Académique Documents
Professionnel Documents
Culture Documents
Working Problem 5 - Semester 1, 2016: Medical Science
Transféré par
dannyboy_588Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Working Problem 5 - Semester 1, 2016: Medical Science
Transféré par
dannyboy_588Droits d'auteur :
Formats disponibles
BMed JMP Year 1 Learning Targets
Working Problem 5 Semester 1, 2016
Pt: Alison Mahon Paracetamol OD (48 units), w/ 250ml of 40% AbV ETOH
Medical Science
How does the body deal with ingested toxins and drugs? What role does the liver play? - Liver Function Tests.
What is the normal structure, substructure and function of the liver? (Anatomy, Physiology) - Liver
Function Tests.
Heaviest gland in the body
Location: inferior to the diaphragm, occupying most of right hypochondriac and part of
epigastric region
http://swissknifev.files.wordpress.com/2009/01/liver-abdomen.jpg
http://media-2.web.britannica.com/eb-media/13/74313-004-31BFAEEC.jpg
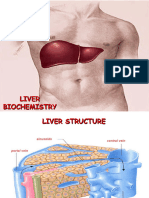
Gross Anatomy
Right lobe- larger
Quadrate lobe (inferior part)
Caudate lobe (posterior
Anatomically part of right lobe but histologically more
like left lobe
Left lobe- smaller
Falciform ligament divides left and right lobe- originates
at inferior surface of the diaphragm between the 2 lobes
and inserts on superior surface of the liver
blood supply:
portal vein- deoxygenated, nutrient rich blood from GI
brings blood to be detoxified and screened for foreign
materials
hepatic artery- brings oxygenated blood for hepatic cell
respiration
hepatic vein- after liver cleaned blood flows through
hepatic vein into vena cava, just superior to diaphragm
BMed JMP Year 1 Learning Targets
Histology
1. Hepatocytes
Specialized (differentiated) epithelial cells- major functional cells of
5-12 sides- 3D arrangement called hepatic laminae
Make up 80% of the liver mass
Secrete bile into bile canaliculi for release into duodenum
Pericential hepatocytes in distal zone (III) secrete enzymes which
metabolize, detoxify or inactivate compounds
Exogenous compounds- drugs and toxins
Endogenous compounds- steroid hormones
Protein synthesis
Cholesterol synthesis
Synthesis of bile salts and phospholipids
Glycogenesis- conversion of glucose to glycogen for storage
Glycogenolysis- conversion of stored glycogen to usable energy
Arrangement of Hepatocytes
Hepatic acinus is latest and most up to date hypothesis
http://www.nature.com/nri/journal/v6/n3/images/nri1784-f1.jpg
http://www.hakeem-sy.com/main/files/acinus_liver.jpg
2. Bile Canaliculi
small ducts between hepatocytes which collect secreted bile and channel from liver
Bile canaliculi bile ductules bile ducts left and right converge common hepatic duct
cystic duct (usually, if being stored in gall bladder temporarily) sphincter of Oddi bile duct
duodenum
3. Hepatic sinusoids
Highly permeable blood vessels between rows of hepatocytes
Brings oxygenated blood for respiration from hepatic artery
Brings blood for filtering by hepatocytes from portal veins
converge to central veins hepatic vein vena cava
Phagocytic Kupffer cells part of immunological defence.
