Académique Documents
Professionnel Documents
Culture Documents
Astm Acido Sulfurico
Transféré par
Armando PerezCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Astm Acido Sulfurico
Transféré par
Armando PerezDroits d'auteur :
Formats disponibles
~-
A S T M E223 93
0759530 0533783 T73
This is a 3 page(s) preview of total 9
41)
Designation: E 223 - 93
AMERICAN SOCIEPI M R TESTING AND MATERIALS
1916 Race St. Philadelphia. Pa 19103
Reprinted from the Annual B w k of ASTM Standards. CopyrgMASTM
If not listed in the current combined inden will appear in the nexi edition
Standard Test Methods for
Analysis of Sulfuric Acid'
This standard is issued under the fixed designation E 223;the number immediately following the designation indicates the year of
ori~naiadoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last rrapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense. Consult the DoD Index of Spect)cations and
Standardsfor the spec,$c year of issue which has been adopted by the Depanment of Defme.
1. Scope
I. 1 These test methods cover the analysis of sulfuric acid.
1.2 The analytical procedures appear in the following
'
order:
Total Acidity.. .......................................
Baum Gravity .......................................
Nonvolatile Matter.. ..................................
Iron . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Sulfur Dioxide .......................................
Arsenic .............................................
Sections
8 to 16
17 to 26
27 to 33
34 to 43
44 to 5 1
52 to 61
1.3 This standard does not purport to address all of the
safety problems, any, associated with its use. It is the
responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific hazards
statements are given in Section 5 .
2. Referenced Documents
2.1 ASTM Standards:
D 1193 Specification for Reagent Wate9
E 1 Specification for ASTM Thermometers3
E 60 Practice for Photometric and Spectrophotometric
Methods for Chemical Analysis of Metals4
E 180 Practice for Determining the Precision of ASTM
Methods for Analysis and Testing of Industrial
Chemicals'
E 200 Practice for Preparation, Standardization,and Storage of Standard and Reagent Solutions for Chemical
Analysis5
--`,,`,,,`,,,,,,,,``,```,,``,```-`-`,,`,,`,`,,`---
3. Significance and Use
3.1 These test methods provide for the classification of
various grades of sulfuric acid and for the determination of
various impurities. Acid strength and impurity levels are
important factors in many uses of sulfuric acid.
4. Purity of Reagents
4.1 Purity ofReagents-Reagent
grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended
1 These test methods are under the jurisdiction of ASTM Committee E-15 on
Industrial Chemicals and are the direct responsibility of Subcommittee E15.51 on
Acids.
Current edition approved June 15, 1993. Published August 1993. Onginally
published as E 223 - 65 T.Last previous edition E 223 - 88.
2 Annual Book ofASTM Siandards, Vol 1 1.01.
Annual Book ofASTM Standards, Vol 14.03.
Annual Book of ASTM Standards, Vol 03.05.
Annual Book of ASTM Standards, Vol 15.05.
www.TeraStandard.com
that all reagents shall conform to the specifications of the
Committee on Analytical Reagents of the American Chemical Society, where such specifications are
Other
grades may be used, provided it is first ascertained that the
reagent is of sufnciently high purity to permit its use without
lessening the accuracy of the determination.
4.2 Purity of Water-Unless otherwise indicated, references to water shall be understood to mean Type II or Type
III reagent water conforming to Specification D 1193.
5. Hazards
5.1 Sulfuric acid is a strong corrosive acid and is dangerous if improperly handed.Avoid any skin or eye contact.
5.2 Clean up all spills immediately by covering the spill
with vermiculite or some other inert absorbent material and
sweeping into a pan. Dispose of the absorbent by flooding
with water and discarding in a suitable container. Flush the
area with water.
6. Photometers and Photometric Practice
6.1 Photometers and photometric practice used in these
methods shal conform to Practice E 60.
7. Sampling
7.1 Sampling of sulfuric acid is not within the scope of
these methods.
7.2 The sample to be analyzed shall be considered to be
that sample in a single bottle submitted to the analytkal
laboratory.
7.3 The size of the sample shall be sufficient to perform ail
analyses without the reuse of any portion of the sample.
