Académique Documents
Professionnel Documents
Culture Documents
Quizz Week 4 (Number 2)
Transféré par
AbdulHaseebArifCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Quizz Week 4 (Number 2)
Transféré par
AbdulHaseebArifDroits d'auteur :
Formats disponibles
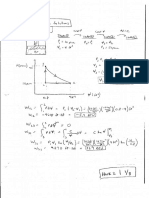
Problem 2.
27
Carbon dioxide (CO2) gas within a piston-cylinder assembly undergoes a process from a state
where p1 = 5 lbf/in.2, V1 = 2.5 ft3 to a state where p2 = 20 lbf/in.2, V2 = 0.5 ft3. The relationship
between pressure and volume during the process is given by p = 23.75 7.5V, where V is in ft3
and p is in lbf/in.2 Determine the work for the process, in Btu.
KNOWN: CO2 gas within a piston-cylinder assembly undergoes a process where the p-V
relation is given. The initial and final states are specified.
FIND: Determine the work for the process.
SCHEMATIC AND GIVEN DATA:
25
2 p = 23/75 7.5 V
p1 = 5 lbf/in.2
V1 = 2.5 ft3
20 .
p (lbf/in^2)
15
p2 = 20 lbf/in. 2 CO2
10
V2 = 0.5 ft3 5
W
.
1
ENGINEERING MODEL: (1) The CO2 is the 0
0 0.5 1 1.5 2 2.5 3
closed system. (2) The p-V relation during the V (ft^3)
process is linear. (3) Volume change is the only
work mode.
ANALYSIS: The given p-V relation can be used with Eq. 2.17 as follows:
= -3600 ftlbf
= (negative sign denotes energy transfer in.)
Alternative Solution
Since the p-V relation is linear, W can also be evaluated geometrically as the area under the
process line:
= -4.63 Btu
Vous aimerez peut-être aussi
- Solution Manual Refrigeration and Airconditioning (Stoecker and Jones) (Ed-2)Document161 pagesSolution Manual Refrigeration and Airconditioning (Stoecker and Jones) (Ed-2)anon_909426932100% (5)
- Solution Manual for an Introduction to Equilibrium ThermodynamicsD'EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsPas encore d'évaluation
- Edr 2 ElarmoDocument15 pagesEdr 2 ElarmoArfel Marie FuentesPas encore d'évaluation
- Shaft PressurizationDocument9 pagesShaft PressurizationDesigner ForeverPas encore d'évaluation
- F1. SCT - SN-Steel Formwork Design - GTG BEAM FORMWORKDocument32 pagesF1. SCT - SN-Steel Formwork Design - GTG BEAM FORMWORKshihabPas encore d'évaluation
- Example 2: Work Done ExamplesDocument3 pagesExample 2: Work Done ExamplesKenneth OkoyePas encore d'évaluation
- EEEQ 221 Fluid Mechanics 7B - Notes For 2nd February 2018Document44 pagesEEEQ 221 Fluid Mechanics 7B - Notes For 2nd February 2018Ellie AwiPas encore d'évaluation
- Solution Manual Refrigeration and Airconditioning Stoecker and Jonesed 2 CompressDocument161 pagesSolution Manual Refrigeration and Airconditioning Stoecker and Jonesed 2 CompressskyyyxsagePas encore d'évaluation
- F1. SCT - SN-Steel Formwork Design - ST COLUMN FORMWORKDocument18 pagesF1. SCT - SN-Steel Formwork Design - ST COLUMN FORMWORKshihabPas encore d'évaluation
- Prob Set 2 SolutionsDocument12 pagesProb Set 2 SolutionsAbdulHaseebArif100% (1)
- Simple Addition Permits Voltage Control of DC-DC Converter's OutputDocument4 pagesSimple Addition Permits Voltage Control of DC-DC Converter's OutputCARLOSPas encore d'évaluation
- Analysis of Steady Flow in Pipelines2Document12 pagesAnalysis of Steady Flow in Pipelines2Shafika AliaPas encore d'évaluation
- Duct Design STDocument7 pagesDuct Design STmiladparsmanPas encore d'évaluation
- Wind LoadDocument15 pagesWind Loadmanikandan4strlPas encore d'évaluation
- Thermo HW SolutionsDocument35 pagesThermo HW SolutionsekantikdevoteePas encore d'évaluation
- Determine The Total Head Loss For Multy Story BuildingDocument16 pagesDetermine The Total Head Loss For Multy Story BuildingORINCOREPas encore d'évaluation
- 2 - Flow in Pipes Closed Conduits (Compatibility Mode)Document28 pages2 - Flow in Pipes Closed Conduits (Compatibility Mode)Eddy BongPas encore d'évaluation
- Boyles LawDocument10 pagesBoyles LawJohn VicPas encore d'évaluation
- Chapter-12 PumpDocument39 pagesChapter-12 PumpSalehin AnamPas encore d'évaluation
