Académique Documents
Professionnel Documents
Culture Documents
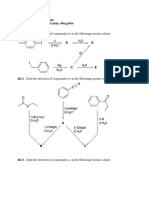
Reaction Summary: Enolates & Enamines
Transféré par
Jamira JarrettCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Reaction Summary: Enolates & Enamines
Transféré par
Jamira JarrettDroits d'auteur :
Formats disponibles
Chapter 19 reaction summary
Reaction Comments
O
O
NaOH Difficult to stop before
Aldol 2 H
H dehydration
OH
O
O
Aldol with NaOH
2 H
dehydration
H
O O
O The aldol acceptor cannot
Mixed aldol H + NaOH
have -hydrogens
O
1. NaOEt O O
Claisen 2
OEt 2. H 3O+
OEt
O O
OEt 1. NaOEt
Dieckmann EtO CO2Et Cyclic Claisen reaction
2. H 3O+
O
O O
1. NaOEt O O
+
Acylation of ketones
EtO OEt 2. H 3O+ OEt
O
N
Alkylation of 1. Br Better method to alkylate a
enamines ketone
2. H 3O+
O O
N O
Acylation of 1.
Cl
enamines
2. H 3O+
O O
O O Can hydrolyze ester to
Acetoacetic ester 1. NaOEt
OEt carboxylic acid then
synthesis OEt 2. Br decarboxylate
O O O O
Acetoacetic ester 1. NaOEt Can hydrolyze ester to
synthesis OEt OEt carboxylic acid then
2. Br
Dialkylation decarboxylate
O O
O O Can hydrolyze ester to
Malonic ester 1. NaOEt
EtO OEt carboxylic acid then
synthesis EtO OEt 2. Br decarboxylate
O O O O
Malonic ester 1. NaOEt Can hydrolyze ester to
synthesis EtO OEt EtO OEt carboxylic acid then
2. Br
Dialkylation decarboxylate
O CO2Et Stabilized carbanions add
1. NaOEt
+ EtO 2C CO2Et 1,4 to ,-unsaturated
Michael addition 2. H 3O+ EtO 2C carbonyl. Carbanion can be
O more than malonic ester
Chapter 19 reaction summary
Reaction Comments
O
1. R 2CuLi R O Cuprates (unlike Grignards)
Cuprate addition add 1,4 to ,-unsaturated
2. H 3O+ carbonyl
O R O
1. R 2CuLi
Cuprate addition with 2. Br
Can couple cuprate 1,4-
-alkylation additon with alkylation
Vous aimerez peut-être aussi
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Edexcel IAL Chemistry A-Level: Topic 20: Organic SynthesisDocument8 pagesEdexcel IAL Chemistry A-Level: Topic 20: Organic SynthesisCornflake 25Pas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Silicones Solúveis - Liberados para Low E No PooDocument6 pagesSilicones Solúveis - Liberados para Low E No PooBeatrice RibeiroPas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Preparation and Reactions of Simple Organic Compounds: Organic Chemistry (Engg.) CHEM2101 1Document52 pagesPreparation and Reactions of Simple Organic Compounds: Organic Chemistry (Engg.) CHEM2101 1حنين الخميسيPas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Analytical Reagents (80001-116000)Document736 pagesAnalytical Reagents (80001-116000)SwissHuge HugePas encore d'évaluation
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Chemistry Notes For Class 12 Chapter 13 Amines: StructureDocument17 pagesChemistry Notes For Class 12 Chapter 13 Amines: Structureharsh vardhanPas encore d'évaluation
- Exercise - II: (One or More Than One Option Correct)Document3 pagesExercise - II: (One or More Than One Option Correct)mehul pantPas encore d'évaluation
- Homework AnswersDocument6 pagesHomework AnswerssaraheaartzPas encore d'évaluation
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Benzene MilanaDocument61 pagesBenzene MilanaMilana WalujoPas encore d'évaluation
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Carboxylic Acids - DerivativesDocument24 pagesCarboxylic Acids - DerivativesRit WickPas encore d'évaluation
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- 13 HydrocarbonsDocument2 pages13 HydrocarbonsPadhai tak : by Dr.Aditya guptaPas encore d'évaluation
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Summary of Organic ReactionsDocument6 pagesSummary of Organic ReactionsAbudi Alsagoff100% (5)
- Mesomeric EffectDocument21 pagesMesomeric EffectAwais Arshad100% (2)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Infrared Spectroscopy: WWU ChemistryDocument34 pagesInfrared Spectroscopy: WWU ChemistryTrung HoPas encore d'évaluation
- Aldehydes, Ketones, and Carboxylic Acids PDFDocument5 pagesAldehydes, Ketones, and Carboxylic Acids PDFmadhurima maityPas encore d'évaluation
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Solvent Miscibility TableDocument1 pageSolvent Miscibility Tablewdsbarros100% (2)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Crown Ether Notes - I MSC Chemistry (CBCS)Document8 pagesCrown Ether Notes - I MSC Chemistry (CBCS)Abhay KanaujiaPas encore d'évaluation
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- IUPAC Nomenclature of Organic Chemistry - WikipediaDocument26 pagesIUPAC Nomenclature of Organic Chemistry - WikipediaYa seen khanPas encore d'évaluation
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- Rapid and Catalyst‐Free α‐Halogenation of Ketones using N‐Halosuccinamides in DMSODocument9 pagesRapid and Catalyst‐Free α‐Halogenation of Ketones using N‐Halosuccinamides in DMSOthegreatgbrothersPas encore d'évaluation
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Tds Gaa - India - BP MalaysiaDocument1 pageTds Gaa - India - BP MalaysiaErik YerzyPas encore d'évaluation
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Worksheet AminesDocument2 pagesWorksheet AminesHarshit ParmarPas encore d'évaluation
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Chap 15-24Document49 pagesChap 15-24Linh NguyễnPas encore d'évaluation
- Chapter 4 Benzene and Its DerivativesDocument36 pagesChapter 4 Benzene and Its DerivativesBARRY EPHRAIM PELICANOPas encore d'évaluation
- Tugas Kimia Organik 2 Jane Nizar RahmanDocument11 pagesTugas Kimia Organik 2 Jane Nizar Rahmanjane nizar rahmanPas encore d'évaluation
- Solvent Naphtha (Petroleum), Heavy Arom. - Registration Dossier - ECHA PDFDocument36 pagesSolvent Naphtha (Petroleum), Heavy Arom. - Registration Dossier - ECHA PDFNestor Armando Marin SolanoPas encore d'évaluation
- SodaPDF-converted-chapter 3 NutritionDocument25 pagesSodaPDF-converted-chapter 3 NutritionFidelia AlvesPas encore d'évaluation
- Module 1 - Organic ChemistryDocument12 pagesModule 1 - Organic ChemistrySelena MoonPas encore d'évaluation
- 08 Anexa 10Document7 pages08 Anexa 10Diana OlvediPas encore d'évaluation
- Second Self-Assessment TestDocument2 pagesSecond Self-Assessment TestAbhay VishwakarmaPas encore d'évaluation
- Poly Halo Al KanesDocument3 pagesPoly Halo Al KanesNabin JoshiPas encore d'évaluation
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- 12 Chemistry Notes ch11 Alcohols Phenols and EthersDocument8 pages12 Chemistry Notes ch11 Alcohols Phenols and Ethersmv7602456Pas encore d'évaluation
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)