TOTAL ACIDITY
8. Scope
8. I This test method covers the determination of the total
acidity of 75 to 99 % suifiuic acid. Two methods are given
for weighing the sample, namely, the Dely tube and the
snake tube methods.
9. Summary of Test Method
9.1 A weighed sample of acid is diluted in water and
titrated with standardized 0.5 N sodium hydroxide solution,
6 Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC.For suggestions on the testing of reagents not
listed by the Amencan Chemical Society, see Atular Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and Nriiional Formulary, US. Phamaccutical Convention, Inc. (USPC),
Rockville, MD.
ASTM E223 93
0759530 0533982 908
This is a 3 page(s) preview of total 9
--`,,`,,,`,,,,,,,,``,```,,``,```-`-`,,`,,`,`,,`---
E223
FIG. 2 SnakeTube
E
E
the bend of the short arm. Attach the short arm to an
elevated water reservoir by means of a rubber tube closed
near the lower end with a pinch clamp. Insert the long arm of
the Dely tube into 400-mL glass beaker containing approximately 1O0 mL of water. Open the pinch clamp and flush the
sample into the beaker. Continue the flow of water until all
acid is washed from the Dely Tube (Notes 2 and 3). Wash the
long end of the Dely tube, collecting the washings in the
beaker. Add 3 to 5 drops of phenolphthalein indicator
solution. Record the temperature of the 0.5 N NaOH
solution, and then titrate the sample to a pink end point.
Record the titration to the nearest 0.02 mL.
FIG. 1 Dely Tube
using phenolphthalein as the indicator.
NOTE 1-The Dely tube can be marked at points equivalent to
weights given in Table 1.
NOTE2-The presence of acid in the Dely tube may be detected by
coloring the water in the reservou with phenolphthalein indicator and
the minimiurn amount of dilute NaOH solution that will produce a
slight pink. The water flowing through the tube is dicolorired as long as
acid is present, and the appearance of a pink color indicates the absence
of acid.
NOTE3-The acid and water are separated by a bubble of air.
10. Interferences
10.1 Acids other than sulfuric and compounds that consume sodium hydroxide will affect the accuracy of this test
method.
11. Apparatus
11.1 Dely Tube (Fig. I) or Snake Tube (Fig. 2
).'
11.2 Burel, 100-mL, Class A, bulb-type.
13.2 Snake Tube Method-Invert the sample bottle several times. (Hold the stopper in tight). Insert the capillary end
of a dry, weighed snake tube and withdraw by suction a
convenient size sample depending upon the acid strength as
given in Table 1. Invert the tube so that the double bend is in
a horizontal position. Wipe the acid from the capillary with
disposable tissue severai layers thick. Discard the tissue
immediately to avoid burning the fingers. Reweigh to the
nearest 0.0001 g and record the weight of the sample.
Submerge the capillary of the tube in approximately 100 mL
of water contained in the 400-mL beaker. Force the weighed
sample from the tube by a stream of water from a wash bottie
by placing the delivery tip in the exposed end of the snake
tube (Note 4). Wash the tube with 50 to 70 mL of water.
Remove the tube and wash the outside free of acid. Swirl the
12. Reagents
12.1 Phenolphthalein indicator Solution ( 10 g/L)-Dissolve 1 g of phenolphthalein in 100 mL of ethanol (95 %),
methanol, or isopr~panol.~
12.2 Sodium Hydroxide, Standard Solution (0.5 N)-See
Practice E 200.
13. Procedure
13.1 Dely Tube Method-Invert the sample bottle several
times. (Hold the stopper in tight.) Insert the long arm of a
dry, weighed Dely tube and withdraw by suction a convenient size sample depending upon the acid strength as given
in Table 1 (Note 1). Invert the Dely tube and wipe the acid
from the long arm with disposable tissue severai layers thick.
Discard the tissue immediately to avoid burning the fingers.
Reweigh to the nearest O.OOO1 g and record the weight of the
sample. Incline the tube so that the acid runs back nearly to
TABLE 1 Sample Size for Tobt Acidity
HzSO., %
98
94
90
85
80
'Suitable Dely and snake tubes are available from Coming Glass Works,
Coming, NY.
