Académique Documents
Professionnel Documents
Culture Documents
Goldenhar PDF
Transféré par
Daniel FernandezTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Goldenhar PDF
Transféré par
Daniel FernandezDroits d'auteur :
Formats disponibles
Hindawi Publishing Corporation
Case Reports in Ophthalmological Medicine
Volume 2015, Article ID 435967, 3 pages
http://dx.doi.org/10.1155/2015/435967
Case Report
Craniofacial Microsomia: Goldenhar Syndrome in
Association with Bilateral Congenital Cataract
U. D. Shrestha1,2 and S. Adhikari1,2
1
National Academy of Medical Sciences, Kathmandu, Nepal
Tilganga Institute of Ophthalmology, P.O. Box 561, Gaushala, Kathmandu, Nepal
Correspondence should be addressed to U. D. Shrestha; ujjowala@gmail.com
Received 6 July 2015; Accepted 17 September 2015
Academic Editor: Vishal Jhanji
Copyright 2015 U. D. Shrestha and S. Adhikari. This is an open access article distributed under the Creative Commons
Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is
properly cited.
Craniofacial microsomia (CFM) includes a spectrum of malformations primarily involving structures derived from the first and
second branchial arches. Patients with hemifacial microsomia and epibulbar dermoids are said to have Goldenhar syndrome (GHS).
Four-month-old boy with whitish pupillary reflex presented with the features of GHS in pediatric ophthalmology clinic. The child
had ocular and auricular manifestations. There were no vertebral anomalies, but he had bilateral congenital cataract. The peculiarity
of this case is the presence of the bilateral total congenital cataract, in association with CFM. There is absence of epibulbar dermoid
or lipodermoid in the eyes, although the child had features of GHS. In addition to it, anesthetic intubation was smooth in this case.
Any case diagnosed with CFM and/or GHS needs treatment through multidisciplinary approach, consultation in ophthalmology
department is one of them.
1. Introduction
CFM includes a spectrum of malformations primarily involving structures derived from the first and second branchial
arches. Characteristic findings include facial asymmetry
resulting from maxillary and/or mandibular hypoplasia;
preauricular or facial tags; ear malformations that can include
microtia (hypoplasia of the external ear), anotia (absence of
the external ear), or aural atresia (absence of the external ear
canal); and hearing loss and ocular abnormalities. Hemifacial
microsomia often refers to craniofacial microsomia with
maxillary or mandibular hypoplasia. People with hemifacial
microsomia and epibulbar dermoids may be said to have
GHS or oculoauricular dysplasia. The pathognomonic triad
of GHS includes CFM, dermolipoma, and vertebra skeletal
anomalies [1].
2. Case Presentation
2.1. Chief Complaint. Four-month-old baby was referred with
the chief complaint of white pupillary reflex in both the eyes
on 14 July, 2013. The antenatal history was uneventful. The
patient was born at 36 weeks of gestation and was delivered by
normal spontaneous vaginal delivery (NSVD). The child did
not have major illness after the delivery. He was not admitted
in intensive care unit (ICU). There was no family history of
facial syndromes or ocular conditions.
2.2. Ocular Examination. The child followed the light with
both eyes (OU). Eyelids were normal OU.
Extraocular motility was full in all cardinal positions of
gaze. Nystagmus was present. Anterior segment examination
revealed normal conjunctiva and cornea. Pupils were round,
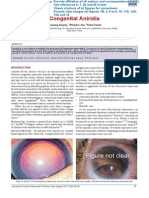
regular, and reacting, OU. On the lens, total cataract was seen,
OU (Figures 1 and 2). Fundus was not visible. Hence ultrasound was done. There were no echo dense opacities, OU. On
facial examination the child had facial asymmetry, presence
of skin tags on left cheek. Preauricular tags were present on
both sides (Figures 2 and 3). He had high arched palate.
For both the eyes, lens aspiration with anterior vitrectomy
was planned under general anesthesia, as the child was
only four months old. Left eye was operated on 9 August,
2013. Right eye was operated on 30 August, 2013. Difficult
intubation is expected in GHS patients because of facial and
Case Reports in Ophthalmological Medicine
Figure 1: Child with cataract in the right eye.
Figure 2: Child with cataract in the left eye and preauricular tag.
oral abnormalities especially mandibular hypoplasia and limitation of neck movement resulting from vertebral anomalies.
