Académique Documents
Professionnel Documents
Culture Documents
1968 - Feher - The Supercritical Thermodynamic Power Cycle
Transféré par
DilipTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
1968 - Feher - The Supercritical Thermodynamic Power Cycle
Transféré par
DilipDroits d'auteur :
Formats disponibles
Energy Conversion.
V o l . 8, p p . 8 5 - 9 0 .
F e r g a m o n Pre~s, 1968.
Printed in Great B r i t a i n
The SupercriticalThermodynamicPower Cycle
E, G, FEHERt
(Received 13 January 1968)
1. Introduction
2. Description of the Cycle
Thermodynamic power cycles most commonly used
for closed cycle engines today are the Rankine Cycle and
the recuperated Brayton Cycle. Both are characterized
by two constant pressure and two isentropic processes.
The Rankine Cycle operates mainly in the saturated
region of its working fluid whereas the Brayton Cycle
processes are located entirely in the superheat or gas
region.
The simple Rankine Cycle is inherently efficient. Heat
is added and rejected isothermally and therefore the
ideal cycle can achieve over 90 per cent of Carnot
efficiency between the same temperatures. Pressure rise
in the cycle is accomplished by pumping a liquid, which
is an efficient process requiring small energy input.
The ratio of net work output to gross work in the cycle
is large. Among the limitations of the cycle are the
following:
For the thermodynamic analysis of the Supercritical
Cycle, the properties of its working fluid are represented
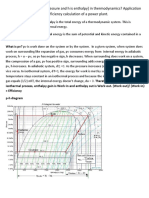
in Figs. 1 and 2. A pure substance (such as water or
carbon dioxide) is shown on a temperature-entropy
diagram and on an enthalpy-entropy diagram. Also
(i) The temperature range of the cycle is severely
limited by the nature of the working fluid. Adding
superheat in an attempt to circumvent this will depart the
cycle from isothermal heat addition. Increasing the
temperature range without superheat leads to excessive
moisture content in the turbines, resulting in blade
erosion.
(ii) Simple recuperator cannot be employed to recover
heat from the turbine exhaust.
(iii) Expansion ratio of the cycle is usually large,
requiring in some cases more than 30 turbine stages,
Entropy
The recuperated Brayton Cycle adds heat at constant
pressure over a temperature range. The temperature level
is independent of the pressure level. No blade erosion
occurs in the turbine. The pressure ratio is low, therefore
one or two turbine stages are adequate. A simple recuperator can recover much of the turbine exhaust heat.
Some of the limitations of the cycle are:
Fig. 1. Temperature-entropy diagram for the supercritieal
cycle.
(i) The compression process requires large energy
input, therefore the net work to gross work ratio is small.
(ii) The cycle is very sensitive to compressor efficiency
and pressure drop.
(iii) Heat transfer surfaces are large for pressure levels
that are typical for current Brayton engines.
.......
A thermodynamic power cycle has been devised which
avoids most of the problems of these cycles and yet retains
many of their advantages. This cycle operates entirely
above the critical pressure of its working fluid; it is the
Supercritical Cycle.
t Astropower Laboratory Missile and Space Systems Division,
Douglas Aircraft Co., Inc., 2121 Campus Drive, Newport Beach,
California.
,~mV'f C ~ P o i n J
--
Entropy
Fig. 2. Enthalpy-entropy diagram for the supercritical cycle.
85
86
E.~ G; FEllER .
shown are lines of constant pressure, constant enthalpy,
constant temperature, and the saturation line and critical
point.
The ideal cycle processes are shown by line segments
ab, bd, de, and ea. Segment ab represents an isentropic
compression of the subcooled liquid from pressure Pt'to
p2. Segment b d represents heat addition at constant pressure p2 to the highest temperature of the cycle at point d.
From d to e, isentropic expansion occurs from pressure
pz to pl, with accompanying work output. Heat is extracted from e to a along constant pressure line pl. A
portion of this heat, represented by enthalpy drop from
e to f at constant pressure pl, is transferred back to the
fluid, raising its enthalpy from b to c at constant pressure
p2. Net heat rejected is indicated by the enthalpy dro p
f r o m f t o a at constant pressurepl. Point a is at the lowest
temperature of the cycle and above the temperature of
the heat-receiving reservoir. Net heat input to the cycle
occurs from c to d at constant pressure p2. Net work
output is the difference between work output from d
to e and pump work input from a to b.
A pseudo-Supercritical Cycle has been employed for
steam power plants previously. In this combination of the
Rankine and Brayton Cycles the working fluid is pumped
from the saturated state to a supercritical pressure. (See
Fig. 3.) Heat is added in a constant pressure process over
3. Cycle Performance Analysis
Referring to Fig. 2, the ideal thermal efficiency of the
Supercritical Cycle is defined as
~t-
Wout _ (ha - - he) - - (ho - - ha)
Qin
h a - hc
'
(1)
where h is the value of the enthalpy at the point indicated
by the subscript.
The efficiency of the cycle is high because, (i) the pump
work is a small fraction of the total work output, and
(ii) most of the heat extracted from the cycle can be transferred back to lhe working fluid by regeneration, thus
reducing the total energy rejected from the cycle.
