Académique Documents
Professionnel Documents
Culture Documents
Closon
Transféré par
rahi6055Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Closon
Transféré par
rahi6055Droits d'auteur :
Formats disponibles
Closon
Betamethasone dipropionate BP 0.5mg + Clotrimazole BP 10mg
COMPOSITION
Closon cream : Each gram cream contains 0.50 mg Betamethasone as Dipropionate and 10 mg
clotrimazole.
PHARMACOLOGY
Clotrimazole is a broad-spectrum antifungal agent used for the treatment of superficial infections
caused by species of pathogenic dermatophytes, yeasts and Malassezia furfur. The mechanism of
action involves inhibition of the synthesis of ergosterol, a major sterol in the fungal cell
membrane. This leads to instability of the cell membrane and eventual death of the fungus.
Betamethasone dipropionate is a corticosteroid with anti-inflammatory, antipruritic, and
vasoconstrictive properties. But the exact mechanism of action of corticosteroids is not clearly
known.
INDICATION
Closoncream is indicated for the topical treatment of inflammatory dermal infections like tinea
pedis, tinea cruris, tinea corporis, etc.
DOSAGE AND ADMINISTRATION
Sufficient cream should be applied onto the affected and surrounding skin areas twice a day, in
the morning and evening, for 2 weeks in tinea cruris and tinea corporis and for 4 weeks in tinea
pedis. The use of cream for longer than four weeks is not recommended.
CONTRAINDICATION AND PRECAUTION
The preparation is contraindicated to those patients who are sensitive to any of its components or
to other corticosteroids or to imidazoles. If irritation or sensitization develops with the use of the
cream, treatment should be discontinued and appropriate therapy instituted.
The cream is contraindicated in facial rosacea, acne vulgaris, perioral dermatits, perianal and
genital pruritus, napkin eruptions and bacterial or viral infections. Systemic absorption of topical
corticosteroides can produce reversible hypothalmic-pituitary-adrenal (HPA) axis suppression. If
HPA axis suppression is noted, an attempt should be made to withdraw the drug or to reduce the
frequency of application. Pediatric patients may be more susceptible to systemic toxicity from
equivalent doses due to their large skin surface to body mass ratios.
SIDE EFFECT
Adverse reactions reported for the preparation in clinical trials were paresthesia in 1.9% of
patients, rash, edema and secondary infection, each in less than 1% of patients. Other adverse
reactions reported with the preparation were burning and dry skin in 1.6% of patients and
stinging in less than 1% of patients.
USE IN PREGNANCY AND LACTATION
There is inadequate evidence of safety in pregnancy. Clotrimazole has no teratogenic effect in
animals, but is foetotoxic at high oral doses. Topical administration of corticosteroids to pregnant
animals can cause abnormalities of fetal development. Hence the cream should only be used in
pregnancy, if the benefit justifies the potential risk to the fetus and such use should not be
extensive, i.e. in large amounts or for long periods. It is not known whether the components of
the preparation are excreted in human milk and therefore caution should be exercised when
treating nursing mothers.
USE IN CHILDREN
The safety and effectiveness of the preparation has not been established in children below the age
of 12 years.
OVERDOSE
Acute overdose with the cream is unlikely and would not be expected to lead to a life-threatening
situation. The cream should not be used for longer than the prescribed time period.
STORAGE CONDITION
Store at or below 30C. Do not freeze.
HOW SUPPLIED
Closon cream: Each pack has a tube containing 10 gm of the cream.
Manufactured by:
MEDICON Pharmaceuticals Ltd.