* This reagent is also described in Practice E 200.
77
75
2
www.TeraStandard.com
*e,
1.9 to 2.2
2.0 to 2.3
2.1 to 2.4
2.2 io 2.6
2.3 io 2.7
2.4 io 2.8
2.5 to 2.9
AST!
E223 9 3
= 0759510 0533983 8Li4
This is a 3 page(s) preview of total 9
E223
performed duplicate determinations and repeated one day later, for a
total of I20 determinations. Practice E I80 - 90 was used in developing
these precision estimates.
contents of the beaker gently while washing. Accumulate all
washings in the beaker and add 3 to 5 drops of
phenolphthalein indicator solution. Record the temperature
of the 0.5 N NaOH solution, and then titrate the sample to a
pink end point. Record the titration to the nearest 0.02 mL.
NOTE4-Do not introduce the water into the snake tube too rapidly,
16.2 Since there is no accepted reference material for
determining the bias for measuring the total acidity of
sulfuric acid, the bias of this test method has not been
determined.
as this will cause spattering.
BAUM$ GRAVITY
14. Calculation
17. Scope
17.1 This test method covers the determination of the
Baum gravity of concentrated sulfuric acid by means of a
giass hydrometer in the range from 57 to 66.2' Baum. The
Baum gravity is determined at 15.5'C (60'F). This method
is not applicable to readings above 66.2 Baum gravity units.
14. I If necessary, correct the buret reading for calibration
errors and record the volume of titrant as V and the
temperature as t.
14.2 Correct the normality of the sodium hydroxide
standard solution for any difference in temperature between
time of standardization and time of use according to the
following equation:
= N,
18. Summary of Test Method
18.1 A sample of sulfuric acid is placed in a hydrometer
cylinder and when the temperature is constant, the Baum
gravity is read from the giass hydrometer.
+ 0.00014 (S - i )
where:
N = normality of NaOH solution at temperature t during
u=,
N, = normality of NaOH solution at temperature s during
standardization,
s = temperature of NaOH solution during standardization, and
t = temperature of NaOH solution during analysis.
14.3 Calculate the total acidity as percentage of sulfuric
acid as foliows:
(VNx 0.04904) ,oo
sulfuric acid, wt. % =
19. Significance and Use
19.1 The Baum gravity is used to classify various grades
of sulfuric acid. This test method is not applicable for
accurate determinations of the concentration of sulfuric acid.
20. Definition
20.1 Baum Gruvit-a
unit of density based on specific
gravity and defined by the foliowing equation:
Baum gravity = 145
where:
V =corrected millilitre of NaOH solution required for
titration of the sample,
N = normality of the NaOH solution, and
W = grams of sample used.
21. Apparatus
2 1.1 Hydrometer: streamline or torpedo design, precision
grade for liquids heavier than water in ranges fiom 57 to 62'
B and 63 to 67' B. The total length shaii be approximately
12 in. (305 mm) divided to 0.05' B over a 6-in. (152-mm)
(approximate) scale and standardized at 15.5/15.5'C (60/
60'F) with a tolerance of 0.05' B throughout. The modulus
is
15. Report
15.1 Report the percentage of sulfuric acid to the nearest
0.01 %. Duplicate runs that agree within O. 19 % absolute are
acceptable for averaging (95 % probability). This limit is
based on an estimated standard deviation of 0.0671 %
absolute at 60 DF (see Note 5).
B = 145 - [145/sp gr] at 15.5/15.5'C (60/60'F)
Each of the hydrometers shall show on the scale the
modulus.
2 1.2 Thermometer, having a range from -2 to +8VC (30
to 180'F) and conforming to the requirements for Thermometer 15C (1%) as prescribed in Specification E 1.
21.3 Cylinder, Hydrometer, giass, with or without lip,
diameter 38 to 40 mm, height 325 to 375 mm.
16. Precision and Bias
16.1 The following criteria should be used for judging the
acceptibility of results (Note 5):
16.1.1 Repeatability (Single Analyst)-The standard deviation of results (each the average of duplicates), obtained by
the same analyst on different days, has been estimated to be
O. 103 % absolute at 29 DF. The 95 % limit for the difference
between two such averages is 0.29 96 absolute.