In this case the anesthetic intubation was smooth. There was
no difficulty in airway management [2]. No single airway
test can provide a high index of sensitivity and specificity for
prediction of difficult airway in patients of GHS. The airway
and anesthetic management of GHS patients depend on the
type, extent, and severity of craniofacial-vertebral anomalies,
associated cardiovascular problems, and nature of surgery.
Postoperatively, retinoscopy and refraction were done in
four weeks after the cataract surgery.
He was prescribed the aphakic glass of +16.00 D. On 3month follow-up, the child had alternate fixation with the
glasses.
3. Discussion
CFM describes the spectrum of abnormalities that primarily
affect the cranial and facial development before birth. CFM
patients have microsomia, facial asymmetry between the
two sides. In about two-thirds of cases, facial asymmetry is
bilateral. The facial characteristics in CFM typically include
unilateral maxillary or mandibular hypoplasia. It results in
dental problem and breathing, feeding, and speech difficulty.
GHS is also known as oculoauricular-vertebral spectrum
or variant of hemifacial microsomia. It is a developmental
anomaly of maxillofacial skeleton, apparent at birth. It results
in macrostomia and lateral facial clefts. GHS is dysmorphogenesis which occurs due to aberrant development of the first
Figure 3: Child with preauricular tag and the skin tag in the cheek.
and second branchial arches [3]. It has prevalence from 1 to
9/100000 live births and incidence rate is 1 per 2500045000
births, with a male to female ratio of 3 : 2.
It is mostly unilateral (7080%). Anomalies in patients
with this syndrome include preauricular skin tags (90%),
hemifacial microsomia (77%), vertebral anomalies of different size and shape (70%), microtia (52%), CNS malformations
(47%), cardiac malformations (39%), epibulbar dermoid cyst
(39%), unilateral maxillary hypoplasia, and genitourinary
anomalies [4, 5]. Oral cavity malformations include high arch
or cleft palate and tongue abnormalities. This patient had the
high arched palate [6, 7].
Other ocular anomalies include microphthalmos, microcornea, and anophthalmos. Deficient neural crest migration
into the developing eye can lead to coloboma which can be
present in eyelid, iris, and choroid [8]. Corneal hypoesthesia,
extraocular motility disorders like strabismus, blepharoptosis, anomalous lacrimal drainage system, polar cataract, and
retinal and optic nerve anomalies may be present.
Microtia can result from aberrant migration of neural
crest cells into the first and second branchial arches during
early embryonic development.
GHS is known for its classical triad of epibulbar dermoids
or lipodermoids, auricular appendages, and pretragal fistulas
[8]. However, this child did not have any epibulbar dermoid.
According to Baum and Feingold, 24% of cases do not have
any epibulbar dermoids, 23% have bilateral dermoid, and 53%
have unilateral epibulbar dermoid [9]. Polydactyl is one of the
unusual associations in GHS [10].
This is the case report to describe GHS in association with
bilateral congenital total cataract. Congenital cataract is the
opacification of the lens and its capsule. The aetiology of the
cataract is: in one-third cases-idiopathic, in one-third casesmaternal infections and the rest of one-third casesgenetic and
hereditary.
Developmental genes PAX 6 and PITX3 affect the lens
development [11]. Any problem during embryonic lens development leads to congenital cataract formation.
The most common aetiologies of all pediatric cataract
include intrauterine infections, metabolic disorders, and
genetically transmitted syndromes [12]. However, CFM most
frequently occurs as a simplex case with unknown etiology.
It is unclear what genes are involved in CFM. It is not well
Case Reports in Ophthalmological Medicine
understood why certain disruptions to development affect the
first and second pharyngeal arches in particular.
Although the etiology of this disease is not fully understood, autosomal recessive or dominant inheritance is possible. The majority of patients with hemifacial microsomia
including GHS are sporadic cases [13].
CFM and GHS are diagnosed clinically. They are the
diagnoses of exclusion. Genetic study is important nowadays.
However, till now no definite genetic test has been described
to diagnose CFM and GHS yet [14].
4. Conclusion
The peculiarity of this case is the presence of the bilateral
total congenital cataract, in association with CFM. There is
absence of epibulbar dermoid or lipodermoid in the eyes,
although the child had features of GHS. In addition to it,
anesthetic intubation was smooth in this case. Congenital
cataract should be considered as one associations of the
GHS. Any case diagnosed with CFM and/or GHS needs
treatment through multidisciplinary approach, consultation
in ophthalmology department is one of them.
