Académique Documents
Professionnel Documents
Culture Documents
Unsteady State Heat Transfer
Transféré par
Vivaan Sharma0 évaluation0% ont trouvé ce document utile (0 vote)
15 vues17 pagessfgsdfgsdfg
Copyright
© © All Rights Reserved
Formats disponibles
PDF ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentsfgsdfgsdfg
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
15 vues17 pagesUnsteady State Heat Transfer
Transféré par
Vivaan Sharmasfgsdfgsdfg
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 17
UNSTEADY STATE HEAT
TRANSFER
=I
| Contents|
Part-I
Aim
Theory
Description
Experimental Procedure
Specification
Formulee.
Observation & Calculation
Nomenclature
Part-II
‘Sample Calculation
UNSTEADY STATE HEAT TRANSFER UNIT
Department of Chemical Engineering, IT Guwah;
Au;
‘To study unsteady state heat transfer by the lumped capacitance
In many situations where steady state is not prevalent, analysis becomes much more
difficult. {1 is in these situations where unsteady heat flaw causes temperature and other
variables to change with time, However, in some unsteady situations, for which a certain
criterion is met, the use of the lumped capacitance theory greatly simplifies the analysis, The
criterion is based on the assumption that temperature gradients within a solid are negligible
compared to the temperature gradicnts between the solid and fluid, To verity whether this
assumption is trac oF not depends on the value of Biot number (Bi),
‘To understand the lumped heat capecity theory we consider a hot metal block that is
submerged in water. The basic concept of this theory is that the temperature within the solid
block is assumed 10 be spatially uniform at any instant throughout the unsteady heating
process, This implies that the temperature gradient within the solid is negligible compared to
the gradient across the solid-fluid interface.
Heat transfer process that is dependent on time is termed as transient heat ansfer or
unsteady state heat transfer. Such processes are analyzed by solving general heat conduction
equation using some simplified assumption like considering only one directional heat transfer
only.
oo
Bt
Assuming a system with negligible internal resistance i.e, @ system that has infinite
Lar
oo Ot
thermal conductivity (Ideal Case), This assumption is justified when external thermal
resistance between the surface of the system and surrounding medium is very large as
compared to the internal thermal resistance e.g, consider 2 metallic surface at temperature T,
(a1t=0) being suddenly placed in a bath of water where temperature is maintained at T(t >
0), then the energy balance for the metallic body over a small time interval, dt, i
pV Cratidt = ha(T. -7) creel
UN STEADY STATE HEAT TRANSFER UNIT Part Il
Department of Chemical Engineering, 1 Guwahati
Eq-I_ can be written as;
aT _ ha
ae
T,-T aC,v
Integration yields:
ha
in(TeT)=- t+
Applying boundary condition 1
t =0, T=7,
C= In (Te-T)
So, fiom Eqn. 3 we have att=0, T=T, 120
(Fig-1)
CV
OM GE —(4)
Thermal capacitance of the system is given by:
Go= Cepv
‘Thermal resistance is given by:
R= VhA
‘The following dimensionless numbers are defined as:
Biot number, Bj
Fouriernumber, F, =
)
‘Where, s = characteristic dimension
for a cylinder, the characteristic dimension is generally
taken ass=R,
Interms of these two dimensional groups,
Bq -4can be written as:
Tet games
T-7,
(When Bi <0.1, the bouy is ken to have negligible intemal thermal resistence)
UN STEADY STATE HEAT PRANSFER UNIT — Part I
Department of Chemical Engineering. 1'Y Guwahati
Instantaneous heat flow rate to solid cylinder is given by:
Q=pCrv (aTiaty
Oi Ae mC MMGy = HACE TI EM
and total heat gained during time ty is
Cop V (Te-T) [1-4 Py"
Crp Y (Te -T) -e 7") —. (7)
For B\>0.1,
‘The temperature distribution isa funetion of both time and location and transient temperature
charts are used for their solution.