Mirpur, Dhaka, Bangladesh
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- ABS Rules For Cable SizingDocument2 pagesABS Rules For Cable SizingMohammed JassimPas encore d'évaluation
- Liquid SizingDocument38 pagesLiquid SizingChetan ChuriPas encore d'évaluation
- Daily Lesson Log 4Document5 pagesDaily Lesson Log 4Diane Marr Nicolas Dencio100% (2)
- IncentiveDocument2 pagesIncentiverahi6055Pas encore d'évaluation
- Welcome To The PresentationDocument23 pagesWelcome To The Presentationrahi6055Pas encore d'évaluation
- TrainingDocument2 pagesTrainingrahi6055Pas encore d'évaluation
- Mid Term QuestionsDocument1 pageMid Term Questionsrahi6055Pas encore d'évaluation
- New Student International BrochureDocument2 pagesNew Student International Brochurerahi6055Pas encore d'évaluation
- Cefmix Lit FinalDocument2 pagesCefmix Lit Finalrahi6055Pas encore d'évaluation
- Bsmmu PG Hospital Investigations FeesDocument5 pagesBsmmu PG Hospital Investigations Feesrahi6055Pas encore d'évaluation
- Product Name 03 12 12Document1 pageProduct Name 03 12 12rahi6055Pas encore d'évaluation
- 3 MLDocument1 page3 MLrahi6055Pas encore d'évaluation
- Monicopharma Limited AM, Evaluation Test-2011: Time: 40 Minutes Marks: 40Document3 pagesMonicopharma Limited AM, Evaluation Test-2011: Time: 40 Minutes Marks: 40rahi6055Pas encore d'évaluation
- Bogra Depot Office: 654, Hafizar Rahmanroad, Jaleswaritola, BograDocument1 pageBogra Depot Office: 654, Hafizar Rahmanroad, Jaleswaritola, Bograrahi6055Pas encore d'évaluation
- Azi ThroDocument2 pagesAzi Throrahi6055Pas encore d'évaluation
- Doctor Visit SccheduleDocument1 pageDoctor Visit Scchedulerahi6055Pas encore d'évaluation
- Letter 56Document1 pageLetter 56rahi6055Pas encore d'évaluation
- Fac 451Document1 pageFac 451rahi6055Pas encore d'évaluation
- Implications of SetroDocument2 pagesImplications of Setrorahi6055Pas encore d'évaluation
- ICDDRBDocument1 pageICDDRBrahi6055Pas encore d'évaluation
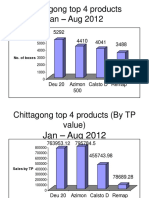
- Chittagong Top 4 Products Jan - Aug 2012: No. of BoxesDocument4 pagesChittagong Top 4 Products Jan - Aug 2012: No. of Boxesrahi6055Pas encore d'évaluation
- Butter Fly Needle Price PropsalDocument1 pageButter Fly Needle Price Propsalrahi6055Pas encore d'évaluation
- Ana Sales FinalDocument1 pageAna Sales Finalrahi6055Pas encore d'évaluation
- Bac and CefurDocument1 pageBac and Cefurrahi6055Pas encore d'évaluation
- Comilla Top 4 ProductsDocument4 pagesComilla Top 4 Productsrahi6055Pas encore d'évaluation
- 23 July 2011Document1 page23 July 2011rahi6055Pas encore d'évaluation
- ImDocument23 pagesImrahi6055Pas encore d'évaluation
- New Microsoft Office Word DocumentDocument1 pageNew Microsoft Office Word Documentrahi6055Pas encore d'évaluation
- Better Health For Better TomorrowDocument1 pageBetter Health For Better Tomorrowrahi6055Pas encore d'évaluation
- BonusDocument2 pagesBonusrahi6055Pas encore d'évaluation
- Training Manual On Kidivit 200mlDocument11 pagesTraining Manual On Kidivit 200mlrahi6055Pas encore d'évaluation
- Employment (Academic) : Application ForDocument7 pagesEmployment (Academic) : Application Forrahi6055Pas encore d'évaluation
- Formulation of TabletDocument1 pageFormulation of Tabletrahi6055Pas encore d'évaluation