In the actual cycle, the greatest loss will occur in the
expansion process, which will proceed along the polytropic line d e ' instead of the isentropic line de. The
efficiency of the turbine may be defined as
et --
ha-
he"
ha - - he
--
AH~
AHt"
(2)
The total work output is reduced because of the lowered
turbine efficiency. The thermal efficiency of the cycle is
also loWered. However, some of the energy not available
as work, is available as heat and can be transferred to
the heat input process of the cycle. In other words, the
enthalpy drop he" - - he is utilized in raising the enthalpy
from he to he', thus reducing the net heat input from
ha - - he to ha - - h e .
The pressure drops in the system are reflected as an
increase in pump work. The liquid pump has high effiCiency, and the magnitude of the enthalpy difference
between ideal and actual pump work is small. The
efficiency of the pump may be defined as
ho - - ha
ev - - hb' - - ha
AHv
AH"r "
(3)
The actual thermal efficiency of a real cycle becomes
Vlat :
(ha - - he') - - (hb" - - ha)
ha - - he"
(4)
4. Regeneration
Entropy
Fig. 3. Temperature-entropy diagram for a pseudo-supercritical
cycle,
a temperature range. The same cycle, using carbon dioxide as the working fluid has been :discussed by the
Russian, V. L. Dektiarev [1], and the Italian,.G. Angelino
[2]. This CO2 cycle was discussed by the author, without
knowledge of the above works, in connection with the
initial disclosure of the Supercritical Cycle in an Engineering Report [3].
The regeneration process is essential to the achievement of high thermal efficiency in the Supercritical
Cycle. The process is more complicated than that for
the Brayton Cycle due to the large deviation of fluid
properties from the ideal in the vicinity of the critical
point. For the pure substance, the specific heat at constant
pressure increases without bounds at the critical point.
By definition, the specific heat at constant pressure is
the ratio of increase in enthalpy in a constant pressure
process to the corresponding increase in temperature,
expressed as a derivative. Symbolically,
C,=(O0~I,)~.
(5)
If the properties of a pure substance are represented
on a rectangular coordinate system, with enthalpy as the
The Supercritical Thermodynamic Power Cycle
87
where he - - he = h ! form,
hb = AHr. Expressed in another
1 -- AHv/AHt
1 + AHr/AHt"
~. =
5' ]
(7)
From this relation it is evident that ~,t can be increased
by reducing AHr.
If AH for a higher set of pressures, say 4500 and 5500
psia is plotted (see Fig. 5) a lower A H r is obtained. It is
evident that as the pressure level (above the critical
~, ~ 2OO
-* - 100
oC,)
J"
<"
7L,,3
800
'
900
',~-----lOOO
HOO
1500
1200
I ur~?clat:,rc Io[j
Fig. 4. Enthaipy-temperaturediagram--high A H r .
ordinate, temperature as the abscissa, and lines of
constant pressure are drawn, then the slope at any point
is proportional to the value Of the Specific heat. Figure 4
shows such a set of curves for water. On the critical
pressure line the maximum slope is infinite and occurs
at the critical point. The maximum slope is finite at
higher pressures and its value decreases with increasing
pressure.
To illustrate the effect of the pressure level and pressure
ratio on the regeneration process let the lower and upper
pressures Of an ideal supercritical cycle be 3500 psia and
4500 psia, respectively. In Fig. 4 the enthalpy difference
at constant temperature for these pressures is plotted as a
function of temperature and labeled AH. The constant
temperature line at the maximum point of the AH curve,
AHr, is drawn. This line intersects the 3500 psia and
4500 psia constant pressure lines at ao and b0, respectively.
Now, equal increments of enthalpy are taken on the two
pressure lines in the directions of both increasing and
decreasing enthalpy. The corresponding points are
labeled a~, bl, all, bll, a2, b2, a22, b22, etc. It can be seen
that for constant enthalpy increments in both directions
the temperature increment is increasing. The increase is
the greatest on the lower pressure line in the increasing
enthalpy direction and on the higher pressure line in the
decreasing enthalpy direction. The existence of this
positive temperature gradient allows heat transfer to
take place in the supercritical region from the lower to
the higher pressure.
Of the two aspects of the enthalpy~-temperature
relations, namely the existence of a maximum AH
between the constant pressure relation and the increasing
temperature increment with constant enthalpy increment,
the former is a detriment to cycle efficiency and the
latter is an aid in reducing recuperator size. The former
is an index of the unavailability of heat energy for
conversion to work. As such, it leads to an alternate but
equivalent definition of cycle thermal efficiency,
lit
(ha - - he) - - (hb - - ha)
(hd -- he) + (he - - he)
(ha -- he) -- (ho --
-- (ha --
he) +
(hi --
ha)
hb)'
(6)
Critical Point
~- -t'}i '~] 3 "~ - "-U0
]l IBII.IiIb!
all;
500
500
;-"/
700
600
!
800
Ternperatu re
- 900
i
1000
b
II00
i -0
1200
lF}
Fig. 5. Enthalpy-temperaturediagram--low AHr.
pressure) is increased (within undetermined limits) and
as the pressure ratio is decreased, the A H r is decreased.
The value of the untransferable enthalpy is A H r in an
ideal recuperator. At that point the AT across the recuperator is zero and theoretically an infinite surface
would be required for heat transfer. In a real recuperator
the AT has a positive value ATp~ and is called the "pinch
temperature". The surface required for heat transfer
becomes smaller as AT is increased. An increase in AT
results in an increase in A H r to some value AH~,
according to the relation
A H 'r - - A H r :
Cp-avg >( ATpi.