Conflict of Interests
The authors declare that there is no conflict of interests
regarding the publication of this paper.
References
[1] U. D. Shrestha and S. Adhikari, Goldenhar syndrome in association with duane syndrome, Journal of the Nepal Medical
Association, vol. 52, no. 185, pp. 3335, 2012.
[2] F. Roodneshin and M. Agah, Management of anesthesia in
Goldenhar syndrome: case-series study, Tanaffos, vol. 8, no. 4,
pp. 4350, 2009.
[3] E. Senggen, T. Laswed, J.-Y. Meuwly et al., First and second
branchial arch syndromes: multimodality approach, Pediatric
Radiology, vol. 41, no. 5, pp. 549561, 2011.
[4] S. Bayraktar, S. T. Bayraktar, E. Ataoglu, A. Ayaz, and M. Elevli,
Goldenhars syndrome associated with multiple congenital
abnormalities, Journal of Tropical Pediatrics, vol. 51, no. 6, pp.
377379, 2005.
[5] J. Cohen and N. C. Schanen, Branchial cleft anomaly, congenital heart disease, and biliary atresia: Goldenhar complex or
Lambert syndrome? Genetic Counseling, vol. 11, no. 2, pp. 153
156, 2000.
[6] D. S. Chauhan and Y. Guruprasad, Goldenhar syndrome with
Tessiers 7 cleft: report of a case, Journal of Maxillofacial and
Oral Surgery, vol. 14, supplement 1, pp. 4246, 2015.
[7] O. Engiz, S. Balci, M. Unsal, S. Ozer, K. K. Oguz, and D. Aktas,
31 cases with oculoauriculovertebral dysplasia (Goldenhar
syndrome): clinical, neuroradiologic, audiologic and cytogenetic findings, Genetic Counseling, vol. 18, no. 3, pp. 277288,
2007.
[8] O. Villanueva, D. S. Atkinson, and S. R. Lambert, Trigeminal
nerve hypoplasia and aplasia in children with goldenhar syndrome and corneal hypoesthesia, Journal of American Association for Pediatric Ophthalmology and Strabismus, vol. 9, no. 2,
pp. 202204, 2005.
3
[9] J. L. Baum and M. Feingold, Ocular aspects of Goldenhars
syndrome, American Journal of Ophthalmology, vol. 75, no. 2,
pp. 250257, 1973.
[10] D. Mishra, B. P. Sinha, and R. Kumar, Goldenhar syndrome
with unusual association of pre-axial polydactyly, European
Journal of Ophthalmology, vol. 19, no. 6, pp. 10631064, 2009.
[11] G. Halder, P. Callaerts, and W. J. Gehring, Induction of ectopic
eyes by targeted expression of the eyeless gene in Drosophila,
Science, vol. 267, no. 5205, pp. 17881792, 1995.
[12] S. Lambert, Lens, in Paediatric Ophthalmology, D. Taylor, Ed.,
p. 455, Blackwell Publishing, London, UK, 2nd edition, 1997.
[13] U. Burck, Genetic aspects of hemifacial microsomia, Human
Genetics, vol. 64, no. 3, pp. 291296, 1983.
[14] C. L. Heike, D. V. Luquetti, and A. V. Hing, Craniofacial microsomia overview, in GeneReviews, University of Washington,
Seattle, Wash, USA, 2014.
Copyright of Case Reports in Ophthalmological Medicine is the property of Hindawi
Publishing Corporation and its content may not be copied or emailed to multiple sites or
posted to a listserv without the copyright holder's express written permission. However, users
may print, download, or email articles for individual use.