- 18.1 Outline The Mechanisms Which: Chemotherapy Target Dividing CellsDocument8 pages18.1 Outline The Mechanisms Which: Chemotherapy Target Dividing CellsSenthereng MoaisiPas encore d'évaluation
- The Foods of Italy BookDocument92 pagesThe Foods of Italy BookmanupopPas encore d'évaluation
- Easy Guide For Fujitsu T901 LaptopDocument141 pagesEasy Guide For Fujitsu T901 LaptopElainePas encore d'évaluation
- Vein Type DepositDocument7 pagesVein Type DepositHarisArmadiPas encore d'évaluation
- 96-09302-00-01 Reva Technical Manual Inogen One G5Document18 pages96-09302-00-01 Reva Technical Manual Inogen One G5Paula Andrea MarulandaPas encore d'évaluation
- HISTOPATHDocument38 pagesHISTOPATHDennis Louis Montepio BrazaPas encore d'évaluation
- IJHIM 6 - Nur Husnina (36 SD 42)Document7 pagesIJHIM 6 - Nur Husnina (36 SD 42)RSU Sayang BundaPas encore d'évaluation
- 10 de Thi Tieng Anh Hướng Dẫn Giải Chi TiếtDocument145 pages10 de Thi Tieng Anh Hướng Dẫn Giải Chi TiếtVuong DiepPas encore d'évaluation
- Testo-Flue Gas in Industry 3-27-2008Document149 pagesTesto-Flue Gas in Industry 3-27-2008leruaitesPas encore d'évaluation
- Nasua NasuaDocument9 pagesNasua NasuaJetsabellGutiérrezPas encore d'évaluation
- Barge 180Ft Deck Load Capacity & Strength-Rev1Document52 pagesBarge 180Ft Deck Load Capacity & Strength-Rev1Wahyu Codyr86% (7)
- Pre Post Tests For HPPDocument3 pagesPre Post Tests For HPPapi-434982019Pas encore d'évaluation
- Measuring Salinity in Crude Oils Evaluation of MetDocument9 pagesMeasuring Salinity in Crude Oils Evaluation of Metarmando fuentesPas encore d'évaluation
- Scan&SolveDocument24 pagesScan&SolveAtul ChauhanPas encore d'évaluation
- Role of Packaging in Sales of FMCG Products and Its TrendsDocument57 pagesRole of Packaging in Sales of FMCG Products and Its TrendsSaurabh0% (1)
- ASD Fan CalculatorsDocument14 pagesASD Fan CalculatorslubricacionPas encore d'évaluation
- 2004 - Quality of Life in Romania I MargineanDocument206 pages2004 - Quality of Life in Romania I Margineandale_titiPas encore d'évaluation
- Antenatally Diagnosed Kidney AnomaliesDocument17 pagesAntenatally Diagnosed Kidney AnomalieslauraPas encore d'évaluation
- CEBUANO ERNESTO CODINA (Astonaut Hardware Designer)Document1 pageCEBUANO ERNESTO CODINA (Astonaut Hardware Designer)Dessirea FurigayPas encore d'évaluation
- Taiwan API Manufacturer ListDocument4 pagesTaiwan API Manufacturer Listkalyani dynamicsPas encore d'évaluation
- Buku Murid Bahasa Inggris - Student's Book My Next Word For Elementary School Unit 10 - Fase BDocument8 pagesBuku Murid Bahasa Inggris - Student's Book My Next Word For Elementary School Unit 10 - Fase BKeni KenizaPas encore d'évaluation
- Jason Read, "Real Subsumption"Document32 pagesJason Read, "Real Subsumption"Aren Z. AizuraPas encore d'évaluation
- Architect As An Environmental PlannerDocument14 pagesArchitect As An Environmental PlannerJames Adrian MoralPas encore d'évaluation
- Draf Model LC 2024 Non TransferableDocument3 pagesDraf Model LC 2024 Non TransferablepresidenciaPas encore d'évaluation
- HLN Applications enDocument27 pagesHLN Applications enClint TcPas encore d'évaluation
- Fact Sheeton Canola OilDocument15 pagesFact Sheeton Canola OilMonika ThadeaPas encore d'évaluation
- Basic Pancakes Recipe - Martha StewartDocument37 pagesBasic Pancakes Recipe - Martha Stewartkrishna kumarPas encore d'évaluation