(8)
This relation holds where ATp, is less than 15F, which
is a practical value for real applications. Over this
range the differences between Cp_avg and the maximum
and minimum values of Cp are negligibly small.
The efficiency of the recuperator may be defined as
the ratio of the ideal and actual enthalpy differences,
AHr
er :
AH~
(9)
Using Equations (2), (3), (7) and (9), the actual thermal
efficiency of a real cycle becomes
~at
1 -- AHp/AHtete v
i-~- A-Hr-/AHteter"
(10)
5. Working Fluids
In principle, the Supercritical Cycle can be operated
with any fluid, just as a Brayton Cycle can be operated
with any gas. In practice, the choice of working fluid
controls the range of cycle operating pressures and
temperatures. Table 1 lists critical properties of some
88
E . G . FEHER
Table 1. Critical constants of working fluids
Name
Formula
Ammonia
Carbon dioxide
Hexafluorobenzene
Perfluoropropane
Sulfur dioxide
Sulfur hexafluoride
Water
Xenon
0"65 -
Critical
temperature
(F)
Critical
pressure
(psia)
271"2
87.8
460
161"4
315"5
114
705
61.9
1636
1072
402
388
1143
546
3206
853
NH3
CO2
C6F6
CaFa
SO2
SF6
H20
Xe
of the working fluids which can be used in practical
applications.
For initial investigations carbon dioxide was selected
as the working fluid for several reasons. First, its critical
pressure is one third that of water, allowing lower
operating pressures. Second, it is known to be a stable
and inert material throughout the temperature range of
interest. Third, there is a considerable body of literature
on the properties of carbon dioxide, hence cycle analysis
is based on reasonably firm data. And finally, carbon
dioxide is abundant, non-toxic and relatively inexpensive.
The thermodynamic and transport properties of carbon
dioxide were assembled from several sources, notably
from R. L. Sweigert et al. [4], G. C. Kennedy [5], D.
Price [6], D. M. Newitt et aL [7], and L. H. Chen [8].
This data covers the temperature range from 32F to
1800F and the pressure range from 0 to 500 atm.
6. Effect of Component Parameters
Inspection of Equation (7) reveals the influence on
ideal cycle efficiency of turbine work, pump work and
the unavailable energy in the regeneration process.
Figure 6 is a plot of cycle efficiency as a function of the
independent variables of Equation (7), AH~/AHt and
AHr/AHt. Superimposed on this plot are some typical
ideal carbon dioxide cycles operating between practical
temperature and pressure limits. It is evident that typical
values of AHp/AHt range from 0.07 to 0.20 and that
the values of AHr/AHt usually lie between 1/3 and 1.
Figure 7 shows the change in all variables of Equation
1-
0.8
P~IPI =2"2"~
~
-0"8
0.6 f
f
-0"5
~, 0.55.
g
Turbine InldTemp, ).300OF
0"5- - Pump Inlet Temp, 6~'F
Pump Inlet Press, L~O00psia
Cycle Press Drop - 0
q-%-er.l
Cnrn0t Efficleney- 0.70
0.45- -W0rklng Fluid: C02
-0.4
-0-2
0.41"5
-O
3.5
2
2.5
3
Pump Discharge Pressure
Pump inlet Pressure
.
Fig. 7. Cycle efficiency vs. pressure ratio.
(7) as a function of pressure ratio, for a pump inlet
pressure of 2000 psia. Ideal cycle efficiency is also
compared to Carnot efficiency. The highest efficiency
occurs for a pressure ratio of more than 3.5, but it is
significant that the efficiency remains almost constant
down to a pressure ratio of 2.
The difference between ideal and actual thermal
efficiencies as represented by Equations (7) and (10),
respectively, is due to the efficiencies of the three major
components of an engine operating on the Supercritical
Cycle. These components are the pump, turbine and
recuperator.
Figure 8 shows the recuperator efficiency and cycle
efficiency as a function of pressure ratio and pinch
temperature ATtn. Here the degrading influence of the
TurbineInletTemp.
1600%- u
1200F- o
800F=~ - -
~P21PI *.I'25
'0.6
~0"4
, o,2.~
1
O"
0.2
0"4
0.6
Z~Hptz' ~Ht
O.B
F i g . 8.
Fig. 6. Cycle efficiency vs. pump work to turbine work ratio.
1.5
2
2"5
Pump Discharge Pressure
Pump Inlet Pressure
3"5
Cycle efficiency and recuperator efficiency vs. pressure ratio
and pinch temperature.
The Supercritical Thermodynamic Power Cycle
shown in Fig. 10. Peak cycle efficiency occurs at progressively lower pressure ratios as component efficiencies
decrease.
In a practical engine, the total pressure drop around
the cycle also has a degrading effect on cycle efficiency.
Total pressure drop can be referred to the pump as
increase in pump pressure ratio over the turbine pressure
ratio.
Symbolically
APc~ = APv -- APt
(11)
0,8-
Turbine Inlet Temp, - ~300F . ~
Pump Inlet Temp, - 58 F
0.7- Turbine Inlet Press, 40(X)psia Turbine Outlet Press, - 20(]0psia
=CyclelPress" Drop- 0 _ ~
er
0.6- WorkingFluid: CO2
0.4"G
89
rbin (ep = 1)
0.3-
and expressed as a ratio,
(3.2 ~ - - ~ _ _
0.1
APp
APt
APt
I.