Portal triads
Collective name for a branch of hepatic artery, vein and a bile duct grouped together
Functions of the Liver
the liver
BMed JMP Year 1 Learning Targets
1. Carbohydrate metabolism
carbohydrates are hydrolysed by enzymes in the digestive process into simple monosaccharides
80% glucose
20% fructose and galactose- most of which is converted to glucose by hepatocytes in liver
Converts lactic acid to glucose
1.2 Glucose Catabolism (respiration)
Step 1: Glycolysis
one molecule of glucose (6 carbon chain) converted into 2 molecules of pyruvic acid (3 carbon chains)
10 chemical reactions- regulated by at least 1 specific enzyme
Anaerobic
Glucose + 2ADP + 2PO4 (2-)
2 pyruvic acid + 2ATP + 4H
Step 2: Formation of Acetyl conenzyme A
prepares pyruvic acid for Krebs cycle- for conversion to energy
CO2 and 2H+ released
Produces NADH + H+ and CO2
Step 3: Krebs Cycle
series of reactions in which acetyl portion of acetyl coenzyme A is degraded to CO2 and H
NADH + H+ (energy containing) or FADH2, ATP and CO2 produced
Step 4: Electron transfer chain reactions
NADH +H+/ FADH2 oxidised, transferring electrons and releasing energy from ATP
Formula:
C6H12O6 + 6O2 + 36/38 ADP + 36/38 Phosphate 6CO2+ 6H2O+ 36/38 ATP
1.3 Glucose Anabolism
Glucose Storage: Glycogenesis
hepatocytes and skeletal muscle cells stimulated by insulin to begin storing when glucose for respiration is in excess
glucose monomers converted to polysaccharide glycogen and stored I hepatocytes
Glucose Release: Glycogenolysis
when needed stored glycogen in hepatocytes can be released directly into bloodstream, skeletal muscle cells also store but indirect
release to blood
lactic acid produced, most converted by liver to glucose also
Formation on Glucose from fats and proteins
certain amino acids, lactic acid, triglycerides, adipose and glycerol can be converted by hepatocytes into glucose
BMed JMP Year 1 Learning Targets
2. Lipid Metabolism
Excess triglyceride can be converted to adipose for storage
Fatty acids broken down to ATP for energy- lipolysis
Bile helps emulsify triglycerides in SI for increased surface area for absorption
Synthesis of phospholipids- which transport fatty acids, cholesterol and triglycerides
Synthesise cholesterol
3. Protein Metabolism
hepatocytes deaminate (remove NH2) from amino acids- inactivates proteins, enzymes, hormones etc
amino acids used for ATP production or converted to carbohydrates or fat
hepatocytes also synthesise most plasma proteins:
alpha globulin
beta globulin
albumin- controls osmotic pressure in plasma, therefore important for plasma to carry molecules
fibrinogen
coagulation factors (clotting factors)
prothrombin
4. Processing of Drugs and Alcohol
pericential hepatocytes metabolize, detoxify and inactivate compounds (exo and endogenous)
Specific method for different compounds- deamination, enzymes
Main filtering mechanism for newly nutrient absorbed blood
5. Excretion of Bilirubin
* bilirubin has no known physiological use
Main pigment in bile
Derived as waste product of haem from broken down erythrocytes (liver destroys aged blood cells)
Added to bile and secreted to SI where it is digested and excreted as faeces
6. Bile Production
bile salt and acid synthesized by hepatocytes and secreted in initial bile form into bile canaliculi
Flows into common ducts where additional watery solution of sodium and bicarbonate ions are added, stimulated from secretory
epithelial cells by secretin hormone
Diverted (usually) for temporary storage in gall bladder
Concentrated in gall bladder- small electrolytes reabsorbed, concentrating bile:
Cholesterol
BMed JMP Year 1 Learning Targets
Bilirubin
Lecithin- phospholipid
Bile salts
Na+
K+
HNO3 Released into duodenum by rhythmic contraction through sphincter of Oddi when stimulated by cholecystokinin
Bile Functions:
Excretion of bilirubin
Emulsification- aids breakdown of large lipid globules into smaller globules, increasing the surface area that lipase can breakdown lipids
to fatty acids for absorption
7. Storage
Vitamins A, B12, D, E, K
Glycogen
Minerals (iron, copper)
8. Phagocytosis
Kupffer cells abundant in hepatic sinusoids
Phagocytic
Scan newly absorbed, nutrient rich blood for pathogens and phagocytoses
What are some of the mechanisms by which the liver processes normal (e.g. amino acids, fatty acids, glucose) and abnormal (e.g. ingested drugs
and toxins) molecules? (Biochemistry, Pharmacology) - Liver Function Tests.
How do you assess liver function? (Biochemistry) - Liver Function Tests.
The liver biochemical and function tests can be categorized as follows:
Tests that detect injury to hepatocytes Most of these tests measure the activity of hepatic enzymes, such as the aminotransferases, in the circulation.
These enzymes are normally intracellular, but are released when hepatocytes are injured.
BMed JMP Year 1 Learning Targets
Tests of the liver's capacity to transport organic anions and metabolize drugs These tests measure the liver's ability to clear endogenous or
exogenous substances from the circulation. The best studied include serum measurements of bilirubin, bile acids, caffeine, and lidocaine metabolites, a
variety of breath tests, and clearance tests such as bromsulphalein (BSP) and indocyanine green (ICG).
