Académique Documents
Professionnel Documents
Culture Documents
Linear Programming Part II
Transféré par
Shelly Theresse Catian Novo0 évaluation0% ont trouvé ce document utile (0 vote)
8 vues1 pageCopyright
© © All Rights Reserved
Formats disponibles
DOCX, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
8 vues1 pageLinear Programming Part II
Transféré par
Shelly Theresse Catian NovoDroits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 1
Problem 8.
One method for the manufacture of synthesis gas depends on the vapor-phase
catalytic reaction of methane with steam according to the equation below.
CH4 + H2O CO + 3H2
Rxn 1
The water-gas shift reaction also is important.
CO + H2O CO2 + H2
Rxn 2
Based on stoichiometric feed of steam and methane, i.e. steam to methane ratio
(S/M) is equal to 1, compute the equilibrium composition at 600 K and 1300 K, and
1 and 100 bars.
Perform similar calculations for S/M of 2, 3, and 4. What is the effect of changing
S/M on the extent of of the 2 reactions?
Vous aimerez peut-être aussi
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- 28 Psychrometric ProcessesDocument19 pages28 Psychrometric ProcessesPRASAD326100% (7)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Binary Distillation in A Bubble Cap Tray ColumnDocument3 pagesBinary Distillation in A Bubble Cap Tray ColumnShelly Theresse Catian NovoPas encore d'évaluation
- Present Worth Analysis PDFDocument24 pagesPresent Worth Analysis PDFShelly Theresse Catian NovoPas encore d'évaluation
- Drying Temperature and Water Content-Strength Correlations - Brendan O'Kelly - AcademiaDocument14 pagesDrying Temperature and Water Content-Strength Correlations - Brendan O'Kelly - AcademiaShelly Theresse Catian NovoPas encore d'évaluation
- Linear Programming ProblemsDocument2 pagesLinear Programming ProblemsShelly Theresse Catian Novo100% (1)
- HazardsDocument3 pagesHazardsShelly Theresse Catian NovoPas encore d'évaluation
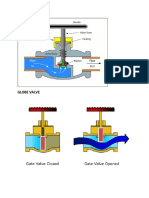
- Valves Fittings Von Karman From FoustDocument9 pagesValves Fittings Von Karman From FoustShelly Theresse Catian NovoPas encore d'évaluation
- Take Home Quiz - Higher OrderDocument1 pageTake Home Quiz - Higher OrderShelly Theresse Catian NovoPas encore d'évaluation
- Exam 1 SolutionDocument5 pagesExam 1 SolutionShelly Theresse Catian NovoPas encore d'évaluation
- Estimation of Friction Loss FROM FOUSTDocument18 pagesEstimation of Friction Loss FROM FOUSTShelly Theresse Catian NovoPas encore d'évaluation
- Sludge-To-Cement ConversionDocument4 pagesSludge-To-Cement ConversionShelly Theresse Catian NovoPas encore d'évaluation
- Fluid Motive DevicesDocument31 pagesFluid Motive DevicesShelly Theresse Catian NovoPas encore d'évaluation
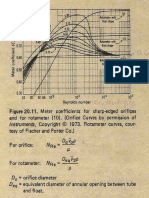
- Flow Meter Charts and Assignments On Flow Meters PDFDocument3 pagesFlow Meter Charts and Assignments On Flow Meters PDFMona BreyPas encore d'évaluation
- Project ProposalDocument1 pageProject ProposalShelly Theresse Catian NovoPas encore d'évaluation
- SocioDocument3 pagesSocioShelly Theresse Catian NovoPas encore d'évaluation
- Project ProposalDocument1 pageProject ProposalShelly Theresse Catian NovoPas encore d'évaluation