(12)
The effect of increasing pressure drop on cycle efficiency
at several pump and turbine efficiencies is shown in
Fig. 11.
0-
0"2
0,4
0,6
0,8
Turbine and PumpEfficiency
APey
Fig, 9. Cycle efficiency vs. turbine and pump efficiency.
recuperator efficiency on cycle efficiency is shown
quantitatively. Increasing AT~ represents decreasing size
recuperators. It is to be noted that the higher the pinch
temperature, the more marked the effect of pressure
ratio on cycle efficiency.
The effect of pump and turbine efficiencies is shown
in Fig. 9. As would be expected, the pump efficiency h a s
the smaller effect, producing less than 10 per cent
degradation in cycle efficiency for a drop from 100 per
cent to 60 per cent pump efficiency. The effect of turbine
efficiency is similar to that for a regenerated Brayton
Cycle.
The combined effect on cycle efficiency of the three
component efficiencies as a function of pressure ratio is
Turbine Inlet Temp. 1300OF
Pump Inlet Temp. - 68F
Turbine Inlet Press. - 4000psia
Turbine Outlet Press, - 2000psia
0,6 - - - O r . ,90
Pincll Temp. - 8OF(Approx.)
CarnotEfficiency - 0,70
Working Fluid: CO2
i
~ e t - ep 1 ]
0.55
/ - - e o e = 0.9
~
0,5
0.45
i
~ , ~
0.4 ~
Fet=ep:0"7
!
~
0.025
0.05
0.35
J
0.075
~P
CyclePressure Drop, ~ -
0,1
Fig. 11. Cycle efficiency vs. cycle pressure drop.
o.6-~
!- ~
I !
I
App i
[CyclePress'Drp"~t "1
.31
0'35 ~
r
1:5
z
2;5
3
Pump DischargePressure
Pump Inlet Pressure
3;3
Fig. 10. Cycle efficiency vs. pressure ratio and component
efficiency.
o . 3 ~
I000
----~
nlet Press. = 3000psie i
Turbine OutletPress, = 2000psia
Pump Inlet Temp. - 68OF
et ~
ep = e r
- 0.8
~,'orkmg
Fluid: CO2
1100 1200 1300 1400 1500
Turbine Inlet Temperature,OF
1600
Fig. 12. Cycle efficiency vs. turbine inlet temperature.
90
E . G . FEHER
o.~
--~-!__
0"45
The reasons for the neglect of the Supercritical Cycle
until now are not known. It can be conjectured that its
introduction to use has been delayed by the engineering
requirements of some of the components. It is clear,
however, that the level of today's technology is adequate
for the successful practical utilization of the Supercritical
Cycle.
~-,
,~Pp
~_
0.35---
,/
I//
'
~/~1~/~
o-/,1 /
'l--',t
I
0.3-
Nomenclature
Turbine InletTemp. !
0.2~-
Critical Temperature
.2 .....
40
60
80
Cp
Turbine Inlet Press. - 30~Opsia I
Turbine Outlet Press. - 2000 psiaj
~ et - e. - e r - 0-8
--$ .....
d
! Worki~n~j Fluid. CO-~
I
100
120
140
160
180
Pump Inlet Temperature, OF
Fig. 13. Cycle efficiency vs. pump inlet temperature.
Among the several cycle parameters that influence
cycle efficiency there remains the turbine and pump
inlet temperatures to consider. The effect of turbine inlet
temperature on cycle efficiency at several cycle pressure
drops is shown in Fig. 12. The variation is much the
same as for the regenerated Brayton Cycle. Figure 13
shows the effect of pump inlet temperature on cycle
efficiency at several cycle pressure drops. The cycle
efficiency variation here is quite different from that of
a Brayton Cycle. At temperatures lower than the critical
temperature the effect is small. A change of 40F in
pump inlet temperature causes a 1 percentage point
change in cycle efficiency. At temperatures higher than
about 15F above the critical temperature the effect is
suddenly large. A change of about 8F causes a 1 percentage point change. At temperatures farther above the
critical temperature the cycle behaves much as a high
pressure Brayton Cycle because the working fluid there
is a high density compressible gas.
7. Conclusion
The Supercritical Cycle offers the followfng characteristics, which are desirable in a practical application.
High thermal efficiency, low volume to power ratio, no
blade erosion in the turbine, no cavitation in the pump,
single stage turbine and pump, single phase fluid in the
heat rejection process, and insensitivity to compression
efficiency.
Some applications for a supercritical engine are:
electric power generation for space,
terrestrial electric power generation (stationary and
portable),
shaft power for marine propulsion (surface and
sub-surface).
With carbon dioxide as the working fluid and a
nuclear reactor as the heat source, the supercritical
engine can be a compact, portable electric power
generator.
