Académique Documents
Professionnel Documents
Culture Documents
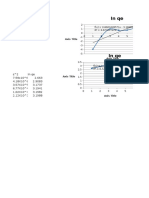
Kimfis 2
Transféré par
Apriyanti TindageTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Kimfis 2
Transféré par
Apriyanti TindageDroits d'auteur :
Formats disponibles
Adsorption 3, 189-195 (1997)
1997 KluwerAcademicPublishers. Manufacturedin The Netherlands.
Theoretical Basis for the Dubinin-Radushkevitch (D-R) Adsorption
Isotherm Equation
NICK D. HUTSON AND RALPH T. YANG
Department of Chemical Engineering, University of Michigan, Ann Arbor, M148109
Received March 18, 1996; Revised June 26, 1996; Accepted July 10, 1996
Abstract. The Dubinin-Radushkevitch (D-R) equation, which was originally proposed as an empirical adaptation
~d'the Polanyi adsorption potential theory, has been the fundamental equation to quantitatively describe the adsorption
td gases and vapors by microporous sorbents. The equation, based on the postulate that the mechanism for adsorp-
tion in micropores is that of pore-filling rather than layer-by-layer surface coverage, generally applies well to
adsorption systems involving only van der Waals forces and is especially useful to describe adsorption on activated
carbon. The ability of the D-R equation to describe gas adsorption on porous materials has inspired many to
undertake studies, both experimental and theoretical, to explain the source of the success of the D-R equation in
terms of molecular properties at the gas-solid interface. In many cases, these studies have led to extensions or
modifications of the original D-R equation. Many of these attempts and the resulting extensions are reviewed and
discussed here. Recently, an isotherm equation was derived for adsorption of gases and vapors on microporous
solids from statistical mechanical principles. It was shown that the D-R equation is an approximated form of this
potential theory isotherm. This development is also reviewed and discussed.
Keywords: Dubinin-Radushkevitch equation, Dubinin-Astakhov equation, adsorption, micropore adsorption
Introduction Development of the D-R Equation
Background The development of this equation was prompted by the
failure to extend the theories governing adsorption on
Most adsorbents used in modern technology, such as non-porous solids to that on real, porous solids such
activated carbons, synthetic and natural zeolites, sil- as active carbons, synthetic zeolites, and dehydrated
ica and other inorganic gels, etc., are microporous in inorganic gels (Dubinin, 1966, 1975). This lack of
nature (i.e., they contain pores with equivalent radii success led Dubinin and co-workers to focus their in-
less than 6-7 A). And while these sorbents may con- vestigations on the problem of physical adsorption on
tain larger pore varieties (e.g., mesopores and macrop- porous materials and resulted in the Dubinin-Polanyi
ores), it is the micropores that determine the adsorptive theory of micropore filling (also known as the theory
nature of the material. Despite intensive study, much of volume filling of micropores (TVFM)). This theory
of it by Dubinin and coworkers, there are still funda- is based on the postulate that the mechanism for ad-
mental questions in the science of adsorption of gases sorption in micropores is that of pore filling rather than
and vapors by microporous solids which remain unan- a layer-by-layer formation of a film on the walls of the
swered (Rudzinski and Everett, 1992). Nonetheless, pores.
for nearly a half-century, the Dubinin-Radushkevitch The D-R equation is an adaptation of the earlier
equation has been used to effectively describe the ad- Polanyi potential theory of adsorption (Polanyi, 1914,
sorption of vapors and gases by microporous solids. 1932; Goldmann and Polanyi, 1928; Gregg and Sing,
190 Hutsonand Yang
1982). An essential parameter of this theory is the constant (where 3 = 1 for the reference vapor). The
quantity A, defined by reason for grouping A/E is simply to express it as a di-
mensionless unit. On the assumption that the pore size
distribution is Gaussian, Dubinin and Radushkevitch
A = RT ln(~-~-) (1)
(1947) arrived at the following expression:
where P is the equilibrium pressure at temperature T,
pO is the saturated vapor pressure, and A is referred
to, by Polanyi, as the adsorption potential. Although,
o=
A 2
] (6)
the development of this theory requires an assumption which is the commonly used form of the D-R equation.
of layer-by-layer coverage of the pore surfaces with Combining Eqs. (2), (3) and (6) results in the following
the formation of a condensed adsorption film on the expression:
walls of the micropores and thus contradicts Dubinin's
fundamental postulate, the basic principle of the theory
concerning the temperature invariance of the parameter
A was shown to be true for microporous adsorbents but
W=Woexp- i ( Rr_Co.8pOX2m
y } Ij (7)
not, to a satisfactory degree, for large-pore and non-
or
porous adsorbents (Dubinin, 1975).