Tests of the liver's biosynthetic capacity The most commonly performed tests to assess the biosynthetic capacity of the liver are the serum albumin
and the prothrombin time (which requires the presence of clotting factors produced in the liver). Other tests which have been use are the serum
concentrations of lipoproteins, ceruloplasmin, ferritin, and alpha-1 antitrypsin.
Tests that detect chronic inflammation in the liver, altered immunoregulation, or viral hepatitis These tests include the immunoglobulins,
hepatitis serologies, and specific autoantibodies. Most of these substances are proteins made by B lymphocytes, not by hepatocytes. However, some are
quite specific for certain liver diseases, such as antimitochondrial antibodies in primary biliary cirrhosis.
What are the effects of paracetamol and ethanol in excess on the liver? (Pharmacology) - Liver Function Tests.
Paracetamol (acetaminophen) overdose:
At therapeutic doses, 90 percent of acetaminophen is metabolized in the liver to sulfate and glucuronide conjugates, which are then excreted in the urine. Approximately
2 percent is excreted in the urine unchanged. The remaining acetaminophen is metabolized via the hepatic cytochrome P450 (CYP2E1, CYP1A2, CYP3A4 subfamilies)
mixed function oxidase pathway into a toxic, highly reactive, electrophilic intermediate, N-acetyl-p-benzoquinoneimine (NAPQI).
Appropriate acetaminophen doses produce a small amount of NAPQI which is rapidly conjugated with hepatic glutathione, forming nontoxic cysteine and mercaptate
compounds that are excreted in the urine. However, with toxic doses of a acetaminophen the sulfation and glucuronidation pathways are saturated, and more
acetaminophen is metabolized to NAPQI via the cytochrome P450 enzymes. When hepatic glutathione stores are depleted by approximately 70 to 80 percent, NAPQI
begins to react with hepatocytes, and injury ensues.
NAPQI arylates and binds covalently to the cysteine groups on hepatic macromolecules, forming NAPQI-protein adducts. This process is irreversible and leads to
oxidative injury and hepatocellular centrilobular necrosis. Although not fully characterized, lipid peroxidation and mitochondrial injury likely play a role in the
progression of hepatocellular injury. In addition, it appears that the release of cytokines and reactive nitrogen and oxygen species from damaged hepatocytes also play a
role in the spread of hepatic injury. Cytokine release from hepatocytes may initiate a secondary inflammatory response from Kupffer cells and other inflammatory cells,
extending the zone of hepatic injury. This secondary injury occurs during stage II of clinical toxicity.
Acute alcohol ingestion Acute alcohol ingestion is NOT a risk factor for hepatotoxicity and may even be protective by competing
with acetaminophen for CYP2E1 and, thereby, decreasing the amount of NAPQI produced.
BMed JMP Year 1 Learning Targets
What is cellular necrosis, what can cause it? (Anatomical Pathology) - Liver Function Tests.
Professional Practice
Clinical Ethics
What do you need to consider when treating an adolescent? Trigger.
Age at which medical treatment can be sought independently.
Confidentiality
Refusal of consent.
How do you assess mental competence?
Duty of care for the intoxicated patient.
Duty of care for mentally incompetent/suicidal patient (consider schedules)
What are the attitudes of health professionals to people who self harm?
Communication Skills
What skills are required to facilitate patients telling their story? - Doc.com and medical consultation skills tutes.
Where are Specialist medical records used? - Refer to Toxicology Sheet notes, particular areas of risk.
How do clinical charts aid in decision making? - e.g. Paracetamol poisoning nomogram.
Risk assessment
What special precautions need to be taken in managing patients with decreased level of consciousness? - Pt has decreased level of consciousness
on arrival.
BMed JMP Year 1 Learning Targets
Health Systems
What mental Health services are available and how do people access them? - Pt referred to psychiatrist, family use of community mental health
support.