Vous aimerez peut-être aussi
- Ultrasound Diagnosis of Facial Anomalies (Diagnosis Usg Untuk Kelainan Wajah)Document7 pagesUltrasound Diagnosis of Facial Anomalies (Diagnosis Usg Untuk Kelainan Wajah)Puspita SariPas encore d'évaluation
- Intraocular Lenses and Clinical Treatment in Paediatric CataractDocument5 pagesIntraocular Lenses and Clinical Treatment in Paediatric CataractLinna LetoPas encore d'évaluation
- Anophthalmia and MicrophthalmiaDocument8 pagesAnophthalmia and MicrophthalmiaLjubomirErdoglijaPas encore d'évaluation
- 2161 1122 SR196 PDFDocument3 pages2161 1122 SR196 PDFKarenWillowPas encore d'évaluation
- 46 Onankpa EtalDocument3 pages46 Onankpa EtaleditorijmrhsPas encore d'évaluation
- Clinical Manifestations of Ocular Toxoplasmosis in Yogyakarta, Indonesia: A Clinical Review of 173 CasesDocument7 pagesClinical Manifestations of Ocular Toxoplasmosis in Yogyakarta, Indonesia: A Clinical Review of 173 CasesRiza Haida WardhaniPas encore d'évaluation
- Childhood Glaucoma: An Overview: Parul Singh, Yogesh Kumar, Manoj Tyagi, Krishna Kuldeep, Parmeshwari Das SharmaDocument7 pagesChildhood Glaucoma: An Overview: Parul Singh, Yogesh Kumar, Manoj Tyagi, Krishna Kuldeep, Parmeshwari Das SharmaResa_G1A110068Pas encore d'évaluation
- Ocular and Adnexal Anomalies in Craniofacial MicrosomiaDocument8 pagesOcular and Adnexal Anomalies in Craniofacial MicrosomiaEerrmmaa Ssaarrell Rroommaann PpaarraaPas encore d'évaluation
- Ankyloblepharon Filiforme Adnatum: A Case ReportDocument4 pagesAnkyloblepharon Filiforme Adnatum: A Case ReportGheavita Chandra DewiPas encore d'évaluation
- 47 Shubhangi EtalDocument3 pages47 Shubhangi EtaleditorijmrhsPas encore d'évaluation
- 34tupe EtalDocument5 pages34tupe EtaleditorijmrhsPas encore d'évaluation
- Phthisis Bulbi: Clinical and Pathologic Findings in RetinoblastomaDocument6 pagesPhthisis Bulbi: Clinical and Pathologic Findings in RetinoblastomaEstmar ValentinoPas encore d'évaluation
- Prenatal Diagnosis of Harlequin Ichthyosis: A Case ReportDocument4 pagesPrenatal Diagnosis of Harlequin Ichthyosis: A Case Reportcitra annisa fitriPas encore d'évaluation
- Goldenhar Syndrome An Unusual Case ReportDocument4 pagesGoldenhar Syndrome An Unusual Case ReportInternational Journal of Innovative Science and Research TechnologyPas encore d'évaluation
- Jurnal Pedo IzzahDocument5 pagesJurnal Pedo IzzahSuper LonelyPas encore d'évaluation
- Orthod Craniofacial Res - 2022 - Kuu Karkku - Craniofacial Microsomia More Than A Structural MalformationDocument6 pagesOrthod Craniofacial Res - 2022 - Kuu Karkku - Craniofacial Microsomia More Than A Structural MalformationVanessa KowalskaPas encore d'évaluation
- Goldenhar Syndrome - A Literature Review: JSM DentistryDocument4 pagesGoldenhar Syndrome - A Literature Review: JSM DentistryKaren PinemPas encore d'évaluation
- Anophthalmia WikiDocument7 pagesAnophthalmia WikiUnggul YudhaPas encore d'évaluation
- Goldenhar Syndrome: Current Perspectives: Katarzyna Bogusiak, Aleksandra Puch, Piotr ArkuszewskiDocument11 pagesGoldenhar Syndrome: Current Perspectives: Katarzyna Bogusiak, Aleksandra Puch, Piotr ArkuszewskiDIANA PAOLA FONTECHA GONZÁLEZPas encore d'évaluation
- Medicina 59 01660 v2Document10 pagesMedicina 59 01660 v2Maria CorbuPas encore d'évaluation
- Jurnal MataDocument8 pagesJurnal MatamarinarizkiPas encore d'évaluation
- Final Case Report FinalDocument7 pagesFinal Case Report FinalPawan JarwalPas encore d'évaluation
- Cong GlaucomaDocument20 pagesCong GlaucomapratikandpPas encore d'évaluation
- Congenital Rubella Syndrome Global IssueDocument1 pageCongenital Rubella Syndrome Global IssueAnonymous mhsYKgCPas encore d'évaluation
- Candidate Genes of High Myopia NT Fix YetDocument18 pagesCandidate Genes of High Myopia NT Fix YetFitria RizkyPas encore d'évaluation
- JPNR - S04 - 233Document6 pagesJPNR - S04 - 233Ferisa paraswatiPas encore d'évaluation
- Jurnal Glaukoma PrintDocument15 pagesJurnal Glaukoma PrintrafikaPas encore d'évaluation