Dubinin and coworkers adopted a thermodynamic
interpretation of the Polanyi adsorption potential, re- W = W0 exp - B log (8)
ferring to it as the differential molar work of adsorp-
tion and expressing it as the negative differential free where E0 and B are related by the following expression:
energy of adsorption (AG):
E0 = 0.001915~/-(]-/B) (9)
A = - - A G = RTIn(P-~) (2)
Uses and Limitations of the D-R Equation
Since Dubinin considered microporous adsorption
to be a process of volume filling, a second parameter, The D-R and D-A equations have been effectively used
the degree of filling of the micropores 0 was inlxoduced to describe adsorption by microporous solids (Huber
and defined by et al., 1978). The D-R and D-A equations seem to
be particularly useful in describing adsorption by mi-
W croporous carbonaceous sorbents or activated carbons
0 = -- (3)
Wo (Dubinin and Stoeckli, 1980). In general, the TVFM
seems to be most applicable for microporous solids
where W0 is the total volume of the micropore system
with practically homogeneous or uniform micropore
and W is the volume that has been filled when the
structures (Dubinin, 1981; Dubinin and Kadlec, 1987;
relative pressure is p/pO. A fundamental postulate in
Jaroniec et al., 1989) and least applicable for micro-
the development of this theory is that 0 is a function of
porous adsorbents with a wide micropore distribution
A such that
(Dubinin, 1981).
The principal deficiency of the D-R equation is its
0 = f(A/E) (4)
behavior in the limit of zero loading (i.e., as 0 --+ 0)
Hacskaylo and LeVan (1985) and Dubinin (1975) has
or
pointed out that the validity of the TVFM (and thus the
D-R equation) is limited to degrees of micropore filling
f A
higher than 0.15. The D-R isotherm does not reduce to
Henry's law at low pressures, which is a necessity for
where A is the differential molar work of adsorption, a thermodynamically consistent isotherm (Chen and
E is the characteristic energy of adsorption, E0 is the Yang, 1994). In the low loading region, a thermody-
.characteristic energy of adsorption for a reference va- namically consistent adsorption isotherm should give
por (usually benzene), and fl is termed the similarity a finite Henry's law slope. The D-R equation, however
Theoretical Basis for the Dubinin-Radushkevitch (D-R) 191
gives a Henry's law slope equal to zero in this region which simplifies to the Dubinin-Astakhov equation:
(Kapoor et al., 1989).
Theoretical Basis of the D-R Equation
0 =. exp -
I(aT] (13)
or
The studies of Hobson (1961) revealed the ability of
the D-R equation to describe gas adsorption on non- 0 = exp[- A n
porous, macroporous, and mesoporous adsorbents. (14)
This work inspired many others to undertake studies,
both experimental and theoretical, in an effort to ex- The D-A equation is simply a more generalized ver-
plain the source of the success of the D-R equation sion of the D-R equation (where the parameter n = 2).
(Jaroniec and Madey, 1988). In many cases, these stud- Experimental evidence reveals that n ~-, 2 for activated
ies have led to extensions or modifications of the orig- carbonaceous sorbents (Dubinin, 1975) and approxi-
inal D-R equations. A few of these are summarized mately 4-6 for zeolites. The term n has been referred
below. to as a "heterogeneity factor" (Rudzinski and Everett,
1992); and, it has been suggested that it is related in
some way to the heterogeneity (with respect to the pore
Dubinir~-Astakhov (D-A) Equation distribution) of the surface.