Vous aimerez peut-être aussi
- Chapter 44 LiverDocument17 pagesChapter 44 LiverLiteriaPas encore d'évaluation
- Biochemistry of Specialized Tissues (Liver)Document24 pagesBiochemistry of Specialized Tissues (Liver)Uloko ChristopherPas encore d'évaluation
- Physiology of The Liver and Biliary System M.GDocument54 pagesPhysiology of The Liver and Biliary System M.GTsega WesenPas encore d'évaluation
- Metabolism OverviewDocument43 pagesMetabolism OverviewWinnet MaruzaPas encore d'évaluation
- Case 5, Sec CDocument7 pagesCase 5, Sec CRitz CelsoPas encore d'évaluation
- Biochemistry of Liver and KidneyDocument24 pagesBiochemistry of Liver and KidneyAnastasiafynnPas encore d'évaluation
- Biochemical Functions of LiverDocument94 pagesBiochemical Functions of LiverMi PatelPas encore d'évaluation
- Lipid Metabolism Dental and PhysiotherapyDocument43 pagesLipid Metabolism Dental and PhysiotherapyNada Atef KoraitemPas encore d'évaluation
- Energy Production 2. Energy Storage 3. Energy UtilizationDocument14 pagesEnergy Production 2. Energy Storage 3. Energy UtilizationLALITH SAI KPas encore d'évaluation
- Amino Acids Metabol Synth of UreaDocument32 pagesAmino Acids Metabol Synth of UreaAnastasiafynn100% (1)
- Lecture 12. Liver Biochemistry. Pigmentary Metabolism. JaundicesDocument43 pagesLecture 12. Liver Biochemistry. Pigmentary Metabolism. JaundicesВіталій Михайлович НечипорукPas encore d'évaluation
- Bio Section - Liver Function TestsDocument22 pagesBio Section - Liver Function Testsislam.o.walyPas encore d'évaluation
- Diagnostic EnzymologyDocument20 pagesDiagnostic Enzymologyshalendra150100% (1)
- Unit 8 Digestion and AbsorptionDocument33 pagesUnit 8 Digestion and AbsorptionGlory MuedaPas encore d'évaluation
- Toxicity of The Liver Lecture 20-21Document28 pagesToxicity of The Liver Lecture 20-21h9g886qdnpPas encore d'évaluation
- GruczołyDocument33 pagesGruczołynatalie muyengwaPas encore d'évaluation
- Biochemistry of Liver: Alice SkoumalováDocument37 pagesBiochemistry of Liver: Alice Skoumalováandreas kevinPas encore d'évaluation
- Tissue MetabolismDocument13 pagesTissue MetabolismTonny MutisyaPas encore d'évaluation
- CB CH 05 LiverDocument25 pagesCB CH 05 LiverDeemaPas encore d'évaluation
- Department of Kriyasharir: Mahatma Gandhi Ayurved College Hospital & Research Centre Salod (H), WardhaDocument39 pagesDepartment of Kriyasharir: Mahatma Gandhi Ayurved College Hospital & Research Centre Salod (H), WardhaAvanti ShendurjanePas encore d'évaluation
- Biochemistry - Protein and LipidsDocument31 pagesBiochemistry - Protein and LipidsGiorgi TamazashviliPas encore d'évaluation
- Lecture 5 - Organ Function DisordersDocument68 pagesLecture 5 - Organ Function Disordersjonesweb5Pas encore d'évaluation
- Biochemical Functions of LiverDocument82 pagesBiochemical Functions of Liversiwap34656Pas encore d'évaluation
- LFT FunctionDocument18 pagesLFT FunctionDeepika RathnaPas encore d'évaluation
- Metabolism and EndocrineDocument156 pagesMetabolism and EndocrineGren May Angeli MagsakayPas encore d'évaluation
- Biochemistry: Proteins and Amino AcidsDocument8 pagesBiochemistry: Proteins and Amino AcidsrajanOrajanPas encore d'évaluation
- Clinical Chemistry HomeworkDocument4 pagesClinical Chemistry Homeworkعرفات ابوعبيدةPas encore d'évaluation
- Cholesterol SphingolipidsDocument31 pagesCholesterol SphingolipidsMaryam KPas encore d'évaluation
- Briefly Summarize The Involving Steps in The ATP SynthesisDocument7 pagesBriefly Summarize The Involving Steps in The ATP SynthesisXuân ViPas encore d'évaluation
- Ac. OrgansDocument22 pagesAc. OrgansJoan G. MachonPas encore d'évaluation