- An Insight Into - Hemifacial MicrosomiaDocument6 pagesAn Insight Into - Hemifacial MicrosomiaIJAR JOURNALPas encore d'évaluation
- Bilateral Blindness Due To Pterygium - A Case ReportDocument3 pagesBilateral Blindness Due To Pterygium - A Case ReportDavid Al HavizPas encore d'évaluation
- Intracranial Tumors: An Ophthalmic Perspective: DR M Hemanandini, DR P Sumathi, DR P A KochamiDocument3 pagesIntracranial Tumors: An Ophthalmic Perspective: DR M Hemanandini, DR P Sumathi, DR P A Kochamiwolfang2001Pas encore d'évaluation
- High Myopia Caused by A Mutation in LEPREL1, Encoding Prolyl 3-Hydroxylase 2Document8 pagesHigh Myopia Caused by A Mutation in LEPREL1, Encoding Prolyl 3-Hydroxylase 2Fiolita NatasyaPas encore d'évaluation
- International Journal of Research in Pharmaceutical SciencesDocument12 pagesInternational Journal of Research in Pharmaceutical SciencesMagfirah AliyyaPas encore d'évaluation
- Prenatal Diagnosis of Cleft Lip and Cleft Lip Palate Case ReportDocument5 pagesPrenatal Diagnosis of Cleft Lip and Cleft Lip Palate Case ReportManjirou SanoPas encore d'évaluation
- Congenital Cataracts: CRYBB3 Beaded Filament Structural Protein 1 (BFSP1) Gap FunctionDocument2 pagesCongenital Cataracts: CRYBB3 Beaded Filament Structural Protein 1 (BFSP1) Gap FunctionKuchai BaruPas encore d'évaluation
- Congenital AniridiaDocument16 pagesCongenital AniridiaManish MahabirPas encore d'évaluation
- Children CataractDocument15 pagesChildren CataractMerlose PlacePas encore d'évaluation
- Cataracts Pathophysiology and Managements: Abdulrahman Zaid AlshamraniDocument4 pagesCataracts Pathophysiology and Managements: Abdulrahman Zaid AlshamraniOcha24 TupamahuPas encore d'évaluation
- CebocephalyDocument12 pagesCebocephalyUser AbPas encore d'évaluation
- Brain TumorsDocument12 pagesBrain Tumors36Kushal saNdHuPas encore d'évaluation
- Update On Congenital Glaucoma PrintDocument15 pagesUpdate On Congenital Glaucoma Printalfath rezaPas encore d'évaluation
- Etiology of Proptosis in Children Sindhu 1998Document3 pagesEtiology of Proptosis in Children Sindhu 1998Putri Wulan SukmawatiPas encore d'évaluation
- Community Outreach: An Indicator For Assessment of Prevalence of AmblyopiaDocument11 pagesCommunity Outreach: An Indicator For Assessment of Prevalence of AmblyopiaMusdalipaPas encore d'évaluation
- Congenital Glaucoma Epidemiological Clinical and Therapeutic Aspects About 414 EyesDocument5 pagesCongenital Glaucoma Epidemiological Clinical and Therapeutic Aspects About 414 Eyessiti rumaisaPas encore d'évaluation
- Pediatric Glaucoma PDFDocument8 pagesPediatric Glaucoma PDFDr.Nalini PavuluriPas encore d'évaluation
- Management of Facial AsymmetryDocument28 pagesManagement of Facial AsymmetrykrazeedoctorPas encore d'évaluation
- Achondroplasia: September 2012Document7 pagesAchondroplasia: September 2012ancillaagrayn100% (1)
- Double Trouble - A Rarest of Rare ScenarioDocument2 pagesDouble Trouble - A Rarest of Rare ScenarioBOHR International Journal of Current Research in Optometry and Ophthalmology (BIJCROO)Pas encore d'évaluation
- 156-Article Text-557-2-10-20200909Document5 pages156-Article Text-557-2-10-20200909sagarPas encore d'évaluation
- Paediatric Catarct Review ArticleDocument8 pagesPaediatric Catarct Review ArticleSana ParveenPas encore d'évaluation
- Lip and Palate Reconstruction On Median Cerebrofacial Malformation PatientDocument5 pagesLip and Palate Reconstruction On Median Cerebrofacial Malformation PatientAgus MarsyalPas encore d'évaluation
- Secondary Buphthalmos: A Case SeriesDocument2 pagesSecondary Buphthalmos: A Case SeriesdrheriPas encore d'évaluation
- Cerebral Palsy Oral Manifestations and Dental Mana PDFDocument7 pagesCerebral Palsy Oral Manifestations and Dental Mana PDFnatasha WhisbergPas encore d'évaluation
- Cleft Lip and PalateDocument5 pagesCleft Lip and PalatedilaPas encore d'évaluation
- Congenital Ptosis - JurnalDocument28 pagesCongenital Ptosis - JurnalJohanes Arie SetiawanPas encore d'évaluation
- John WicterDocument8 pagesJohn WicteraldybebikaPas encore d'évaluation