Dubinin and Astakhov (1971) further expanded Eq. (4)
with the addition of an additional parameter n, such Extensions by Cerofolini and Stoeckli
that
Cerofolini (1972, 1974) postulated a relationship be-
0 = f ( A / E , n). (I0) tween the heterogeneity of the sorbent surface and the
adsorptive properties of that surface. He assumed that
heterogeneous surfaces are comprised of numerous ho-
The parameter n was included in this development mogeneous "patches" and that the overall isotherm (0t)
since most distribution functions in the normalized for the adsorptive system is related to a local isotherm
form are characterized by two parameters (Dubinin, (Or) for the patches and a distribution of adsorptive en-
1975). Assuming the temperature invariance of Eq. (10) ergies R(~) by the following:
and the Weibull distribution known in mathematical
statistics, Dubinin and Astakhov (1971) obtained an
equation of adsorption in an analytical form. Weibull's Or(T; P) = Or(T; P; E)R(E)dE (15)
distribution function F(A) in normalized form is fEi)
expressed as:
where E0 is > 0 and corresponds to the local minimum of
F(A)= l - exp -
I (A/] (11)
the adsorption potential resulting from intermolecular
forces.
Cerofolini (1974) showed that Eq. (15) reduces to
From Eqs. (11) and (2), one can see that at A = 0
(i.e., p/pO = 1), F(A) = 0. On the other hand, as Or(T; P) = Rc(E) de (16)
I
p/pO decreases to low equilibrium relative pressures,
A reaches very high values, and F(A) tends to zero. or
Therefore, F(A) can be assumed to express the portion
of the unfilled adsorption volume of the micropores
RcCE) = - - ~ / ~ E (17)
(i.e., 1 - 0). So, then
using a technique called the condensation approxima-
F(A) = 1 - 0 = 1 -- exp - [ (12) tion (CA), This formed the.foundation for a proposal
by Stoeckli (1981) for the theoretical basis of the D-A
192 Hutsonand Yang
equation (and thus the D-R equation). Stoeckli pro- D-R equation applies only to structurally homogeneous
posed that the D-R and D-A equations, which were systems, i.e., micropores with the same dimension. As
proposed as semi-empirical relationships with some a result, Stoeckli (1977) and Huber et al. (1978) at-
thermodynamic considerations, could be based on a tempted to generalize the D-R equation to adsorbents
theoretical model involving adsorption energies and with inhomogeneous microporous structures. For this
their distribution (Stoeckli, 1990). In the develop- purpose they assumed a normal Gaussian distribution
ment of this theory, Stoeckli postulated that if the local of micropores Wo with respect to the parameter B of
isotherm (Or)is the Langmuir type, an overall isotherm the D-R Eq. (8):
(Or)of the D-R type
dWo = f(B) = W exp I Bo - B 1 (22)
dB /,,/~ -~E2- /
0,= exp(-B(RT In -~ ) 2) (18)
where W is the total micropore volume, Bo is the value
corresponds to the energy distribution of the parameter B for the minimum of the distribution
curve, and A is the dispersion (or variance). One can
I'lc(e) = 2B(~ - E0) exp(--B(E -- E0)2) (19) write the following equation for an adsorbent with an
inhomogeneous microporous structure in accordance
where, by the condensation approximation, with Eqs. (8) and (22):
E-e0 = RTln(~-~) (20) W= f(B)exp[-By]dB. (23)
f0
So, in a generalization of this technique, using the fol- where
lowing assumptions:
y= log (24)
(1) the overall isotherm is the D-A equation (Eq. (14)),
(2) the local isotherm in the micropore is of the Integrating (23) yields the adsorption equation:
Langmuir type,
(3) P m = pO, W = W[exp(-Boy)] exp[0.5y2A2]0.5[ 1 -- erf(z)]
(4) for carbons Eo is close to the adsorption energy on (25)
the open surface,
where
the condensation approximation method leads to the
following approximate energy distribution associated ( B0 "~..A_~ (26)
with the D-A equation: Z= Y--A2]~,'~"
(E - Eo)n-1 /' /'E - Eo'~n'X Equation (25) is referred to as the Dubinin-
b~c(E)=n (~E--~ e x p ~ , - ~ , ' - ' ~ o ) J" (21) Radushkevitch-Stoeckli (D-R-S) equation. Equation
(23) can be rewritten as follows:
In this approach, the exponent n reflects the width of
the energy distribution and is related to the pore size 0=fo exp[ - A 2 (27)
distribution (Stoeckli, 1990).