Cp-avg
e
h
P
Qin
T
Wout
~Tat
r/ey
~Tit
AH
AH'
AT
AT~
specific heat at constant pressure
average specific heat at constant pressure
component thermal efficiency
enthalpy
pressure
heat input
temperature
work output
actual thermal efficiency
cycle thermal efficiency
ideal thermal efficiency
ideal enthalpy difference
actual enthalpy difference
temperature difference
pinch temperature (difference)
Subscripts
a to f
state points
p
pump
r
recuperator
t
turbine
Superscripts
'
actual point or process (as opposed to ideal)
Acknowledgments Work presented herein was conducted at the
Astropower Laboratory, Missile and Space Systems Division,
Douglas Aircraft Company, Inc. under company-sponsored
Research and Development funds.
References
[1] V. L. Dekhtiarev, On designing a large, highly economical
carbon dioxide power installation. Elecrtichenskie Stantskii,
5: 1-6, May 1962.
[2] G. Angelino, Perspectives for the Liquid Phase Compression Gas
Turbine. ASME Paper No. 66--GT-111, 13-17 March 1966.
[3] E. G. Feher, Supercritical Thermodynamic Cycles for External
and Internal Combustion Engines. Astropower, Inc. Engineering
Report May 1962.
[4] R. L. Sweigert, P. Weber and R. L. Allen, Ind. Engng. Chem.
38, 185 (1946).
[5l G. C. Kennedy, P - V - T relations in CO2 at elevated temperatures and pressures. Am. J. Sci. 2$2, 225-241 (1954).
[6] D. Price, The Thermodynamic Properties of Carbon Dioxide up
to 1000C and 1400 bars. Navord Report 3846, Nov. 1954.
[7] D. M. Newitt, N. V. Pal, N. R. Kuloor and J. A. W. Huggill,
Thermodynamic Functions of Gases, Vol. 1 tEd. F. Din). Butterworth, London (1956).
[8] L. Chela, Thermodynamic and transport properties of gaseous
carbon dioxide, in the A.S.M.E. book Thermodynamic and
Transport Properties of Gases, Liquids and Solids. McGraw-Hill
(1959).
Vous aimerez peut-être aussi
- High-Pressure Fluid Phase Equilibria: Phenomenology and ComputationD'EverandHigh-Pressure Fluid Phase Equilibria: Phenomenology and ComputationPas encore d'évaluation
- Supercritical Co2 Power Cycle Developments and Commercialization Why Sco2 Can Displace SteamDocument15 pagesSupercritical Co2 Power Cycle Developments and Commercialization Why Sco2 Can Displace Steamvivek pandeyPas encore d'évaluation
- 2.2. 2nd LawDocument75 pages2.2. 2nd LawPalesa Maope100% (1)
- Cascading Vapour Absorption Cycle With Organic Rankine Cycle For Enhancing Geothermal Power Generation PDFDocument13 pagesCascading Vapour Absorption Cycle With Organic Rankine Cycle For Enhancing Geothermal Power Generation PDFM Rama Yudhanto100% (1)
- Progress in Refrigeration Science and Technology: Proceedings of the XIth International Congress of Refrigeration, Munich, 1963D'EverandProgress in Refrigeration Science and Technology: Proceedings of the XIth International Congress of Refrigeration, Munich, 1963Pas encore d'évaluation
- Status of Geothermal Industry in AustraliaDocument8 pagesStatus of Geothermal Industry in AustraliaRyan Hofmann100% (1)
- Problems in the Design and Development of 750 MW Turbogenerators: International Series of Monographs on Electronics and InstrumentationD'EverandProblems in the Design and Development of 750 MW Turbogenerators: International Series of Monographs on Electronics and InstrumentationPas encore d'évaluation
- Heat Cycles, Heat Engines, & Real Devices: John Jechura - Jjechura@mines - Edu Updated: January 4, 2015Document21 pagesHeat Cycles, Heat Engines, & Real Devices: John Jechura - Jjechura@mines - Edu Updated: January 4, 2015Jennifer Sophia Tapia Huamani100% (1)
- Chap. 4.2 Desigh and Rating of Tube Bundle Heat ExchangersDocument22 pagesChap. 4.2 Desigh and Rating of Tube Bundle Heat ExchangersMeshal Al-mutairiPas encore d'évaluation
- Indop Product Catalogue 2016 EnglishDocument16 pagesIndop Product Catalogue 2016 EnglishIndop100% (2)
- Tutorial SCO2 WilkesDocument99 pagesTutorial SCO2 WilkesAshraf Zoubi100% (1)
- Regeneration Flow Pumps Blowers and CompressorDocument22 pagesRegeneration Flow Pumps Blowers and CompressorTalo Talo100% (1)
- Design Co2 EvaporatorsDocument7 pagesDesign Co2 EvaporatorsYutt Watt100% (1)
- 10 1016@j Enconman 2018 08 042 PDFDocument15 pages10 1016@j Enconman 2018 08 042 PDFteorikdeliPas encore d'évaluation
- Power Cycle Lect - 3Document17 pagesPower Cycle Lect - 3tensor9000100% (1)
- Dostal - SUPERCRITICAL CARBON DIOXIDE CYCLE THERMODYNAMIC ANALYSIS AND COMPARISON PDFDocument12 pagesDostal - SUPERCRITICAL CARBON DIOXIDE CYCLE THERMODYNAMIC ANALYSIS AND COMPARISON PDFJahangir MalikPas encore d'évaluation