- Goldenhar Syndrome An Ophthalmological Perspective: A Case SeriesDocument6 pagesGoldenhar Syndrome An Ophthalmological Perspective: A Case SeriesInternational Journal of Innovative Science and Research TechnologyPas encore d'évaluation
- Otogenic Brain Abscess Our ExperienceDocument5 pagesOtogenic Brain Abscess Our ExperienceNeysaAzaliaEfrimaisaPas encore d'évaluation
- Infantile Hemangiomas: A Review: Pediatric Ophthalmology UpdateDocument9 pagesInfantile Hemangiomas: A Review: Pediatric Ophthalmology UpdateYipno Wanhar El MawardiPas encore d'évaluation
- Case Analysis-Aravind Eye HospitalDocument7 pagesCase Analysis-Aravind Eye HospitalAchintya Goel100% (1)
- 7.1 Gradual Loss of VisionDocument19 pages7.1 Gradual Loss of VisionparugandooPas encore d'évaluation
- Management of CataractDocument25 pagesManagement of Cataractapi-3742497Pas encore d'évaluation
- Cbleenpl 11Document12 pagesCbleenpl 11Jeeva JohnPas encore d'évaluation
- Cataract Case PresentationDocument7 pagesCataract Case PresentationShahbaz AAnsariPas encore d'évaluation
- Surgical Outcome of Corneal Triple Procedure in The Management of Cases of Corneal and Lenticular Opacities in A Tertiary Care HospitalDocument4 pagesSurgical Outcome of Corneal Triple Procedure in The Management of Cases of Corneal and Lenticular Opacities in A Tertiary Care HospitalIJAR JOURNALPas encore d'évaluation
- My SeminarDocument12 pagesMy SeminarAdeleye SeunPas encore d'évaluation
- Ophthalmology (Revised)Document4 pagesOphthalmology (Revised)Tanishka KoyandePas encore d'évaluation
- Diseases of LensDocument42 pagesDiseases of LensAmmad ShahidPas encore d'évaluation
- Awareness and Knowledge of Common Eye Diseases Among Health Care Students After InterventionDocument6 pagesAwareness and Knowledge of Common Eye Diseases Among Health Care Students After InterventionInternational Journal of Innovative Science and Research TechnologyPas encore d'évaluation
- Final TouchDocument49 pagesFinal TouchmalathiPas encore d'évaluation
- Alcon AcrySof IQ Lens ImplantDocument2 pagesAlcon AcrySof IQ Lens ImplantAbdelmonem HamedPas encore d'évaluation
- Senile Cataract (Age-Related Cataract) - Practice Essentials, Background, PathophysiologyDocument5 pagesSenile Cataract (Age-Related Cataract) - Practice Essentials, Background, PathophysiologyAhmad FahroziPas encore d'évaluation
- New DelhiDocument186 pagesNew DelhiBala KrishnanPas encore d'évaluation
- Diseases of Lens DR D.JLDocument113 pagesDiseases of Lens DR D.JLNithya GunasekaranPas encore d'évaluation
- Histogram of Oriented Gradient Based Automatic Detection of Eye DiseasesDocument5 pagesHistogram of Oriented Gradient Based Automatic Detection of Eye DiseasesDean FitrahPas encore d'évaluation
- Case Review - EndophthalmitisDocument4 pagesCase Review - EndophthalmitisDr Shawgat Ul Karim KhanPas encore d'évaluation
- NCM 0114 Module 3 - Nursing Care Management of The Older Person With Chronic IllnessDocument84 pagesNCM 0114 Module 3 - Nursing Care Management of The Older Person With Chronic IllnessKristine Kim100% (1)
- Visudyne (Verteporfin For Injection) : Adverse Reactions (6) )Document2 pagesVisudyne (Verteporfin For Injection) : Adverse Reactions (6) )Upik MoritaPas encore d'évaluation
- Jurnal ReadingDocument4 pagesJurnal ReadingArum DiannitasariPas encore d'évaluation
- Opt Halm OlogyDocument87 pagesOpt Halm OlogyAya MahmoudPas encore d'évaluation
- BCQ Seq Paper A Ophthalmology 2015Document7 pagesBCQ Seq Paper A Ophthalmology 2015Hira Panhwer0% (1)
- HESI Prep - Health AssessmentDocument248 pagesHESI Prep - Health Assessmentmeeeenon100% (4)
- Contar ActDocument5 pagesContar ActMalik Abdul HananPas encore d'évaluation
- Ophtalmology Multiple Question Choices For 4 Year Course Moscow State Medical UniversityDocument26 pagesOphtalmology Multiple Question Choices For 4 Year Course Moscow State Medical UniversitysharenPas encore d'évaluation
- 4 Powerful Eye Exercises For Rapidly Improving Your VisionDocument8 pages4 Powerful Eye Exercises For Rapidly Improving Your VisionDoraemon Noby100% (1)