16.1.2 Reproducibility (Multilaboratoryl-The standard
deviation of results (each the average of duplicates), obtained
by analysts in different laboratories, has been estimated to be
O. 142 9% absolute at 9 DF. The 95 % limit for the difference
between two such averages is 0.40 % absolute.
NOTE5-The above precision estimates are based on an
22. Temperature of Test
22.1 Baum gravity shaii be determined at 15.5 f 0.3'C
(60 i OS'F).
23. Procedure
23.1 Rinse a clean hydrometer cylinder with the sample to
be tested, add the sample, and adjust the temperature to 15.5
f 0.3% (60 f 0.5'F). Place the cylinder in a vertical position
interlaboratory study on three samples, containing approximately 80,
90, and 95 % sulfuric acid. One analyst in each of ten laboratories
Suitable hydrometers arc avaiiable from Walter H. Kesrlcr, Inc.. 160 Hicks
h,Westbury, L.I.. NY 11590.
3
www.TeraStandard.com
- [145/sp gr] at 15.5/15.5%(60/60'F)
--`,,`,,,`,,,,,,,,``,```,,``,```-`-`,,`,,`,`,,`---
Vous aimerez peut-être aussi
- D888 12 Standard Test Methods For Dissolved Oxygen in WaterDocument12 pagesD888 12 Standard Test Methods For Dissolved Oxygen in WaterAnonymous FZNn6rBPas encore d'évaluation
- MT 158 Determination of Mercury On Treated Seeds: Miscellaneous Techniques and ImpuritiesDocument18 pagesMT 158 Determination of Mercury On Treated Seeds: Miscellaneous Techniques and ImpuritiesFelipe Navarrete100% (1)
- CIPAC - MT 171 - 1 Dustiness of Granular Products 2015Document7 pagesCIPAC - MT 171 - 1 Dustiness of Granular Products 2015Antonio Viso AcostaPas encore d'évaluation
- AstmDocument14 pagesAstmAsmaningrum SetyawatiPas encore d'évaluation
- ASTM G31-1972 Standard Practice For Lab Immersion CorrosionDocument8 pagesASTM G31-1972 Standard Practice For Lab Immersion CorrosionPlinio Lavinas100% (1)
- UV Spectrum Analysis of EBDC CompoundsDocument29 pagesUV Spectrum Analysis of EBDC CompoundsFelipe NavarretePas encore d'évaluation
- MT 59 Sieve Analysis: Miscellaneous Techniques and ImpuritiesDocument13 pagesMT 59 Sieve Analysis: Miscellaneous Techniques and ImpuritiesFelipe Navarrete100% (1)
- MP-627 Y 628 HerzogDocument4 pagesMP-627 Y 628 HerzogNavi Morales0% (1)
- MT 53 Wettability: Miscellaneous Techniques and ImpuritiesDocument7 pagesMT 53 Wettability: Miscellaneous Techniques and ImpuritiesFelipe Navarrete100% (1)
- Saponification Value of Drying Oils, Fatty Acids, and Polymerized Fatty AcidsDocument2 pagesSaponification Value of Drying Oils, Fatty Acids, and Polymerized Fatty Acidsjunigu abdulPas encore d'évaluation
- Astm - D2196.12408Document5 pagesAstm - D2196.12408Bryan de BarrosPas encore d'évaluation
- Acid Value and Amine Value of Fatty Quaternary Ammonium ChloridesDocument3 pagesAcid Value and Amine Value of Fatty Quaternary Ammonium ChloridesShaker Qaidi100% (1)
- ASTM D2281-10 Standard Test Method For Evaluation of Wetting Agents by Skein TestDocument3 pagesASTM D2281-10 Standard Test Method For Evaluation of Wetting Agents by Skein TestDerek Vaughn100% (1)
- Redicote E-7000: Versatile Emulsifier: Provides Both AniDocument1 pageRedicote E-7000: Versatile Emulsifier: Provides Both AniRabin Bera0% (1)