Descrrrioy:
‘Water bath is used for heating the test picce and @ programmable temperature
controller controls heater input. In this experiment heat i allowed to flow through the
surfiee, The change in tempersior is indicated on digital temperature indicator and we note
itas itrises with respect to time.
Exrerimental PRoceure:
~The water bath is filled with water up to the desited level,
‘The desired bath temperature is set with the help of DTC (,)
The heater is switched on and mixer is started.
The bath is allowed to reach the desired temperature as set,
The initial temperature T; for the cylinder is noted.
The brass cylinder is dipped into the hot water bath and start noting the data for
“emperature Vs time ie, we take readings of temperature at the vente of the cylinder at
intervals of time equal to 10 seconds,
‘The above steps are repeated for another temperature of the water bath and corresponding
teadings noted,
+ The above steps are repeated for SS cylinder and comparative results drswn,
ADY STATE HEAT TRANSFER UNIT Part Il
Speciesrion:
Constant Temperature Water Bath: Stainless Steel Capacity-8 lit. (approx)
Stitter for Bath Slainless Steel Impeller with shaft coupled to a
FHP motor.
Heater Nichrome wire 1 K.W,
‘Test Cylinder Material: S.S./Brass,
Control Panel comprises of
Digital Temperature Controller 9.200%,
(For Water Bath)
Digital Temperature Indicator 0.200°C,
‘Temperature Sensors RTD PT-100 type
Formutae:
1. The energy balance for the metallic body over a small time interval, dt, is :
ETL gaapent
TT,
‘Thermal capacitance of the system is given by :
CG = Cppyv
‘Thermal resistance is given by:
Ro = Wha
Defining the following dimensionless numbers as:
Biotnumber, By = 2a (for a cylinder)
Fourier number, Fy = (for a eylinder)
a
Ry
In terms of these two dimensional groups,
‘The energy balance ean be writien as:-
(When Bi <0.1, the body has negligible internal thermal resistance)
6. Instantancous heat flow rate to solid eylinder is iven by:
UN STEADY STATE HEAT TRANSFER UNIT
Department of Chemical f ing, HT Guwahati
Q@ = pery (ariary
OF = nA GT. -T et,
and total heat gained during time ty is,
WA (Py -T)) et
Q Cop V (Ta -Ty [1- MPC)
Q Crp V (ToT) [1-7]
Onservarioy & CaLcunston:
‘The physical dimension of the est piece are recorded. (S.S or Brass)
R, = Radius of eylinder, m
L. = length of eylinder, m
Material of construction of cylinder ~
Physical Properties
P (density)
Ce (sp. heat)
(thermal conductivity)
(use Perry's Hand book)
kgm)
kg? K
Wim-C
« K! (p Cy) mise.
For brass: For SS:
° 8522 ke/ mé P = 7817 kgm?
Cp 385 Tkg °K c 461 Ske °K
k 110.7 Wim °K k 163 WimeK
We record injtial temperature of the cylinder (1j) and then immerse the test pieve in the
Sonstant temperature bath and position it properly. Starting the stopwatch, we record the
‘emperature of the test piece (at its center) with time.
‘The we tepeat these steps for cylinders of different material and verify whether Lumped
Parameter system model is applicable or not.
Time (t) sec
UN STEADY SUATE HEAT TRANSFER UNIT Part tt
Department of Chemical Engineering, 11 Guwahati
Caer estions
“The Gata in tabulated in the following form:
S.No. Time (t) T-T
sec T.-T,
a = x
eC,
t = Time in see,
1 Plot oe WS Fs ona semiciog eraph paper NN
t
2. Based on physical dimensions ofthe test love we may calculate the following:
Volume of the test piece (V) = TROL
Area of the test piece (A) = one,
Plot Et Ys time (0) on a semilog graph paper,
‘We then draw the best straight tine through the experimental points including the data only
up to the time when steady state just starts
‘The slope is measured from the graph,
aA
Slope = ox
i s ~ PRY stope (W/m)
Calculate Bi No,
Bo- MM
K
1FBi<0.1, the body has negligible interval thermal resistance, IF itis not, then we use
Heisler Chart to estimate Bi No. and then superimpose the plot (Tis) /(T. TT) Vs Fy on
the Heisler Chart and determine the ‘matching Bi. From Bi the heat transfer coefficient is
calculated,
UN
PADY STATE HEAT TRANSFER UNET— Part fh 6
Department of Chi
neering, IT Guwahatt
3. Using following equation, we calculatz Q; for each value of F, and plot Qi Vs Fo on a
ssemi-log graph
"
Q BAT oT) AB 3)
S.No.