- Electronic Devices 9th EditionDocument15 pagesElectronic Devices 9th EditionJoshua GaldonezPas encore d'évaluation
- Solution Manual Physical Chemistry 6th EDocument20 pagesSolution Manual Physical Chemistry 6th EYasmein zweikPas encore d'évaluation
- Appendiks c2 Vacum Pan D (Dny-Smd) Fix (Sampe Halaman 62)Document209 pagesAppendiks c2 Vacum Pan D (Dny-Smd) Fix (Sampe Halaman 62)DimasPas encore d'évaluation
- Hazardous ZonesDocument12 pagesHazardous ZonesIsrael GarciaPas encore d'évaluation
- M.SC Physics PracticalDocument34 pagesM.SC Physics Practicalkapil singhPas encore d'évaluation
- 4952 PDFDocument13 pages4952 PDFSaura Shuvra KumarPas encore d'évaluation
- Bölüm 8 Çalışma SorularıDocument29 pagesBölüm 8 Çalışma SorularıBuse Nur ÇelikPas encore d'évaluation
- Ejemplo 11.1 - Mott. RDocument10 pagesEjemplo 11.1 - Mott. RDylan Navarro LPas encore d'évaluation
- General Input:-: γc= 24.5 KN/m3 fc= 25.5 N/mm2 g= 9.81 m/sec2Document2 pagesGeneral Input:-: γc= 24.5 KN/m3 fc= 25.5 N/mm2 g= 9.81 m/sec2Erika MejiasPas encore d'évaluation
- Conceptests in Chemical Engineering ThermodynamicsDocument96 pagesConceptests in Chemical Engineering ThermodynamicsSrinivas BobbyPas encore d'évaluation
- nz1c00392 Si 001Document9 pagesnz1c00392 Si 001Trần ChứcPas encore d'évaluation
- Pipe Fitting LossesDocument25 pagesPipe Fitting LossesdsdeshpandePas encore d'évaluation
- ECE 4430 - BGDocument14 pagesECE 4430 - BGCuong LaidangPas encore d'évaluation
- SHEET1 - Pipe Friction - 2023-2024Document2 pagesSHEET1 - Pipe Friction - 2023-2024pyoussef109Pas encore d'évaluation
- Middle East Technical University Department of Mechanical Engineering Me 306 Fluid Mechanics Ii (Section 4)Document11 pagesMiddle East Technical University Department of Mechanical Engineering Me 306 Fluid Mechanics Ii (Section 4)Saad KhanPas encore d'évaluation
- Solutions To Exercise Four - Bernoulli's Equation Part II: Mechanical Engineering 390 Fluid MechanicsDocument4 pagesSolutions To Exercise Four - Bernoulli's Equation Part II: Mechanical Engineering 390 Fluid MechanicsCarolina Lobo RamírezPas encore d'évaluation
- Minimum Loads: Thayer's Formula (From Steel Structures 3rd Ed 2012 by Z.A. Siddiqi, p.261)Document25 pagesMinimum Loads: Thayer's Formula (From Steel Structures 3rd Ed 2012 by Z.A. Siddiqi, p.261)JHON CLYDE SEPADAPas encore d'évaluation
- Document PDF 325Document14 pagesDocument PDF 325AYUSH ANANDPas encore d'évaluation
- Minimum Loads: Thayer's Formula (From Steel Structures 3rd Ed 2012 by Z.A. Siddiqi, p.261)Document17 pagesMinimum Loads: Thayer's Formula (From Steel Structures 3rd Ed 2012 by Z.A. Siddiqi, p.261)JHON CLYDE SEPADAPas encore d'évaluation
- Thermodynamics - ch4Document17 pagesThermodynamics - ch4Hassan AzouzPas encore d'évaluation
- Sistemas de Tuberías en Serie - Clase IDocument4 pagesSistemas de Tuberías en Serie - Clase IJair Andres Arevalo BanoyPas encore d'évaluation
- FMV Spreadsheets Master FMVIDocument17 pagesFMV Spreadsheets Master FMVIAlan GamalielPas encore d'évaluation
- Chapter 8 - Internal Flow (Pipe Flow)Document65 pagesChapter 8 - Internal Flow (Pipe Flow)Malisha NursabrinaPas encore d'évaluation
- Chapter 5 - Absorption (Part 1)Document41 pagesChapter 5 - Absorption (Part 1)La Casa Jordan100% (1)
- 2020 TK3082 SOLUSI HW 5 Dimensional Analysis Dan ModelDocument3 pages2020 TK3082 SOLUSI HW 5 Dimensional Analysis Dan ModelRefky NabiilPas encore d'évaluation
- Minimum Loads: Thayer's Formula (From Steel Structures 3rd Ed 2012 by Z.A. Siddiqi, p.261)Document31 pagesMinimum Loads: Thayer's Formula (From Steel Structures 3rd Ed 2012 by Z.A. Siddiqi, p.261)ClydeG.SepadaPas encore d'évaluation
- IEEMA Basics of Dual Ratio Transformers PDFDocument3 pagesIEEMA Basics of Dual Ratio Transformers PDFKelly chatPas encore d'évaluation
- SM CHDocument313 pagesSM CHsandwichnachosPas encore d'évaluation