- Vitrectomy For Primary Symptomatic Vitreous Opacities: An Evidence-Based ReviewDocument11 pagesVitrectomy For Primary Symptomatic Vitreous Opacities: An Evidence-Based ReviewTasya SylviaPas encore d'évaluation
- Fhu PDFDocument3 pagesFhu PDFdanielep1Pas encore d'évaluation
- DT LeukocoriaDocument44 pagesDT LeukocoriahamzahPas encore d'évaluation
- Nevanac Us enDocument3 pagesNevanac Us enBilal Ahmed AttarPas encore d'évaluation
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionD'EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionÉvaluation : 4 sur 5 étoiles4/5 (404)
- The Age of Magical Overthinking: Notes on Modern IrrationalityD'EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityÉvaluation : 4 sur 5 étoiles4/5 (34)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDD'EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDÉvaluation : 5 sur 5 étoiles5/5 (4)
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)D'EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Évaluation : 3 sur 5 étoiles3/5 (1)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsD'EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsÉvaluation : 4 sur 5 étoiles4/5 (4)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedD'EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedÉvaluation : 4.5 sur 5 étoiles4.5/5 (82)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeD'EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (254)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsD'EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsÉvaluation : 4.5 sur 5 étoiles4.5/5 (170)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsD'EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsPas encore d'évaluation
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeD'EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeÉvaluation : 2 sur 5 étoiles2/5 (1)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisD'EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisÉvaluation : 4.5 sur 5 étoiles4.5/5 (44)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsD'EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsÉvaluation : 5 sur 5 étoiles5/5 (1)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaD'EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesD'EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesÉvaluation : 4.5 sur 5 étoiles4.5/5 (1412)
- The Comfort of Crows: A Backyard YearD'EverandThe Comfort of Crows: A Backyard YearÉvaluation : 4.5 sur 5 étoiles4.5/5 (23)
- The Twentysomething Treatment: A Revolutionary Remedy for an Uncertain AgeD'EverandThe Twentysomething Treatment: A Revolutionary Remedy for an Uncertain AgeÉvaluation : 4.5 sur 5 étoiles4.5/5 (2)
- The Obesity Code: Unlocking the Secrets of Weight LossD'EverandThe Obesity Code: Unlocking the Secrets of Weight LossÉvaluation : 4 sur 5 étoiles4/5 (6)
- To Explain the World: The Discovery of Modern ScienceD'EverandTo Explain the World: The Discovery of Modern ScienceÉvaluation : 3.5 sur 5 étoiles3.5/5 (51)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.D'EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Évaluation : 4.5 sur 5 étoiles4.5/5 (110)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisD'EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsD'EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsPas encore d'évaluation
- Hearts of Darkness: Serial Killers, The Behavioral Science Unit, and My Life as a Woman in the FBID'EverandHearts of Darkness: Serial Killers, The Behavioral Science Unit, and My Life as a Woman in the FBIÉvaluation : 4 sur 5 étoiles4/5 (20)
- Why We Die: The New Science of Aging and the Quest for ImmortalityD'EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityÉvaluation : 4.5 sur 5 étoiles4.5/5 (6)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryD'EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryÉvaluation : 4 sur 5 étoiles4/5 (46)