Académique Documents
Professionnel Documents
Culture Documents
Radioactive Decay: Purple
Transféré par
saleem0 évaluation0% ont trouvé ce document utile (0 vote)
18 vues3 pages1. Half-life is the time it takes for half of the radioactive nuclei in a sample to decay.
2. Different radioactive materials have different half-lives, ranging from less than a second to billions of years.
3. The half-life can be measured from a graph showing the decay of radioactivity over time by finding the time when the count rate reaches half of the original value.
Description originale:
life
Titre original
What is
Copyright
© © All Rights Reserved
Formats disponibles
DOCX, PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce document1. Half-life is the time it takes for half of the radioactive nuclei in a sample to decay.
2. Different radioactive materials have different half-lives, ranging from less than a second to billions of years.
3. The half-life can be measured from a graph showing the decay of radioactivity over time by finding the time when the count rate reaches half of the original value.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
18 vues3 pagesRadioactive Decay: Purple
Transféré par
saleem1. Half-life is the time it takes for half of the radioactive nuclei in a sample to decay.
2. Different radioactive materials have different half-lives, ranging from less than a second to billions of years.
3. The half-life can be measured from a graph showing the decay of radioactivity over time by finding the time when the count rate reaches half of the original value.
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme DOCX, PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 3
What is Half-life?
1. Half-life is the time taken for half of the radioactive nuclei to decay.
2. Half-life is the time taken
for the count rate to fall to half of its original reading.
There are a number of ways to define half-life. Remember
one of the above definitions, it may be useful in the exams.
An Explanation of Half-life.
A radioactive material will have some nuclei that are stable
and some that are unstable. The stable nuclei don't change,
that is what stable means. In the picture below,
the unstable nuclei (shown as brown balls) will change
into stable nuclei (shown as purple balls) and emit radioactivity.
Half-life is a measure of the time taken for
the unstable nuclei to change into stable nuclei.
Different substances do this at different rates.
Some do it very quickly and half of the unstable nuclei decay
in less than one second.
For example, lithium-8 has a half-life of only 085 seconds.
Some do it very slowly and half of the unstable nuclei take
billions of years to decay.
For example, uranium-238 has a half-life of 451 billion years.
Remember that half-life is an amount of time.
In the same amount of time, the picture on the right above
will lose half of the remaining unstable nuclei.
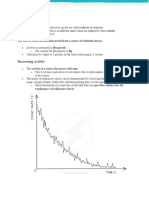
Measuring Half-life from a Graph.
How can Half-life be Measured from a Graph?
A graph showing how
the count rate decreases as time goes by
will have a curve like the one shown in the picture below.
For any particular radioisotope the count rate and time will
be different but the shape of the curve will be the same.
The easiest way to measure the half-life from the graph is
to
1. Read the original count rate at zero days.
On our graph the reading is 1640 counts.
2. Go down to half the original count rate (820 counts)
and draw a horizontal line to the curve.
Then draw a vertical line down from the curve.
You can read off the half-life where
the line crosses the time axis.
On our graph the half-life is 20 days.
Vous aimerez peut-être aussi
- Half LifeDocument11 pagesHalf LifeMarshell JonesPas encore d'évaluation
- Absolute Dating Reading AssignmentDocument5 pagesAbsolute Dating Reading Assignmentapi-251355123Pas encore d'évaluation
- Radioactivity-5 - Activity-And-Half-Life-1 - Online LearningDocument20 pagesRadioactivity-5 - Activity-And-Half-Life-1 - Online LearningShiyamala SubramaniamPas encore d'évaluation