where g(E) is the energy distribution function of the
Dubinin-Radushkevitch-Stoeckli (D-R-S) Equation micropores and E is the local characteristic energy and
empirically, E is related to pore dimension by (Dubinin
Temperature invariance was the basic premise in for-
and Stoeckli, 1980)
mulating the D-R equation. However, it was noted by
Stoeckli (1977), Dubinin and coworkers (1980) that the E = X/x (28)
characteristic energy of adsorption (Eo) decreases with
increasing differential work of adsorption (A). This where x is the half-width of the slit pore and X is an
observation led Stoeckli (1977) to postulate that the empirical constant.
Theoretical Basis for the Dubinin-Radushkevitch (D-R) 193
Extensions by Jaroniec et al. where qr and'qo are the rotational and vibrational par-
tition functions of, the N fluid molecules and are sep-
As mentioned, Dubinin and Stoeckli assumed a arated from the translational partition function; the
Gaussian micropore-size distribution in the develop- superscript s denotes the adsorbed phase. ~. is a con-
ment of the D-R-S equation. Jaroniec and coworkers stant indicating the dimensions of the space (i.e., ~. = 3
(1988a, 1989, 1991) proposed a gamma-type distribu- for three dimensional space and X = 2 for two dimen-
tion of the following form: sional space). For adsorption in micropores, however,
~. may lie between 2 and 3. U(rij) is the potential en-
qn+l
f ( B ) -- - - B n exp(-qB) (29) ergy between two molecules "i" and " j " at distance
F(n + 1) rij, and ~(rk) is the potential energy of molecule "k"
at rk, which arises from the force field in the pore. V"
where q > 0, n > - 1 and 1-" is the gamma function.
is the volume of the system, dr N = d r l d r 2 . . , dru for
The distribution f ( B ) is defined on the domain [0, oo)
all positions, t' = 1/(kT) and
and generates the following micropore-size distribution
(Jaroniec et al., 1989):
A = h/(2rrmkT) 1/2 (34)
n "~n+l
G(x) -
"/c/
i) x 2n+l
exp(-qcx
2
) (30)
is the de Broglie thermal wavelength, h is Planck's
Using.the relationship, constant, m is the molecular weight, k is Boltzmann's
constant, and T is the absolute temperature.
Ot = exp - B f(B)dB (31) Although the potential energy is a function of po-
sition in the pore, Chen and Yang (1994) assumed
that the force field may be approximated by a mean
the distribution f ( B ) in Eq. (31) generates the follow-
value, ~, for all adsorbate molecules in the pore. By
ing simple equation:
using a two-dimensional equation of state to repre-
q ] .+1 sent the adsorbed phase and equating the chemical
O, = q + ~-A/fl)2 j . (32) potentials of the adsorbed and gas phases, the result-
ing adsorption isotherm equation was then derived as
This equation is referred to as the Jaroniec-Ch0ma (or follows:
J-C) equation.
20 + 9 0
Potential Theory Isotherm ln(p~A ) -7 In(1-0)+--
(1 - 0 ) 8 (1-0) 2
Chen and Yang (1994) have derived an isotherm equa- -c (r)rl + = o (35)
tion from statistical mechanical principles which, for
appreciable degrees of pore filling, e.g., 0 > 0.1 re-
duces to the D-A and D-R equations. For 0 ---> 0, the where p" and pg are number densities of the adsorbed
isotherm equation reduces to Henry's law (which is not and fluid phases, respectively. The quantity 0 repre-
true and is the primary flaw of the D-R equation). In the sents the fraction of the surface that is covered (the
derivation of this equation, Chen and Yang considered packing fraction at the surface), A is the de Brogli.e
a system of N fluid molecules exposed to a force field thermal wavelength, k is the Boltzmann constant, and
within a given pore size and geometry. Each molecule a ( T ) is a function of T only.