- 2.1 (BIOCHEMISTRY) Introduction To MetabolismDocument6 pages2.1 (BIOCHEMISTRY) Introduction To Metabolismlovelots1234100% (1)
- Fisio + Jaundice GabunganDocument52 pagesFisio + Jaundice GabunganMufti DewantaraPas encore d'évaluation
- Cholesterol FinalDocument55 pagesCholesterol FinalSobha MatthewPas encore d'évaluation
- Pathophysiology of Acute Liver FailureDocument39 pagesPathophysiology of Acute Liver Failurelefebi6403Pas encore d'évaluation
- Metabolism of ProteinsDocument50 pagesMetabolism of ProteinsAbdur RehmanPas encore d'évaluation
- CyclesDocument32 pagesCyclesSanjana VasistPas encore d'évaluation
- Cholesterol Metabolism 2021Document43 pagesCholesterol Metabolism 2021Eniola abdullahi AduagbaPas encore d'évaluation
- Cholesterol and Steroid MetabolismDocument14 pagesCholesterol and Steroid MetabolismMarkyPas encore d'évaluation
- Structure and Function of Liver and GallbladderDocument21 pagesStructure and Function of Liver and GallbladderKeziaPas encore d'évaluation
- Protein MetabolismDocument6 pagesProtein MetabolismGeline Dela RosaPas encore d'évaluation
- Guc 2705 59 28561 2023-05-18T10 45 27Document47 pagesGuc 2705 59 28561 2023-05-18T10 45 27malak00mohamed229Pas encore d'évaluation
- Liver Function TestsDocument52 pagesLiver Function TestsAhaisibwe GordonPas encore d'évaluation
- Cholesterol and Steroid MetabolismDocument17 pagesCholesterol and Steroid MetabolismMarkyPas encore d'évaluation
- Homeostasis - Maintenance of A Constant Internal Environ., Despite Fluctuations in ExternalDocument4 pagesHomeostasis - Maintenance of A Constant Internal Environ., Despite Fluctuations in ExternalSamuel NafPas encore d'évaluation
- Finals Self-Directed Activity # 3 Liver FunctionDocument7 pagesFinals Self-Directed Activity # 3 Liver FunctionAIRA GIN G. ARELLANOPas encore d'évaluation
- Homeostasis 121117100359 Phpapp02Document68 pagesHomeostasis 121117100359 Phpapp02midahrazalPas encore d'évaluation
- Physiology of Digestive SystemDocument123 pagesPhysiology of Digestive Systemkidusabeje7Pas encore d'évaluation
- GI-PART 2 Lecture For RNDocument19 pagesGI-PART 2 Lecture For RNrichardmd2Pas encore d'évaluation
- Chapter 25 - Introduction To MetabolismDocument5 pagesChapter 25 - Introduction To Metabolismtomorrow.today.yesterday .yesterdayPas encore d'évaluation
- Review: - ACC RegulationDocument41 pagesReview: - ACC RegulationDiana ArwatiPas encore d'évaluation
- Metabolic ProcessesDocument6 pagesMetabolic ProcessesMark Andrian PontanalPas encore d'évaluation
- Hepatic Physiology: Sabina Sabharwal, MD MPH Boston Children's Hospital Sabina - Sabharwal@childrens - Harvard.eduDocument41 pagesHepatic Physiology: Sabina Sabharwal, MD MPH Boston Children's Hospital Sabina - Sabharwal@childrens - Harvard.eduUsmle KidsPas encore d'évaluation
- SGL 7,8Document30 pagesSGL 7,8hasan.hmu2004Pas encore d'évaluation
- Pathology of Liver SamDocument28 pagesPathology of Liver SamJaks RipperPas encore d'évaluation
- Agri-501 Biochemistry 2021-2022: Doc. Regina P. ClavelDocument36 pagesAgri-501 Biochemistry 2021-2022: Doc. Regina P. ClavelIvan Jhon AnamPas encore d'évaluation
- The Biochemistry of Digestion, Absorption and Detoxification by Prof. Dr. Hedef D. El-YassinDocument64 pagesThe Biochemistry of Digestion, Absorption and Detoxification by Prof. Dr. Hedef D. El-YassinSayhidoen CepexPas encore d'évaluation
- Digestive SystemDocument9 pagesDigestive SystemJhomar B, Dela CruzPas encore d'évaluation
- Cellular Metabolism Overview of MetabolismDocument4 pagesCellular Metabolism Overview of MetabolismNathan Louis PalacioPas encore d'évaluation
- Multiple Choice Questions (MCQ) Topic Quiz Biochemistry: Instructions and Answers For TeachersDocument15 pagesMultiple Choice Questions (MCQ) Topic Quiz Biochemistry: Instructions and Answers For TeachersAjitha SPas encore d'évaluation