NOMENCLATURE:
.
UN ST
= Density of material
= Radius of cylinder. m
= length of cylinder, m
= Bath Temperature
= Test Piece Temperature at any time, 1
= Initial Temperature of Test Piece
= Volume of the test piece
= Area of the test piece
= Biot number
= Fourier number
= Thermal capacitance
= ‘Thermal resistance
= specific heat
= thermal conductivity
DY STATE HEAT TRANSFER UNIT Part IL 7
Department of Chemical Engineering, IMT Guwahat,
Unsreapy State Heat TRANSFER UNIT
SAMPLE CALCULATIONS:
Davy
Sample material
Radius of Cylinder (Ro)
Length of Cylinder (L)
Density of Cylinder (p)
Specific Heat of Cylinder (Cr)
‘Thermal Conductivity of Cylinder (k)
‘Thermal Diffusivity of Cylinder (ct)
In
Bath Temperature (Ts)
Volume of the test piece (V)
Area of the test piece (A)
k
eC,
Where,
‘Volume of the test piece (V)
‘Area of the test piece (A)
Onsenvanioy Tanct
For Brass.
No. Time, (see)
1 10
2 20
3 30
4 40
3 50
6 60
UN STEADY STATE HEAT TRANSFER UNUT
ial Temperature of the Cylinder (1)
32
40
46
30.
3
35
Brass solid cylinder
0.01901m
014m
8522 kgim?
385 Ukg-K
110.7 Wim -K
0.000337 msec
24C
60°
0,0001589 m?
0.01672 m*
aRIL
2aR,L
Temp. ,T (°C)
Part I
SS solid cylinder
0.01905m
0.1406 m
7817, kvm’
461 kg -K
163° Wim-K
0.00000452 m*/see
23°C
60°C
0.0001603 m?
0.0168 m*
12
13
Cun
130
(Compute the data in the following form.
(Ta TW Te TO
S.No.
1
6
1
Time . 1 (see)
10
20
30
40
50
60
10
80
90.
100
110
120
130
TT
Plot
1.-T
0.78
0.56
0.39
0.28
0.19
O14
oad
0.08
0.08
0.06
0.06
0.06
0.06
Fo
187
3.23
5.60,
1.47
9.33
11.20
13.07
14.93
16.80
18.67
20.54
22.40
24.27
ys time (t) on a semi-log graph paper. (Graph -1)
2. Plot Tvs oe ona semi-log graph paper. (Graph -2)
From these grapes you will observe that the steady state starts just after time, 100 See. For the
determination of slope include only the data upto this time so that data can be fitted to an
‘equation of exponential form or if plotted on a semi log graph then include only the straight
line portion of the graph and find its slope.
Measure the slope from the graph (Graph -1)
UN STEADY STATE HEAT TRANSEER UNIT
Part I
Slope
hoo =
Bi. No =
a.
eCev
Cr
Department
= -0,0346
— PE” x Slope (W/m? K)=
fx Slope
bR,
2k
of Chemi
Engineerin
1079.47 Wim? K
0.0925. (which is < 0.1)
(Hence « lumped parameter system mode! is valid)