- Absorption Future PDFDocument12 pagesAbsorption Future PDFVaidyanathan KS100% (1)
- Cogeneration Gas TurbineDocument7 pagesCogeneration Gas TurbineAlexzander AdisakPas encore d'évaluation
- Differences Between A Thermostatic Expansion Valve and An Electronic Expansion ValveDocument4 pagesDifferences Between A Thermostatic Expansion Valve and An Electronic Expansion Valvexuyen tran100% (1)
- Heat Pump Combi Unit Pkom: Comfort VentilationDocument16 pagesHeat Pump Combi Unit Pkom: Comfort Ventilationsbattina100% (1)
- Lorentzen1994 PDFDocument10 pagesLorentzen1994 PDFSURAJ NAGPas encore d'évaluation
- Thermodynamic Modelling of A Recompression CO2 Power Cycle For Low Temperature Waste Heat RecoveryDocument12 pagesThermodynamic Modelling of A Recompression CO2 Power Cycle For Low Temperature Waste Heat RecoveryJoão Víctor GermanoPas encore d'évaluation
- 5 Pink PC19Document2 pages5 Pink PC19Amín AC100% (1)
- Mohagheghi - Thermodynamic Optimization of Recuperated S-Co2 Brayton Cycles For Waste Heat Recovery ApplicationsDocument13 pagesMohagheghi - Thermodynamic Optimization of Recuperated S-Co2 Brayton Cycles For Waste Heat Recovery ApplicationsJahangir MalikPas encore d'évaluation
- 108 KAV Consult The World S First Two Stage CO2 Transcritical Refrigeration PlantDocument39 pages108 KAV Consult The World S First Two Stage CO2 Transcritical Refrigeration Plantevrimk100% (1)
- Performance Analysis of Plate Heat Exchangers Used As Refrigerant EvaporatorsDocument295 pagesPerformance Analysis of Plate Heat Exchangers Used As Refrigerant EvaporatorskarthikeyanPas encore d'évaluation
- CO2 A Refrigerant From The Past With Prospects of Being One of The Mai Refrigerants in The FutureDocument12 pagesCO2 A Refrigerant From The Past With Prospects of Being One of The Mai Refrigerants in The FutureOnofreHalber100% (1)
- Analysis of A 10 MW Recompression Supercritical Carbon Dioxide Cycle For Tropical Climatic Conditions Sathish Et AlDocument14 pagesAnalysis of A 10 MW Recompression Supercritical Carbon Dioxide Cycle For Tropical Climatic Conditions Sathish Et AlTanatswa MoyoPas encore d'évaluation
- Hi Pressure Plunger Pump KPDDocument25 pagesHi Pressure Plunger Pump KPDengrtahir100% (1)
- ME 403 CycleDocument40 pagesME 403 CyclesaquibPas encore d'évaluation
- Analysis and Comparison of The Simple and Recompression Supercritical Co2 Cycles PDFDocument8 pagesAnalysis and Comparison of The Simple and Recompression Supercritical Co2 Cycles PDFagneyan dileepPas encore d'évaluation
- TVN Series Trim and Pressure Build VaporizersDocument2 pagesTVN Series Trim and Pressure Build Vaporizersaakhyar_2100% (1)
- Pressure Vs Enthalpy in Thermodynamics - Bell CurvesDocument3 pagesPressure Vs Enthalpy in Thermodynamics - Bell CurvesMahe MahendiranPas encore d'évaluation
- 34 Sample ChapterDocument55 pages34 Sample Chapteralim100% (1)
- Azanechiller Series 2010 (3) Final VersionDocument12 pagesAzanechiller Series 2010 (3) Final VersionRajkumar GulatiPas encore d'évaluation
- Hyundai Diesel Generator 9500w 910228Document23 pagesHyundai Diesel Generator 9500w 910228Danny MarPas encore d'évaluation
- Condensate Cycle: Anshul Agarwal Engr (Opn)Document20 pagesCondensate Cycle: Anshul Agarwal Engr (Opn)Virendra AusarPas encore d'évaluation
- SNZHB4525 2006Document93 pagesSNZHB4525 2006Trent Fearnley100% (1)
- Hybrid Ejector Refrigeration SystemDocument17 pagesHybrid Ejector Refrigeration SystemAbhishek KumarPas encore d'évaluation
- xg-sf5600d gENERATOR mANUALDocument32 pagesxg-sf5600d gENERATOR mANUALTroy100% (1)
- Relation Among Properties & DiagramsDocument23 pagesRelation Among Properties & DiagramsVina Dwita100% (1)
- Chpt14 Chemical Reaction (Combustion) Cengel & BolesDocument40 pagesChpt14 Chemical Reaction (Combustion) Cengel & BolesDocumentos De Interés para IngenieríaPas encore d'évaluation
- Carbon Dioxide As A RefrigerantDocument3 pagesCarbon Dioxide As A Refrigerantjimbzthegr8100% (1)
- Using Standard Pumps As TurbinesDocument7 pagesUsing Standard Pumps As TurbinesLes BennettPas encore d'évaluation
- Basics Process IntegrationDocument42 pagesBasics Process IntegrationJoao MinhoPas encore d'évaluation
- Challenges in Supercritical CO2 Power Cycle Technology and First OperationalDocument16 pagesChallenges in Supercritical CO2 Power Cycle Technology and First OperationalAdven Brilian100% (1)