in this force field has a potential energy of (rk), where In the derivation of the D-R equation, the isotherm
rk is the position coordinate of molecule k in the system. at pressure pO,
The partition function Z~ of the canonical ensemble
of the system can be expressed as (Steele, 1974): n/p~ 21/o 9 r/o
l , o-Vj - ~7 In(1 - . o ) + - - (1 +
- - r/o) 8 (1 - - r/o) 2
s qrqo _fl drN
ZN -- N ! A xN (I)
- a(T)r/o + ~-~ = 0 (36)
exp - V(r j) - y (33)
i<j k ' was subtracted from that at pressure P (as [liven in
194 Hutson and Yang
Eq. (35)). The result expression is as follows: 18.0
16.0. ( ~ F(rl)-F(rl)
ln(P"~+F(o)-F(Oo)=\p~} 'n(~o) (37)
14.0.
\ O KF(rlo)(-ln0)tt2
. .
where 12.0.
7 2._____L_0 10.0.
F(O) = - ~ ln(1 - r/) + (1 - 0)
9 q 8.0
+ ot(T)o (38)
8 (1 - o)2
6.0
and
4.0
p~ P
(39) 2.0
po
0.0
Since, in the D-R and D-A equations, 0 is the frac- 0.0 0.2 0.4 0.6 0.8 1.0
tional pore filling relative to the saturation vapor pres-
sure, the following is true Figure 1. Goodness of approximation for Eq. (41), for the repre-
sentative case of rio = 0.65.
pS 0
- - o (40)
oo
Equation (43) shows that the characteristic energy of
A purely mathematical approximation, that adsorption (Eofl) in the D-R (and D-A) equation is di-
rectly related to the mean potential (~) in the pore.
F(q) - - F(oo) -~ - K F(qo)(-lnO) '/" (41) Since the dependence of @ on pore size can be estab-
lished it is possible to investigate the dependence of
where K and n are constants resulting from the ap-
Eofl on pore size.
proximation and have no physical meaning, is made
and Eq. (37) may be mathematically manipulated to: Nomenclature
In0 = _ kT In A adsorption potential; differential molar work
of adsorption; Helmholtz free energy
B parameter in D-R, D-A equations, related to Eo
=- [ K~--~T0~In ( ~ 0 ) ]" (42) Bo value of the parameter B for the minimum of
the micropore distribution curve in the
D-R-S equation
where No is the Avogadro number. Chen and Yang E characteristic energy of adsorption; energy
(1994) showed that the mathematical approximation Eo characteristic energy of adsorption for
of Eq. (41) is good for0 = 0.2 - 1.0, but breaks down standard vapor
when 0 < 0.1. This is shown in Fig. I where the g(E) energy distribution function
approximations of Eq. (41) and the further case where G(x) micropore size distribution
In 0 is neglected are shown for the representative case h Planck's constant
of 00 = 0.65. Dubinin (1975) and others have pointed k Boltzmann's constant
out this problem for the D-R and D-A equations. K constant from approximation, Eq. (41)
Equation (42) is the form of the Dubinin-Astakhov m molecular weight
equation (or the D-R equation when n = 2) where n parameter in D-A equation
N number of molecules in the system
KNo@ = Eo[3 (43) No Avogadro's number
Theoretical Basis for the Dubinin-Radushkevitch (D-R) 195
P pressure Acknowledgments
P,,, condensation pressure
pO saturated vapor pressure This work was supported by a grant from the National
q parameter of the Gamma micropore Science Foundation (NSF Grant CTS-9520328) and
distribution partially by the BOC Group.
qc parameter of the Gamma micropore
distribution for a reference vapor
qr rotationalpartition function References
qo vibrational partition function
Cerofolini,G.E, J. of Low Temp. Physics, 6, 473 (1972).
R gas constant
Cerofolini,G.E, Thin Solid Films, 23, 129 (1974).
rk position coordinate of molecule"k" Cerofolini,G.F., J. of Colloid and hzterface Sci., 86, 204 (1982).
T temperature Chen, S.G. and R.T. Yang,Langmuir, 10, 4244 (1994).