comparable From B
From graph? you can find the slo
From Graph -2
Slope =
2B, =
B=
R WIk=
ya 08
-2Bi
0.173
0.0865
Bi
6Sx110-7A2 _
0.0191
1007.42 watt /m* K
‘Average heat transfer co ~ efficient
po 1OT9AT481007-42
Yavg
Q@ =
5. No.
zi
143,448 watt m2 K
Using equation calculate Qi for each F,,
WA(Ta-T)e 7°
Fo a
187 345.4135
37 174.6647
5.60 86.55601
747 43,76865
9.33 21.68978
11.20 10.96784
13.07 6.211622
14.94 4.298073
16.80 2.334824
18.67 1.101937
10
UN STEADY STATE HEAT TRANSFER UNIT
i, No. calculate the heat transfer coefficient (h)
Part I
IT Guwahati
pe that is = 2 x Bi; and obtain Bi No. Both Bi, Nos. are
2 iu 20.54 0.780101
2 22.40 0.552262
B 24.27 0.390966
For SS
S.No. Time, t(sec) Temp. (°C)
1 10 2
2 20 33
3 30 29)
4 40 44
5 50 47
6 60) 50.
7 70 52
8 80 sf
9 90 54
10 100 55
uN 110 36
2 120 37
13 130 37
4 140 37
15 150 58
16 160 58
7 170 38
Carern anions
Compute the data in the following form,
1 10 0.89
2 20 073
3 30. 5
4 40 0.43
3S 50 0.35
UN STEADY STATE HEAT TRANSFER UNIT Part I
Depart
io. Time (see) (T2-T)(T2-T).
ent of Chemical En
Fo
0.498
0,996
1.495
1.993
2.491
ngineering. ITE Guwahati
Department of Chemical Engineering, IT Guwa
6 60 027 2.989
“4 0 022 3.487
8 80, 0.16 3.980
9 90 0.16 4.484
i) 100 0.13 4.982
ul 110 oll 5.480
2 120 0.08 5.978
13 130 0.08 6477
4 140 6.08 6.975 \
15 150 0.05 7A73
16 160 0.05 7971
7 170 0.05 8.409
vs time (1) on a semi-log graph paper. (Graph-1)
From these grapes you will observe that the sleady state starts just after time, 100 See,
For the determination of slope include only the data upto this time so that data can be fitted to
an equation of exponential form ox if plotted on a semi log graph thea inclade only the
straight line portion of the graph and find its slope:
Measure the slope from the graph (Graph-1)
ha
SI = = = 0.0243
ope per
h 7 = x Slope (W/m? K)= 835.548 Wim K
hi
Bi. No = “ 0.4877. (whichis < 0.1)
jot No. is > 0.1 then using Heisler Chart to estimate Bi No. Superimpose the plot =
ys Fy on the Heisler Chart and determine the matching Bi
B 0.4165
We see that both values of Bi No are comparable. Using the value of Bi, No. we calculate the
heat transfer coefficient (h)
B, 0.4166
Rb2k= By
UN STEADY STATE HEAT TRANSFER UNIT ~ Part IL 2
al Engineering. HT Guova
Depariment of Chem
0.4166x16.3x2
0.01908
ho = 712.92 Win? K
Average heat transfer co ~ efficient
835.548 471.92
have 7
Dove 774.235 Wii? K
Using equation calculate Q; for each Fy °
Q = baa Tye
S.No. FE, Qa \
1 0.498 264.08
2 0.996 132.88 ;
3 1.495 6357
4 1.993 2979
5 2.491 1489
6 2.989 7.04
a 3.487 347
8 3.986 1.60
9 4.484 0.98
10 4.982 050
ul 5.480 025
2 5.978 oul
3 6477 0.07
14 6975 0.04
18 TAT3 0.02
16 7971 0.01
7 8.469 0.01
UN STEADY STATE HEAT TRANSFER UNIT = Part IL B
—
Department of Chemical Engineering, 11T Guwahati
| GRAPH 1 |
ist : "
' | 0 60 80 100
2 | = 1.165400
| §
S 0.1
2 Toe
|i
. | 2 aRass
61 4 =
‘ L Timet
| GRAPH 2
—o—BRASS
sos4eor 2 |
Dimensionless No.