- A Guide To Energy AuditsDocument46 pagesA Guide To Energy AuditsAtif HussainPas encore d'évaluation
- Vapor Power Cycles Lecture 1Document55 pagesVapor Power Cycles Lecture 1mjunaidPas encore d'évaluation
- Engineering: Designing Green Does Not Have To Cost MoreDocument6 pagesEngineering: Designing Green Does Not Have To Cost MoreVL Koh100% (1)
- 2017 Supercritical Carbon Dioxide Cycles For Power Generation A ReviewDocument32 pages2017 Supercritical Carbon Dioxide Cycles For Power Generation A Reviewjing qiangPas encore d'évaluation
- The Concept of Exergy and Energy Quality - Truls GundersenDocument26 pagesThe Concept of Exergy and Energy Quality - Truls Gundersenuser_account100% (1)
- EGEC Market Report Update ONLINEDocument24 pagesEGEC Market Report Update ONLINELia Badea100% (1)
- Carnot Refrigeration CycleDocument11 pagesCarnot Refrigeration CycleZaimPas encore d'évaluation
- PEPOI Condenser Performance BasicsDocument26 pagesPEPOI Condenser Performance BasicsFranz MonsantoPas encore d'évaluation
- KKI Series 1200 and 7200Document18 pagesKKI Series 1200 and 7200Tabiquera Guadalupe Victoria Texcoco De Mora100% (1)
- An Innovative Approach For The Techno-Economic Optimization of Organic Rankine CyclesDocument352 pagesAn Innovative Approach For The Techno-Economic Optimization of Organic Rankine CyclesMarco Astolfi100% (1)
- XC4418 dataSheetMain PDFDocument1 pageXC4418 dataSheetMain PDFikiakunPas encore d'évaluation
- Powr PlantDocument10 pagesPowr PlantSajjad Ibraheem100% (1)
- ds65 700 PDFDocument4 pagesds65 700 PDFkumar_chemicalPas encore d'évaluation
- 1.2N Logical EquivalenceDocument11 pages1.2N Logical EquivalenceTrang NguyễnPas encore d'évaluation
- Metric Measure For Wood ProductsDocument7 pagesMetric Measure For Wood ProductspopescucvPas encore d'évaluation
- GEC 17 Lesson 1 Introduction To STSDocument15 pagesGEC 17 Lesson 1 Introduction To STSJhupit Ganihay100% (1)
- Chapter1-Propertiesoffluids Semakan2.1Document35 pagesChapter1-Propertiesoffluids Semakan2.1sufi 3393Pas encore d'évaluation
- Ujian Nasional Bahasa Inggris SMA Tahun 1994Document6 pagesUjian Nasional Bahasa Inggris SMA Tahun 1994Andhika A. SetiyonoPas encore d'évaluation
- Fuzzy: When We Say About Certainty of A ThingDocument15 pagesFuzzy: When We Say About Certainty of A ThingRajesh kumarPas encore d'évaluation
- Fundamentals of INdustrial ControlDocument5 pagesFundamentals of INdustrial ControlKirtikumarPas encore d'évaluation
- Bgcse Physics Paper 1 2017Document17 pagesBgcse Physics Paper 1 2017Katlego LebekwePas encore d'évaluation
- Supplee's ParadoxDocument3 pagesSupplee's Paradoxperception888Pas encore d'évaluation
- Questions and Answers in Quantum Mechanics: Q1 .What Is A Simple Harmonic Oscillator (SHO) ?Document12 pagesQuestions and Answers in Quantum Mechanics: Q1 .What Is A Simple Harmonic Oscillator (SHO) ?kalshinokovPas encore d'évaluation
- WLP Creative WritingDocument12 pagesWLP Creative Writingsheena balaisPas encore d'évaluation
- PHYSICS FOR ENGINEERS Chapter 2Document30 pagesPHYSICS FOR ENGINEERS Chapter 2Leo Prince GicanaPas encore d'évaluation
- Bp8-Tension: User Defined Applied Forces at Interface Basic DimensionsDocument4 pagesBp8-Tension: User Defined Applied Forces at Interface Basic DimensionsMallesh NenkatPas encore d'évaluation
- Matlab Fourier Series Signal & SystemDocument15 pagesMatlab Fourier Series Signal & SystemNik Ahmad FaisalPas encore d'évaluation
- EC &LD-Lab ManualDocument50 pagesEC &LD-Lab ManualEk naye din ki shuruwat kroPas encore d'évaluation
- Wopho 13 Prob7-Final PDFDocument3 pagesWopho 13 Prob7-Final PDFPeter JonesPas encore d'évaluation
- AEE CivilDocument16 pagesAEE CivilPhoenix Cruise100% (1)
- Anritsu Understanding OtdrsDocument60 pagesAnritsu Understanding OtdrsMathieu Bolle100% (3)
- Grade 5 Science Practice Test: Nebraska Department of Education 2012Document13 pagesGrade 5 Science Practice Test: Nebraska Department of Education 2012Ria SihombingPas encore d'évaluation
- Astm E2583Document3 pagesAstm E2583Eliana salamancaPas encore d'évaluation
- Microbiology Study GuideDocument9 pagesMicrobiology Study GuideMonica E. AgogoPas encore d'évaluation
- Syllabus - Vishwakarma Institute of TechnologyDocument211 pagesSyllabus - Vishwakarma Institute of TechnologyAditya PophalePas encore d'évaluation
- Pump Specifications Elv Series Submersible Sump Pump With Oiltector ControlDocument7 pagesPump Specifications Elv Series Submersible Sump Pump With Oiltector ControlAnonymous PCsoNCt0mFPas encore d'évaluation