- Para Consulta: Glass Capillary Kinematic ViscometersDocument25 pagesPara Consulta: Glass Capillary Kinematic ViscometersJUAN CARLOSPas encore d'évaluation
- Metodo HACH 8140 - DEHADocument4 pagesMetodo HACH 8140 - DEHAFelix CruzPas encore d'évaluation
- Ic-0014 Kit Refrifluid B Uc-0020-1700 (En)Document2 pagesIc-0014 Kit Refrifluid B Uc-0020-1700 (En)Lluis Cuevas EstradaPas encore d'évaluation
- M789 - Free-Formaldehyde Content Determination ISO 11402Document12 pagesM789 - Free-Formaldehyde Content Determination ISO 11402Abhijit PatilPas encore d'évaluation
- Seta Verification Materials: STVM MTVMDocument2 pagesSeta Verification Materials: STVM MTVMdchyPas encore d'évaluation
- Astm D1208 - 1 (En)Document3 pagesAstm D1208 - 1 (En)rezokaPas encore d'évaluation
- TDS Polartech 7600Document1 pageTDS Polartech 7600Ngũ Viên Gia Các100% (1)
- AOAC 965.08 Kadar Air Pada PupukDocument3 pagesAOAC 965.08 Kadar Air Pada PupukDENI ARIFIYANTOPas encore d'évaluation
- EN 14103 - ThermoDocument4 pagesEN 14103 - ThermoLuciana TrisnaPas encore d'évaluation
- D4281Document6 pagesD4281saifullah629Pas encore d'évaluation
- ALLA FRANCE (Hydrometer)Document1 pageALLA FRANCE (Hydrometer)toan buiPas encore d'évaluation
- MT 23 Miscibility With Hydrocarbon Oil: Miscellaneous Techniques and ImpuritiesDocument6 pagesMT 23 Miscibility With Hydrocarbon Oil: Miscellaneous Techniques and ImpuritiesFelipe Navarrete0% (1)
- Standard Test Method ASTM D1218-02 International Standard ISO 5661:1983Document3 pagesStandard Test Method ASTM D1218-02 International Standard ISO 5661:1983Corina StanculescuPas encore d'évaluation
- AOCS 2d-25-2017Document2 pagesAOCS 2d-25-2017María Renee Quintanilla Vidal100% (1)
- D 7152 - 11 (2016)Document9 pagesD 7152 - 11 (2016)Ippolito Gualdi100% (1)
- ASTM 1882 - 96 R01 - Effect of Cooling System Chemical Solutions On Organic PDFDocument2 pagesASTM 1882 - 96 R01 - Effect of Cooling System Chemical Solutions On Organic PDFPham Thị Thuy HaPas encore d'évaluation
- Astm D5453-93Document6 pagesAstm D5453-93nardenePas encore d'évaluation
- Moisture Volatile OilDocument2 pagesMoisture Volatile OilRobert GilmorePas encore d'évaluation
- D 4191 - 15Document4 pagesD 4191 - 15hydrogenperoksidePas encore d'évaluation
- TDS KH-8011S en 21Document2 pagesTDS KH-8011S en 21Carlos Lizarraga FloresPas encore d'évaluation
- Biodegradability of Alkylbenzene Sulfonates: Standard Test Method ForDocument10 pagesBiodegradability of Alkylbenzene Sulfonates: Standard Test Method ForStuartPas encore d'évaluation
- D1969 - 07 (2012) Standard Specification For 2 - Ethylhexanol PDFDocument2 pagesD1969 - 07 (2012) Standard Specification For 2 - Ethylhexanol PDFJacques BlueqPas encore d'évaluation
- D 8085 - 17Document5 pagesD 8085 - 17Nguyễn Như ThếPas encore d'évaluation
- Solid Fat Content by Low-Resolution NMRDocument13 pagesSolid Fat Content by Low-Resolution NMRMagnolia Cespedes GuzmanPas encore d'évaluation
- D128 PDFDocument11 pagesD128 PDFJuan Diego ArizabalPas encore d'évaluation
- D 2074 - 92 (Reapproved 1998)Document2 pagesD 2074 - 92 (Reapproved 1998)dchy100% (2)
- Total, Primary, Secondary, and Tertiary Amine Values of Fatty Amines by Alternative Indicator MethodDocument3 pagesTotal, Primary, Secondary, and Tertiary Amine Values of Fatty Amines by Alternative Indicator MethodShaker Qaidi100% (1)
