Nuclear Physics: Determining The Half-Life of Ba 137 MPedroPas encore d'évaluation
- Mata Pelajaran Tingkatan Tajuk: Sains Tingkatan 3 Revision FOR PT3 Complete PBSDocument15 pagesMata Pelajaran Tingkatan Tajuk: Sains Tingkatan 3 Revision FOR PT3 Complete PBSmalaomarPas encore d'évaluation
- Nuclear Technology and EnergyDocument4 pagesNuclear Technology and EnergyJessica Anna LuchkaPas encore d'évaluation
- Biological Diversity WSDocument104 pagesBiological Diversity WSSuzy AwadPas encore d'évaluation
- Avhusifhxm &RNFH Tcef (13) Ar Cgef A (Mif Twgufykpäm/: DR Vince Grade 10 Physics: Questions and ProblemsDocument2 pagesAvhusifhxm &RNFH Tcef (13) Ar Cgef A (Mif Twgufykpäm/: DR Vince Grade 10 Physics: Questions and ProblemsPyae Sone KyawPas encore d'évaluation
- PDFDocument38 pagesPDFJohn Paul EspañoPas encore d'évaluation
- Igcse 73 ApplicationsofradioactivityDocument27 pagesIgcse 73 ApplicationsofradioactivityHany ElGezawy100% (1)
- AlphaDocument6 pagesAlphaArdhy ArmawanPas encore d'évaluation
- Review Exercises: Concept ReinforcementDocument4 pagesReview Exercises: Concept Reinforcementlinh nguyenPas encore d'évaluation
- Che311 1Document112 pagesChe311 1flowealthPas encore d'évaluation
- General Mathematics Activity Sheet Week 5 6Document4 pagesGeneral Mathematics Activity Sheet Week 5 6Luke JessieMhar LumberioPas encore d'évaluation