- A Brief History of BioplasticsDocument8 pagesA Brief History of BioplasticsSHANKAR PRINTINGPas encore d'évaluation
- Biomolecules: Molecules of LifeDocument32 pagesBiomolecules: Molecules of LifeEric MabesaPas encore d'évaluation
- Nutrition Sports Gleeson Michael y Jeukendrup Asker E Sport Nutrition Human Kinetics 2019Document1 047 pagesNutrition Sports Gleeson Michael y Jeukendrup Asker E Sport Nutrition Human Kinetics 2019Jhony Rodriguez100% (5)
- Quizlet Chapter 5Document9 pagesQuizlet Chapter 5EUNAH LimPas encore d'évaluation
- CarbsDocument34 pagesCarbsManendra PatwaPas encore d'évaluation
- Fungal AmylaseDocument8 pagesFungal AmylaseangelinaanavarroPas encore d'évaluation
- S 2 Unit 4 Course Book, Work Book Ques and KeysDocument19 pagesS 2 Unit 4 Course Book, Work Book Ques and KeysHein Aung Zin100% (2)
- Zainab Muhammad T.RDocument31 pagesZainab Muhammad T.RAdam AbubakarPas encore d'évaluation
- WCC Fall 2020 v18n3 Final r1 p.40-47 NutritionDocument8 pagesWCC Fall 2020 v18n3 Final r1 p.40-47 NutritionBella PratiwiPas encore d'évaluation
- Laboratory Report Of: Analysis of Food (FST 606)Document13 pagesLaboratory Report Of: Analysis of Food (FST 606)biokimia 2018Pas encore d'évaluation
- E - D Text 2Document52 pagesE - D Text 2CherwinPas encore d'évaluation
- Chemistry Handout (Basic)Document6 pagesChemistry Handout (Basic)Tin SumangaPas encore d'évaluation
- Fitness Manual IFADocument128 pagesFitness Manual IFAAlejandro BalcazarPas encore d'évaluation
- Balance of Carbohydrate and Lipid Utilization During Exercise The Cossover ConceptDocument9 pagesBalance of Carbohydrate and Lipid Utilization During Exercise The Cossover ConceptdbranzaniPas encore d'évaluation
- M1-M3 Biochem LecDocument36 pagesM1-M3 Biochem Lecloreign sinocruzPas encore d'évaluation
- Biomolcules (Week 3) (AutoRecovered)Document6 pagesBiomolcules (Week 3) (AutoRecovered)JoshRobertD.ObaobPas encore d'évaluation
- Physiology of HomeostasisDocument17 pagesPhysiology of HomeostasisRamadan PhysiologyPas encore d'évaluation
- Test Tube & Tube RackDocument4 pagesTest Tube & Tube RackSimmy DhaliwalPas encore d'évaluation
- Simple Non-Ruminants: Principles of Animal Nutrition Dr. Cristine W. MaramagDocument30 pagesSimple Non-Ruminants: Principles of Animal Nutrition Dr. Cristine W. MaramagMc Kjell Dagman RaveloPas encore d'évaluation
- Yeast Group Lab Write UpDocument5 pagesYeast Group Lab Write Upapi-392377025Pas encore d'évaluation
- BIO OlympiadDocument8 pagesBIO OlympiadMilka RahmanPas encore d'évaluation
- Yale Protocol For Insulin DripDocument2 pagesYale Protocol For Insulin DripLiz EscuetaPas encore d'évaluation
- CarbohydratesDocument35 pagesCarbohydratesRaincel Mae WarnacPas encore d'évaluation
- Experiment 1 Qualitative Analysis of CarbohydratesDocument14 pagesExperiment 1 Qualitative Analysis of CarbohydratesEko Nevrian90% (10)
- Anh Chuyen 20.5.docx StudentsDocument18 pagesAnh Chuyen 20.5.docx StudentsQuỳnh TrangPas encore d'évaluation
- Chapter 9: Nutrition & The Human Digestive System: 9.3 Absorption 9.4 AssimilationDocument9 pagesChapter 9: Nutrition & The Human Digestive System: 9.3 Absorption 9.4 Assimilation静宁Pas encore d'évaluation
- Nutrition and MetabolismDocument179 pagesNutrition and MetabolismTuTitPas encore d'évaluation
- DIgestion and Absorption of CarbohydratesDocument35 pagesDIgestion and Absorption of CarbohydratesAtif Amin Baig100% (1)
- Carbohydrate Metabolism BioChem Lec Finals2x1 FormatDocument3 pagesCarbohydrate Metabolism BioChem Lec Finals2x1 FormatSheene ImblarinagPas encore d'évaluation