- Determination of Metals in Lubricating Greases by Inductively Coupled Plasma Atomic Emission SpectrometryDocument7 pagesDetermination of Metals in Lubricating Greases by Inductively Coupled Plasma Atomic Emission SpectrometryThang DoPas encore d'évaluation
- Norma Tension Superficial D1331Document4 pagesNorma Tension Superficial D1331cristiangggPas encore d'évaluation
- Hydroxyl Value of Base Polyol PDFDocument9 pagesHydroxyl Value of Base Polyol PDFswapon kumar shillPas encore d'évaluation
- Needle Penetration of Petroleum Waxes: Standard Test Method ForDocument4 pagesNeedle Penetration of Petroleum Waxes: Standard Test Method Forjdiosbernal10100% (3)
- Total Sulfur in The Analysis Sample of Refuse-Derived Fuel: Standard Test Methods ForDocument4 pagesTotal Sulfur in The Analysis Sample of Refuse-Derived Fuel: Standard Test Methods FornerissalovePas encore d'évaluation
- Technical Data Sheet Arquad MC 210: Guideline Formulation On Arquad MC 210 For Surface DisinfectionDocument2 pagesTechnical Data Sheet Arquad MC 210: Guideline Formulation On Arquad MC 210 For Surface DisinfectionRaida SiagianPas encore d'évaluation
- Determination of Metals in Lubricating Greases by Inductively Coupled Plasma Atomic Emission SpectrometryDocument7 pagesDetermination of Metals in Lubricating Greases by Inductively Coupled Plasma Atomic Emission SpectrometryNguyen Hoang QuanPas encore d'évaluation
- ASTM D 1387 - 89 (Reapproved 2002) Saponification Number (Empirical) of Synthetic and NaturalDocument2 pagesASTM D 1387 - 89 (Reapproved 2002) Saponification Number (Empirical) of Synthetic and Naturalalin2005100% (1)
- D1747Document4 pagesD1747rimi7alPas encore d'évaluation
- Microwave Digestion System With Pressurized Digestion CavityDocument5 pagesMicrowave Digestion System With Pressurized Digestion CavityTank TopPas encore d'évaluation
- Mho/cm. This Procedure Automatically Adjusts Cell ConDocument3 pagesMho/cm. This Procedure Automatically Adjusts Cell ConRonald Figo Torres EchePas encore d'évaluation
- Etil Hexano ASTM D1969Document2 pagesEtil Hexano ASTM D1969Coordinador LaboratorioPas encore d'évaluation
- Grease Thickener Systems and Calcium Sulphonate Complex Greases with Novel Base OilsDocument2 pagesGrease Thickener Systems and Calcium Sulphonate Complex Greases with Novel Base OilsHesham MahdyPas encore d'évaluation
- Standard Test Methods For Analysis of Soda AshDocument9 pagesStandard Test Methods For Analysis of Soda AshWendy Orozco60% (5)
- Conducting Cyclic Humidity Tests Exposures: Standard Test Method Practice ForDocument5 pagesConducting Cyclic Humidity Tests Exposures: Standard Test Method Practice ForCordova RaphaelPas encore d'évaluation
- D 4607 - 94 R99 RDQ2MDCDocument5 pagesD 4607 - 94 R99 RDQ2MDCMoch Arief AlbachronyPas encore d'évaluation
- D 5198 - 92 R97 - Rduxotgtotjsotc - PDFDocument3 pagesD 5198 - 92 R97 - Rduxotgtotjsotc - PDFAnonymous C1jOkuPas encore d'évaluation
- ASTM E291 - TM - Chemical Analysis of Caustic Soda and Caustic PotashDocument14 pagesASTM E291 - TM - Chemical Analysis of Caustic Soda and Caustic Potashphamthuyha50% (2)