- Half-Life - WikipediaDocument1 pageHalf-Life - WikipediaSyed Wajih HaiderPas encore d'évaluation
- Half Life Reactions Discussions and SolutionsDocument20 pagesHalf Life Reactions Discussions and SolutionsDeiparineIrisPas encore d'évaluation
- Nuclear Energy: Nuclear Decay: The NucleusDocument7 pagesNuclear Energy: Nuclear Decay: The NucleusmPas encore d'évaluation
- Half-Life Student Lab Sheet-REVDocument6 pagesHalf-Life Student Lab Sheet-REVElPerritoSapeePas encore d'évaluation
- Phys 91172 Half-LifeDocument2 pagesPhys 91172 Half-LifeRoneel SinghPas encore d'évaluation
- Atoms: Half Life Questions and AnswersDocument6 pagesAtoms: Half Life Questions and AnswersBubuPas encore d'évaluation
- Nuclear Physics NotesDocument9 pagesNuclear Physics NotesNashae Hall-Pass AllenPas encore d'évaluation
- DS-4, English MediumDocument56 pagesDS-4, English MediumRashini AnnePas encore d'évaluation
- Physics 11 - 7.3Document4 pagesPhysics 11 - 7.3danaPas encore d'évaluation
- Geochron. Assmt-Gomy - 2Document12 pagesGeochron. Assmt-Gomy - 2clement ntoriPas encore d'évaluation
- What Is An AtomDocument27 pagesWhat Is An Atomapi-377646239Pas encore d'évaluation
- Atoms and MoleculesDocument46 pagesAtoms and MoleculeskaixiangPas encore d'évaluation
- Properties of MaterialsDocument154 pagesProperties of MaterialsDarryl WHDPas encore d'évaluation
- Lesson Four - Half Lives and Nuclear EquationsDocument11 pagesLesson Four - Half Lives and Nuclear EquationsDeepti PatilPas encore d'évaluation
- Atomic StructureDocument28 pagesAtomic StructureAbed BaalbakiPas encore d'évaluation
- Concepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1D'EverandConcepts of Nuclear Medicine Volume I: Concepts of Nuclear Medicine, #1Pas encore d'évaluation
- Radioactive Isotope TwizzloriumDocument4 pagesRadioactive Isotope Twizzloriumapi-251874912Pas encore d'évaluation
- Everything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksD'EverandEverything You Must Know about Radioactivity 6th Grade Chemistry | Children's Chemistry BooksPas encore d'évaluation
- Nuclear Physics (Lecture 25-27) - Compatibility ModeDocument30 pagesNuclear Physics (Lecture 25-27) - Compatibility ModeBibhu Prasad SahooPas encore d'évaluation
- Physics AssignmentDocument22 pagesPhysics AssignmentanessnabelaPas encore d'évaluation
- Sec 2 Term 1 - 1 NotesDocument35 pagesSec 2 Term 1 - 1 NotesWolfie Saraswathi Santhosham100% (1)
- Chapter 2, 3Document35 pagesChapter 2, 3silvanyangelliong2Pas encore d'évaluation
- Unit 3 Atom and Periodic Table Notes Part 2Document56 pagesUnit 3 Atom and Periodic Table Notes Part 2api-483662721Pas encore d'évaluation
- Band of StabilityDocument8 pagesBand of StabilityDaneilla BanksPas encore d'évaluation
- Radioacitvity Lesson 1Document25 pagesRadioacitvity Lesson 1tanishknandal2009Pas encore d'évaluation
- Chemistry Notes Pt. 1Document108 pagesChemistry Notes Pt. 1weny fidayPas encore d'évaluation
- Special RelativityDocument17 pagesSpecial RelativityShreyansh SinghPas encore d'évaluation
- Science SOL 5.4 PowerPointDocument44 pagesScience SOL 5.4 PowerPointWynonah Merie MendozaPas encore d'évaluation
- (Chemistry) Lab ReviewerDocument3 pages(Chemistry) Lab ReviewerIris Kaye AbelleraPas encore d'évaluation
- Nuclear ChemistryDocument20 pagesNuclear ChemistryRose Belle A. GarciaPas encore d'évaluation
- Radiation and Half-LifeDocument6 pagesRadiation and Half-LifeAbdul NoorPas encore d'évaluation
- Solid State Chapter 1Document38 pagesSolid State Chapter 1Abrish HaremPas encore d'évaluation
- Calculations for Science Fiction Writers/Circular OrbitsD'EverandCalculations for Science Fiction Writers/Circular OrbitsPas encore d'évaluation
- 21 Radioactivity and Nuclear PhysicsDocument8 pages21 Radioactivity and Nuclear PhysicsAman BhuttaPas encore d'évaluation
- Atomic Structure and Periodicity VocabularyDocument4 pagesAtomic Structure and Periodicity Vocabularychrisroldan012696Pas encore d'évaluation
- Number of Original Nuclei) To Drop To Half of Its Initial ValueDocument8 pagesNumber of Original Nuclei) To Drop To Half of Its Initial ValuesalmaPas encore d'évaluation