°
UN STEADY STATE HEAT TRANSFER UNUT~ Part I 4
Department of Chemical Engineering, IF Guysabati
GRAPH 3
—+- BRASS
$8
|
3
30
\
0.001
Fo
UN STEADY STATE HEAT TRANSFER UNIT Part tt 15
Vous aimerez peut-être aussi
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Lect - 16 Thermal RadiationDocument38 pagesLect - 16 Thermal RadiationVivaan SharmaPas encore d'évaluation
- Lect - 21 Evoporation Lecture 1 of 3Document56 pagesLect - 21 Evoporation Lecture 1 of 3Vivaan Sharma100% (1)
- Lect - 20 Heat Exchanger Lecture 4 of 4Document18 pagesLect - 20 Heat Exchanger Lecture 4 of 4Vivaan SharmaPas encore d'évaluation
- Lect - 15 Radiation BasicsDocument16 pagesLect - 15 Radiation BasicsVivaan SharmaPas encore d'évaluation
- Tutorial Slides - Internal Forced Convection & Natural ConvectionDocument31 pagesTutorial Slides - Internal Forced Convection & Natural ConvectionVivaan Sharma75% (4)
- Tutorial Slides (Only Questions) - Internal Forced Convection & Natural ConvectionDocument10 pagesTutorial Slides (Only Questions) - Internal Forced Convection & Natural ConvectionVivaan SharmaPas encore d'évaluation
- Lect - 10 External Forced ConvectionDocument45 pagesLect - 10 External Forced ConvectionVivaan SharmaPas encore d'évaluation
- Lect - 3 Basic Equations of One-Dimensional, Two-Dimensional and Three-Dimensional ConductionDocument17 pagesLect - 3 Basic Equations of One-Dimensional, Two-Dimensional and Three-Dimensional ConductionVivaan SharmaPas encore d'évaluation
- Lect - 12 Natural Convection, Empirical Equations For Free and Forced ConvectionDocument18 pagesLect - 12 Natural Convection, Empirical Equations For Free and Forced ConvectionVivaan SharmaPas encore d'évaluation
- Lect - 7 Examples Transient Conduction (Only Questions)Document15 pagesLect - 7 Examples Transient Conduction (Only Questions)Vivaan SharmaPas encore d'évaluation
- Lect - 3 Basic Equations of One-Dimensional, Two-Dimensional and Three-Dimensional ConductionDocument17 pagesLect - 3 Basic Equations of One-Dimensional, Two-Dimensional and Three-Dimensional ConductionVivaan SharmaPas encore d'évaluation
- Lect - 11 Internal Forced ConvectionDocument44 pagesLect - 11 Internal Forced ConvectionVivaan Sharma100% (2)
- Lect - 5 Examples Steady Conduction in Slabs, Cylinders and Spheres - Critical Thickness of Insulation With SolutionDocument22 pagesLect - 5 Examples Steady Conduction in Slabs, Cylinders and Spheres - Critical Thickness of Insulation With SolutionVivaan Sharma33% (3)
- Lect - 5 Examples Steady Conduction in Slabs, Cylinders and Spheres Critical Thickness of InsulationDocument11 pagesLect - 5 Examples Steady Conduction in Slabs, Cylinders and Spheres Critical Thickness of InsulationVivaan SharmaPas encore d'évaluation
- Heat Transfer College SlidesDocument2 pagesHeat Transfer College SlidesVivaan Sharma100% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Pressure Loss Calculation SheetDocument6 pagesPressure Loss Calculation Sheetchuyen.reePas encore d'évaluation
- Lecture 3 - Laplace Transform-Part1Document12 pagesLecture 3 - Laplace Transform-Part1John AlvarezPas encore d'évaluation
- Lab EmissionSpectraDocument2 pagesLab EmissionSpectraKaren ClementePas encore d'évaluation
- Fluid Mechanics 2019 2020Document67 pagesFluid Mechanics 2019 2020basic langPas encore d'évaluation