U(rij ) potential energy between molecules i and j Dubinin,M.M., Chem. and Phys. of Carbon, EL. Walker,Jr. (Ed.),
Vs surface area Mercel Dekker, New York, 1966.
Dubinin, M.M., Progress in Surface and Membrane Science,
W volume of micropores filled at relative
D.A. Cadenhead et al. (Eds.), Academic Press, New York,
pressure p / pO 1975.
Wo total volume of micropore system Dubinin,M.M., Carbon, 23,373 0985).
x half-width of slit pore Dubinin,M.M. and O. Kadlec, Carbon, 24, 321 (1987).
,Y empirical constant relating characteristic Dubinin,M.M. and V.A.Astakhov,Izv. Akad. Nauk SSR, Ser. Khim.,
p. 5, 1971.
energy to pore size Dubinin, M.M. and L.V. Radushkevitch, Proc. Acad. Sci. USSR.,
Z~ partition function of the canonical ensemble Vol. 55, p. 331, 1947.
Dubinin,M.M. and H.E Stoeckli, J. of Colloid and Interface Sci.,
Greek etc. Symbols 75, 34 (1980).
Goldmann,E and M.M. Polanyi,Z. Phys. Chem., Abt. A, 132, 321
~(T) a function of temperature in Eq. (35) (1928).
Gregg, S.J. and K.S.W.Sing,Adsorption, Surface Area and Porosity,
similarity coefficient 2nd Ed., AcademicPress, London, 1982.
A dispersion (or variance) Hacskaylo,J.J. and M.D. LeVan,Langmuir, 1, 97 (1985).
AGads differential free energy of adsorption Hobson, J.P., J. of Phys. Chem., 34, 1850 (1961).
6 energy Huber, H., H.F. Stoeckli,and J.Ph. Houriet,J. of CoUoid and Inter-
60 m i n i m u m adsorption energy face Sci., 67, 195 (1978).
Jaroniec, M. and J. Choma, Carbon, 26, 747 (1988a).
r] packing fraction of the surface Jaroniec, M. and R. Madley,Physical Adsorption on Heterogeneous
r]0 packing fraction of the surface at P ---=-pO Solids, Elsevier,Amsterdam, 1988b.
V 1/kT Jaroniec, M., R. Madey, J. Choma, B. McEnaney,and T.J. Mays,
F' gamma function Carbon, 27, 77 (1989).
k number of dimension Jaroniec, M., J. Choma, and X. Lu, Chem. Eng. Sci., 46, 3299
(1991).
A de Broglie thermal wavelength
q~ Kapoor, A., J.A. Ritter,and R.T. Yang,Langmuir, 5, 1118 (1989).
potential energy function Polanyi,M., Verb. Deutsch. Physik. Ges., 16, 1012 (1914).
p.," number density of the solid phase Polanyi,M., Trans. Faraday Soc., 28, 316 (1932).
pX' number density of the gas phase Rudzinski,W. and D.H. Everett,Adsorption of Gases on Heteroge-
0 fractional coverage ( = W / W o ) neous Surfaces, AcademicPress, London, 1992.
Steele, W.A.,The Interaction of Ga.~eswith Solid Surfaces, Pergamon
o, overall isotherm for microporous system
Press, Oxford, 1974.
ol local isotherm for microporous system Stoeckli, H.E, J. of Colloid and Interface Sci., 59, 184 (1977).
~(~) energy distribution Stoeckli, H.E, Carbon, 19, 325 (1981).