- Fossil Power Point PresentationDocument25 pagesFossil Power Point PresentationSophia OrtizPas encore d'évaluation
- RadioactivityHalflife 05.09.2021Document25 pagesRadioactivityHalflife 05.09.2021Erdem AltunPas encore d'évaluation
- Radioactive Decay InteractivesDocument2 pagesRadioactive Decay Interactivescool joesPas encore d'évaluation
- Radioactive Decay & Half-Life Calculation: Presenters: Damion Lawrence and Michael WardDocument39 pagesRadioactive Decay & Half-Life Calculation: Presenters: Damion Lawrence and Michael WardKimonie BellanfantePas encore d'évaluation
- Properties of Materials July 24Document10 pagesProperties of Materials July 24Darryl WHDPas encore d'évaluation
- Radioactive DecayDocument28 pagesRadioactive Decayapi-234891239Pas encore d'évaluation
- Atomic Structure & The Periodic TableDocument56 pagesAtomic Structure & The Periodic TableAnas DarwishPas encore d'évaluation
- Uyp 5Document3 pagesUyp 5FATIN NOORPas encore d'évaluation
- Phys 1 - Module 8, 9, 10 & 11Document9 pagesPhys 1 - Module 8, 9, 10 & 11Kent Paul Camara UbayubayPas encore d'évaluation
- Chapter 1 Pearson Science 9Document44 pagesChapter 1 Pearson Science 9abhi0% (1)
- KS4 Radioactive DecayDocument31 pagesKS4 Radioactive DecayJaleel James100% (1)
- Session-09-Periodocity and PracticeDocument43 pagesSession-09-Periodocity and PracticeMojdeh AnbarfamPas encore d'évaluation
- Student Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Document5 pagesStudent Exploration: Half-Life: Prior Knowledge Questions (Do These BEFORE Using The Gizmo.)Kristina33% (3)
- 7.5 Halflife SEDocument5 pages7.5 Halflife SEWyatt KesterPas encore d'évaluation
- PEH Periodic Table (Principles) - Get The Table Organized in Time! Lab Manual (English)Document6 pagesPEH Periodic Table (Principles) - Get The Table Organized in Time! Lab Manual (English)Monette CabugayanPas encore d'évaluation
- Student Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationDocument5 pagesStudent Exploration: Half-Life: Vocabulary: Daughter Atom, Decay, Geiger Counter, Half-Life, Isotope, Neutron, RadiationJahel HerculesPas encore d'évaluation
- The Simple Pendulum Simple Harmonic OscillatorDocument4 pagesThe Simple Pendulum Simple Harmonic OscillatortpsoftxPas encore d'évaluation
- Principals 1Document18 pagesPrincipals 1raeiwwisPas encore d'évaluation
- Lecture Notes Nuclear Chemistry-RevisedDocument6 pagesLecture Notes Nuclear Chemistry-RevisedMarj EgiptoPas encore d'évaluation
- 10 1023@a@1022301907161Document8 pages10 1023@a@1022301907161saleemPas encore d'évaluation
- Surface ScienceDocument6 pagesSurface SciencesaleemPas encore d'évaluation
- Thin Solid Films: Guanglei Zhang, Guoqiang Qin, Gang Yu, Qianku Hu, Hua Fu, Changtao ShaoDocument6 pagesThin Solid Films: Guanglei Zhang, Guoqiang Qin, Gang Yu, Qianku Hu, Hua Fu, Changtao ShaosaleemPas encore d'évaluation
- Promotion of Tin Oxide Gas Sensor by Aluminum Doping: Vol. 0 1991 Pergamon Press PLCDocument7 pagesPromotion of Tin Oxide Gas Sensor by Aluminum Doping: Vol. 0 1991 Pergamon Press PLCsaleemPas encore d'évaluation
- Materials Letters: M. Lei, Q.R. Hu, S.L. Wang, W.H. TangDocument3 pagesMaterials Letters: M. Lei, Q.R. Hu, S.L. Wang, W.H. TangsaleemPas encore d'évaluation
- Interstitial Void SpacesDocument4 pagesInterstitial Void SpacessaleemPas encore d'évaluation
- Liu 2014Document5 pagesLiu 2014saleemPas encore d'évaluation
- Nuclear Physics Multiple Choice QuestionsDocument5 pagesNuclear Physics Multiple Choice QuestionssaleemPas encore d'évaluation
- Solid State Communications: Bingyun Ao, Zhengjun Zhang, Tao Tang, Yiping ZhaoDocument5 pagesSolid State Communications: Bingyun Ao, Zhengjun Zhang, Tao Tang, Yiping ZhaosaleemPas encore d'évaluation
- Lab 12 - Radioactivity, Beta, and Gamma RaysDocument18 pagesLab 12 - Radioactivity, Beta, and Gamma RayssaleemPas encore d'évaluation
- LEP 5.2.01 Half-Life and Radioactive Equilibrium: Related ConceptsDocument4 pagesLEP 5.2.01 Half-Life and Radioactive Equilibrium: Related ConceptssaleemPas encore d'évaluation