- Standard methods for the examination of water and sewageD'EverandStandard methods for the examination of water and sewagePas encore d'évaluation
- Sulphuric Acid DataDocument19 pagesSulphuric Acid Datamartinjw100% (1)
- The Synthesis of Azo DyesDocument12 pagesThe Synthesis of Azo DyesJaveed GanaiePas encore d'évaluation
- Sulphuric Acid DataDocument19 pagesSulphuric Acid Datamartinjw100% (1)
- Global Zinc Stearate MarketDocument4 pagesGlobal Zinc Stearate MarketArmando PerezPas encore d'évaluation
- Aluminato de Sodio Oxido de Aluminio 15 - Ac16 PDFDocument12 pagesAluminato de Sodio Oxido de Aluminio 15 - Ac16 PDFArmando PerezPas encore d'évaluation
- Norma de Acido SulfuricoDocument13 pagesNorma de Acido SulfuricoArmando PerezPas encore d'évaluation
- Pe AaDocument5 pagesPe AanedalllPas encore d'évaluation
- Preparation of Sulphanilic AcidDocument4 pagesPreparation of Sulphanilic AcidMuhammad Usman100% (1)
- Ab-313 - 2 - en MetromDocument4 pagesAb-313 - 2 - en MetromArmando PerezPas encore d'évaluation
- DEC09ts PDFDocument19 pagesDEC09ts PDFBoomdayPas encore d'évaluation
- FOLLETO AN-h127 METROHMDocument4 pagesFOLLETO AN-h127 METROHMArmando PerezPas encore d'évaluation
- Acid Content DeterminationDocument3 pagesAcid Content DeterminationArmando PerezPas encore d'évaluation
- Acido Sulfanilico Pub2526Document134 pagesAcido Sulfanilico Pub2526Armando PerezPas encore d'évaluation
- Sulfuric Acid HandbookDocument44 pagesSulfuric Acid Handbookramsrivatsan50% (2)
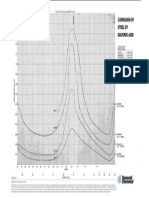
- Sulfuric Acid Corrosion of Steel 4Document1 pageSulfuric Acid Corrosion of Steel 4Emily AgostiniPas encore d'évaluation
- Instrumental Analysis ManualDocument71 pagesInstrumental Analysis ManualArmando PerezPas encore d'évaluation
- Handbook Acid Sulfuric PDFDocument265 pagesHandbook Acid Sulfuric PDFArmando PerezPas encore d'évaluation
- Pe AaDocument5 pagesPe AanedalllPas encore d'évaluation
- Handbook Acid Sulfuric PDFDocument265 pagesHandbook Acid Sulfuric PDFArmando PerezPas encore d'évaluation
- ISO 9001 Guia de TransicionDocument4 pagesISO 9001 Guia de TransicionArmando PerezPas encore d'évaluation
- Acido Sulfanilico Pub2526Document134 pagesAcido Sulfanilico Pub2526Armando PerezPas encore d'évaluation
- The Synthesis of Azo DyesDocument12 pagesThe Synthesis of Azo DyesJaveed GanaiePas encore d'évaluation
- ISO 9001 Guia de TransicionDocument4 pagesISO 9001 Guia de TransicionArmando PerezPas encore d'évaluation
- Fundamentos Del Color Diazotacion PDFDocument304 pagesFundamentos Del Color Diazotacion PDFArmando PerezPas encore d'évaluation
- Fundamentos Del Color Diazotacion PDFDocument304 pagesFundamentos Del Color Diazotacion PDFArmando PerezPas encore d'évaluation
- Manual Usuario Fias Mhs15 PDFDocument142 pagesManual Usuario Fias Mhs15 PDFArmando PerezPas encore d'évaluation
- Guia Electrolito de BateriaDocument15 pagesGuia Electrolito de BateriaArmando PerezPas encore d'évaluation
- Norma de Acido SulfuricoDocument13 pagesNorma de Acido SulfuricoArmando PerezPas encore d'évaluation
- NSF 60 9-20-2011revDocument9 pagesNSF 60 9-20-2011revArmando PerezPas encore d'évaluation
- Awwa b403-98Document24 pagesAwwa b403-98Armando PerezPas encore d'évaluation