- Chapter 7 - F2 - A - Differentiation: Sub-Topics PageDocument1 pageChapter 7 - F2 - A - Differentiation: Sub-Topics PageMuhammad Aminnur Hasmin B. HasminPas encore d'évaluation
- Aits 1718 FT Ii Jeem PDFDocument24 pagesAits 1718 FT Ii Jeem PDFsoumengoswami10Pas encore d'évaluation
- Moving Range Chart LectureDocument1 pageMoving Range Chart LectureJann Paulo MendozaPas encore d'évaluation
- Development of X-Ray-Induced Afterglow Characterization SystemDocument3 pagesDevelopment of X-Ray-Induced Afterglow Characterization SystemneerkePas encore d'évaluation
- Conte R Fort WallDocument30 pagesConte R Fort Wallmirko huaranccaPas encore d'évaluation
- ASTM C50-00 Limestone SamplingDocument8 pagesASTM C50-00 Limestone SamplingDiana NurjanahPas encore d'évaluation
- Brochure Liquid Cylinders DOT 4LDocument8 pagesBrochure Liquid Cylinders DOT 4LShaheer TariqPas encore d'évaluation
- Aluline Pro 38Document2 pagesAluline Pro 38baihakiPas encore d'évaluation
- PGL-Lesson 94-97Document17 pagesPGL-Lesson 94-97Jomabelle UbaldoPas encore d'évaluation
- TriennaleDocument129 pagesTriennaleJesus UrrestiPas encore d'évaluation
- Unit-2 BookDocument64 pagesUnit-2 BookPuzzle SolverPas encore d'évaluation
- Paper2 Provisional Answer KeysDocument31 pagesPaper2 Provisional Answer KeysRahul RaviPas encore d'évaluation
- Hunting The Elements Video QuestionsDocument4 pagesHunting The Elements Video Questionsapi-26400457150% (4)
- Activity 7 - Momentum Impulse and Collision 1Document7 pagesActivity 7 - Momentum Impulse and Collision 1frigstacy69Pas encore d'évaluation
- Chapter 06 - Load Path and Load Transfer in Structural ElementsDocument15 pagesChapter 06 - Load Path and Load Transfer in Structural ElementsMohamed AbdPas encore d'évaluation
- Tutorial 2 Superposition and Reflection of Pulses: Instructor: Kazumi TolichDocument13 pagesTutorial 2 Superposition and Reflection of Pulses: Instructor: Kazumi TolichHydeki RyugaPas encore d'évaluation
- ZN5050ADocument44 pagesZN5050ATHiz OCtavvPas encore d'évaluation
- NCSE Math 2018 SolutionDocument15 pagesNCSE Math 2018 SolutionDexter B50% (2)
- Kinegram Measurements by Sezad and Shuvo - RCDocument15 pagesKinegram Measurements by Sezad and Shuvo - RCSilence is BetterPas encore d'évaluation
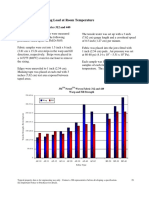
- Warp and Fill Breaking Load at Room Temperature: 3M Nextel Woven Fabrics 312 and 440Document1 pageWarp and Fill Breaking Load at Room Temperature: 3M Nextel Woven Fabrics 312 and 440ninatenaPas encore d'évaluation
- Electronics Engineering, 2nd Edition (2022)Document469 pagesElectronics Engineering, 2nd Edition (2022)Darling Ortiz Huaroto100% (2)
- Extra Practice Week 01Document6 pagesExtra Practice Week 01devikaPas encore d'évaluation
- Saddle AnalysisDocument17 pagesSaddle AnalysiscutefrenzyPas encore d'évaluation
- Micheslon'S Interferometer: ConstructionDocument5 pagesMicheslon'S Interferometer: Construction21MS0073KritarthaPas encore d'évaluation
- Myp4 Ext Summative C DDocument2 pagesMyp4 Ext Summative C DBrian KamuchisaPas encore d'évaluation
- Propagation and Attenuation Characteristics of Various Ground VibrationsDocument12 pagesPropagation and Attenuation Characteristics of Various Ground VibrationsTony Christian Canahua ChoquezaPas encore d'évaluation