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- J0313845 PDFDocument8 pagesJ0313845 PDFApriyanti TindagePas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Site KishimotoDocument7 pagesSite KishimotoApriyanti TindagePas encore d'évaluation
- Latihan Soal KimfisDocument10 pagesLatihan Soal KimfisApriyanti TindagePas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Abs TrakDocument1 pageAbs TrakApriyanti TindagePas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Dubinin-Radushkevich Equation and The UnderlyiDocument10 pagesThe Dubinin-Radushkevich Equation and The UnderlyiApriyanti TindagePas encore d'évaluation
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Evaluasi Proses Pengolahan Limbah Kulit Udang Untuk Meningkatkan Mutu Kitosan Yang DihasilkanDocument8 pagesEvaluasi Proses Pengolahan Limbah Kulit Udang Untuk Meningkatkan Mutu Kitosan Yang DihasilkanNataliakusumaDewiPas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- The Dubinin-Radushkevich Equation and The Underlyi (Kimfis1)Document11 pagesThe Dubinin-Radushkevich Equation and The Underlyi (Kimfis1)Apriyanti TindagePas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Descriptive PDFDocument7 pagesDescriptive PDFApriyanti TindagePas encore d'évaluation
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Pigment From Annato Seeds Contains Carotenoids Bixin and NorbixinDocument1 pagePigment From Annato Seeds Contains Carotenoids Bixin and NorbixinApriyanti TindagePas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Berkumpul Di F 310 (24 &25 Ags 2014) Berkumpul Di BALAIRUNG UKSW (26 Ags 2014) Berkumpul Di AUDITORIUM FTI (27 Ags 2014)Document135 pagesBerkumpul Di F 310 (24 &25 Ags 2014) Berkumpul Di BALAIRUNG UKSW (26 Ags 2014) Berkumpul Di AUDITORIUM FTI (27 Ags 2014)Apriyanti TindagePas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- StatdasDocument3 pagesStatdasApriyanti TindagePas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Efektivitas Antibakteri Nano Kitosan Terhadap PertumbuhanDocument7 pagesEfektivitas Antibakteri Nano Kitosan Terhadap PertumbuhanApriyanti TindagePas encore d'évaluation
- Materials 02 00010Document12 pagesMaterials 02 00010Apriyanti TindagePas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Hanna KatalisDocument13 pagesHanna KatalisApriyanti TindagePas encore d'évaluation
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Caracterisation Chitosan 1Document4 pagesCaracterisation Chitosan 1Ali SulaimanPas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Biomaterials: Weitai Wu, Jing Shen, Probal Banerjee, Shuiqin ZhouDocument11 pagesBiomaterials: Weitai Wu, Jing Shen, Probal Banerjee, Shuiqin ZhouApriyanti TindagePas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- Chitosan in Bio MaterialDocument22 pagesChitosan in Bio Materialsins1984Pas encore d'évaluation
- Descriptive PDFDocument7 pagesDescriptive PDFApriyanti TindagePas encore d'évaluation
- Damir Eposidna SmolaDocument3 pagesDamir Eposidna SmolaOliver RisteskiPas encore d'évaluation
- @face X 0, Ux 0 @face X Width, " Linear Constraint Equation" @face Y 0, Uy 0 @face Y Width, Uy Is Specified @face Z 0, Uz 0 @face Z Width, " Linear Constraint Equation"Document3 pages@face X 0, Ux 0 @face X Width, " Linear Constraint Equation" @face Y 0, Uy 0 @face Y Width, Uy Is Specified @face Z 0, Uz 0 @face Z Width, " Linear Constraint Equation"VijaybalajiPas encore d'évaluation
- Reaction Kinetice and Reactor DESIGN (CH-322) : by H. Scott Fogler Fourth EditionDocument140 pagesReaction Kinetice and Reactor DESIGN (CH-322) : by H. Scott Fogler Fourth EditionTEZ ANALYSIS AND STORIESPas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Elliott Davis Ferreira Mahdi Gorgun Semi Rigid - Structural Eng Part 1Document14 pagesElliott Davis Ferreira Mahdi Gorgun Semi Rigid - Structural Eng Part 1Luciana Barros - LeonardiPas encore d'évaluation