- UCM Question Bank 2 MarksDocument22 pagesUCM Question Bank 2 MarksManivannan JeevaPas encore d'évaluation
- Local Buckling Analysis Based On DNV-OS-F101 2000Document4 pagesLocal Buckling Analysis Based On DNV-OS-F101 2000shervinyPas encore d'évaluation
- Curriculum I Semester: SL - No Subject Code Subject Name Category L T P CreditsDocument23 pagesCurriculum I Semester: SL - No Subject Code Subject Name Category L T P CreditsRathinaKumarPas encore d'évaluation
- 1757 Nursing Foundation Question BankDocument11 pages1757 Nursing Foundation Question BankSovon Samanta100% (1)
- Earth and Life Science Copy (Repaired)Document39 pagesEarth and Life Science Copy (Repaired)Aaron Manuel MunarPas encore d'évaluation
- Introduction To Separation of Oil and WaterDocument30 pagesIntroduction To Separation of Oil and Waterodracir091865100% (1)
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseD'EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseÉvaluation : 4.5 sur 5 étoiles4.5/5 (51)
- The Laws of Thermodynamics: A Very Short IntroductionD'EverandThe Laws of Thermodynamics: A Very Short IntroductionÉvaluation : 4.5 sur 5 étoiles4.5/5 (10)
- Aerodynamics for Engineering StudentsD'EverandAerodynamics for Engineering StudentsÉvaluation : 5 sur 5 étoiles5/5 (5)
- Hyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionD'EverandHyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionÉvaluation : 4.5 sur 5 étoiles4.5/5 (3)
- Centrifugal Pumps: Design and ApplicationD'EverandCentrifugal Pumps: Design and ApplicationÉvaluation : 2 sur 5 étoiles2/5 (7)
- Pilot's Handbook of Aeronautical Knowledge (2024): FAA-H-8083-25CD'EverandPilot's Handbook of Aeronautical Knowledge (2024): FAA-H-8083-25CPas encore d'évaluation
- Pressure Vessels: Design, Formulas, Codes, and Interview Questions & Answers ExplainedD'EverandPressure Vessels: Design, Formulas, Codes, and Interview Questions & Answers ExplainedÉvaluation : 5 sur 5 étoiles5/5 (1)
- Post Weld Heat Treatment PWHT: Standards, Procedures, Applications, and Interview Q&AD'EverandPost Weld Heat Treatment PWHT: Standards, Procedures, Applications, and Interview Q&APas encore d'évaluation
- 1,001 Questions & Answers for the CWI Exam: Welding Metallurgy and Visual Inspection Study GuideD'Everand1,001 Questions & Answers for the CWI Exam: Welding Metallurgy and Visual Inspection Study GuideÉvaluation : 3.5 sur 5 étoiles3.5/5 (7)
- Offshore Mechanics: Structural and Fluid Dynamics for Recent ApplicationsD'EverandOffshore Mechanics: Structural and Fluid Dynamics for Recent ApplicationsPas encore d'évaluation
- Vibration Basics and Machine Reliability Simplified : A Practical Guide to Vibration AnalysisD'EverandVibration Basics and Machine Reliability Simplified : A Practical Guide to Vibration AnalysisÉvaluation : 4 sur 5 étoiles4/5 (2)
- Fuels, Furnaces and Refractories: International Series on Materials Science and TechnologyD'EverandFuels, Furnaces and Refractories: International Series on Materials Science and TechnologyÉvaluation : 5 sur 5 étoiles5/5 (1)
- Handbook of Mechanical and Materials EngineeringD'EverandHandbook of Mechanical and Materials EngineeringÉvaluation : 5 sur 5 étoiles5/5 (4)
- Piping Design for Industrial Facilities: Understanding Codes and StandardsD'EverandPiping Design for Industrial Facilities: Understanding Codes and StandardsÉvaluation : 4 sur 5 étoiles4/5 (1)
- Geotechnical Engineering Calculations and Rules of ThumbD'EverandGeotechnical Engineering Calculations and Rules of ThumbÉvaluation : 4 sur 5 étoiles4/5 (17)
- Mechanical Vibrations and Condition MonitoringD'EverandMechanical Vibrations and Condition MonitoringÉvaluation : 5 sur 5 étoiles5/5 (1)
- Introduction to the Explicit Finite Element Method for Nonlinear Transient DynamicsD'EverandIntroduction to the Explicit Finite Element Method for Nonlinear Transient DynamicsPas encore d'évaluation
- Practical Guides to Testing and Commissioning of Mechanical, Electrical and Plumbing (Mep) InstallationsD'EverandPractical Guides to Testing and Commissioning of Mechanical, Electrical and Plumbing (Mep) InstallationsÉvaluation : 3.5 sur 5 étoiles3.5/5 (3)
- Heat Exchanger Design Guide: A Practical Guide for Planning, Selecting and Designing of Shell and Tube ExchangersD'EverandHeat Exchanger Design Guide: A Practical Guide for Planning, Selecting and Designing of Shell and Tube ExchangersÉvaluation : 4 sur 5 étoiles4/5 (13)