- Unit 4 Laser and Advances in MetrologyDocument74 pagesUnit 4 Laser and Advances in MetrologyS. SARGUNA THAMIZHAN MECHPas encore d'évaluation
- How Thick Is The Copper On A 1 Oz Copper PCB in MMDocument13 pagesHow Thick Is The Copper On A 1 Oz Copper PCB in MMjackPas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- UNC and UNF - Unified Inch Screw ThreadsDocument8 pagesUNC and UNF - Unified Inch Screw ThreadsPatrick FaelPas encore d'évaluation
- SchockDocument32 pagesSchockAndyPas encore d'évaluation
- Cilamce 2013 - 0690Document20 pagesCilamce 2013 - 0690José Guilherme Santos da SilvaPas encore d'évaluation
- Measurement of Directions and AnglesDocument45 pagesMeasurement of Directions and AnglesXiellePas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (120)
- Data Sheet 6ES7297-0AX30-0XA0: General InformationDocument2 pagesData Sheet 6ES7297-0AX30-0XA0: General InformationsebouelletPas encore d'évaluation
- MechanicsDocument171 pagesMechanicsFabian ClarkePas encore d'évaluation
- Higher Mathematics Mathematics: PAPER 5 Mechanics 2 (M2)Document4 pagesHigher Mathematics Mathematics: PAPER 5 Mechanics 2 (M2)JuePas encore d'évaluation
- 1.3 Atomic Mass Atomic Number and Isotopes PDFDocument23 pages1.3 Atomic Mass Atomic Number and Isotopes PDFMARVIN DELA CRUZPas encore d'évaluation
- Lesson #15 Centroid of Plane Areas and of Solids of Revolution-1Document20 pagesLesson #15 Centroid of Plane Areas and of Solids of Revolution-1yveeshPas encore d'évaluation
- Review Module 35 - Hydraulics 1 - Part 2Document1 pageReview Module 35 - Hydraulics 1 - Part 2Mina, KhristinePas encore d'évaluation
- The Particleincell MethodDocument29 pagesThe Particleincell MethodAntonio JuarezPas encore d'évaluation
- Cepheus Journal Issue 007bDocument40 pagesCepheus Journal Issue 007bScott Tay100% (1)
- Tyco Quality Manual 1654241-1CrimpBroGBDocument28 pagesTyco Quality Manual 1654241-1CrimpBroGBdamirPas encore d'évaluation
- Engineering Mechanics DynamicsDocument2 pagesEngineering Mechanics DynamicsMallene EhurangoPas encore d'évaluation
- Relative Dielectric Permittivity in Porous MediaDocument10 pagesRelative Dielectric Permittivity in Porous MediaLazuardhy Vozika FuturPas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Course OutlineDocument3 pagesCourse OutlineLillian MuwinaPas encore d'évaluation
- Geography Skills HandbookDocument16 pagesGeography Skills HandbookGa SunshinePas encore d'évaluation
- Research On Interactive Steering Control Strategy Between Driver and AFS in Different Game Equilibrium Strategies and Information PatternsDocument33 pagesResearch On Interactive Steering Control Strategy Between Driver and AFS in Different Game Equilibrium Strategies and Information PatternsSasi VardhanPas encore d'évaluation
- ABS Dargage Shear BoxDocument3 pagesABS Dargage Shear Boxabdullahikabirnee81Pas encore d'évaluation
- Experimento de Efecto Magnus - Curvatura de La Trayectoria de Una Bola en Caída LibreDocument4 pagesExperimento de Efecto Magnus - Curvatura de La Trayectoria de Una Bola en Caída Librevitevo56Pas encore d'évaluation
- Direct Analysis SchemeDocument4 pagesDirect Analysis SchemeYu PiPas encore d'évaluation
- MECH3423 Building Services Engineering II Experiment 1: Basic Water Cooling TowerDocument6 pagesMECH3423 Building Services Engineering II Experiment 1: Basic Water Cooling TowerMahleh Thabe'khuluPas encore d'évaluation
- Functions and Characteristics of InstrumentsDocument29 pagesFunctions and Characteristics of InstrumentsMarion AlyssaPas encore d'évaluation
- Topic 5 - Curvilinear Motion - 2Document17 pagesTopic 5 - Curvilinear Motion - 2Eugene GarbosaPas encore d'évaluation
- Gut: the new and revised Sunday Times bestsellerD'EverandGut: the new and revised Sunday Times bestsellerÉvaluation : 4 sur 5 étoiles4/5 (392)
- The Comfort of Crows: A Backyard YearD'EverandThe Comfort of Crows: A Backyard YearÉvaluation : 4.5 sur 5 étoiles4.5/5 (23)