- G D V L H: Calculate The Nodal PressureDocument3 pagesG D V L H: Calculate The Nodal PressureHilgan Ya MupetamiPas encore d'évaluation
- Ef152 Lec 4 4Document8 pagesEf152 Lec 4 4Adu GilbertPas encore d'évaluation
- Mast r1Document6 pagesMast r1irshad khanPas encore d'évaluation
- Bernoulis NotesDocument10 pagesBernoulis NotesTalha AmjadPas encore d'évaluation
- AEI405Document17 pagesAEI405api-26787131Pas encore d'évaluation
- Fan Power Pressure Loss CalculationDocument3 pagesFan Power Pressure Loss Calculationmashkhal luqmanPas encore d'évaluation
- ECEG 351 Electronics II Spring 2019Document3 pagesECEG 351 Electronics II Spring 2019KazaValiShaikPas encore d'évaluation
- Experiment 1 - Friction Losses in PipesDocument34 pagesExperiment 1 - Friction Losses in PipesKhairil Ikram33% (3)
- Ch17 SolutionDocument10 pagesCh17 Solutionapi-3700944Pas encore d'évaluation
- Aisc Lrfd-93 Example 002Document7 pagesAisc Lrfd-93 Example 002Rodrigo PereiraPas encore d'évaluation
- (Chapter 6) Examples PDFDocument6 pages(Chapter 6) Examples PDFKarwan GoodPas encore d'évaluation
- Electrical and Electronic Principles 3 Checkbook: The Checkbook SeriesD'EverandElectrical and Electronic Principles 3 Checkbook: The Checkbook SeriesPas encore d'évaluation
- New Text DocumentDocument1 pageNew Text DocumentAbdulHaseebArifPas encore d'évaluation
- 33-005-PCI Datasheet DigitalPendulum MATLAB 10 2013Document2 pages33-005-PCI Datasheet DigitalPendulum MATLAB 10 2013AbdulHaseebArifPas encore d'évaluation
- Serial Number IDM 6.21Document1 pageSerial Number IDM 6.21PapavikaPapavikaPas encore d'évaluation
- Akhil Jose, Clint Augustine, Shinu Mohanan Malola, Keerthi Chacko. 2015. Scientific Research PublishingDocument1 pageAkhil Jose, Clint Augustine, Shinu Mohanan Malola, Keerthi Chacko. 2015. Scientific Research PublishingAbdulHaseebArifPas encore d'évaluation
- Chapter3 PDFDocument52 pagesChapter3 PDFAbdulHaseebArifPas encore d'évaluation
- New Text DocumentDocument1 pageNew Text DocumentAbdulHaseebArifPas encore d'évaluation
- New Text DocumentDocument1 pageNew Text DocumentAbdulHaseebArifPas encore d'évaluation
- PDFDocument5 pagesPDFAbdulHaseebArifPas encore d'évaluation
- Ad Hoc-Channel PDFDocument9 pagesAd Hoc-Channel PDFAbdulHaseebArifPas encore d'évaluation
- Assignment 1 PDFDocument4 pagesAssignment 1 PDFAbdulHaseebArifPas encore d'évaluation
- 1st QuizDocument1 page1st QuizAbdulHaseebArifPas encore d'évaluation
- FDocument2 pagesFAbdulHaseebArifPas encore d'évaluation
- Prob Set 2 SolutionsDocument12 pagesProb Set 2 SolutionsAbdulHaseebArif100% (1)
- PDFDocument6 pagesPDFAbdulHaseebArifPas encore d'évaluation
- Q2 SDocument1 pageQ2 SAbdulHaseebArifPas encore d'évaluation
- Ps 1Document2 pagesPs 1AbdulHaseebArifPas encore d'évaluation
- H2 SDocument10 pagesH2 SAbdulHaseebArifPas encore d'évaluation
- sm2 16Document1 pagesm2 16AbdulHaseebArifPas encore d'évaluation
- SyllabusDocument2 pagesSyllabusAbdulHaseebArifPas encore d'évaluation
- Q1 SDocument1 pageQ1 SAbdulHaseebArifPas encore d'évaluation
- Hwk1Solution PDFDocument14 pagesHwk1Solution PDFAbdulHaseebArifPas encore d'évaluation
- Hwk2Solution PDFDocument13 pagesHwk2Solution PDFAbdulHaseebArifPas encore d'évaluation
- 8esm 02 32 PDFDocument1 page8esm 02 32 PDFAbdulHaseebArifPas encore d'évaluation
- Cammarata L & Yliniemi L (1999) Development of A Self-Tuning Fuzzy Logic Controller (STFLC) For A Rotary Dryer. December 1999 PDFDocument35 pagesCammarata L & Yliniemi L (1999) Development of A Self-Tuning Fuzzy Logic Controller (STFLC) For A Rotary Dryer. December 1999 PDFAbdulHaseebArifPas encore d'évaluation
- 5Document18 pages5Yahya Ben WalidPas encore d'évaluation
- 8 10 2009 Kou Kong PDFDocument155 pages8 10 2009 Kou Kong PDFAbdulHaseebArifPas encore d'évaluation
- 1.engineering Economics Cost Analysis PDFDocument11 pages1.engineering Economics Cost Analysis PDFAbdulHaseebArifPas encore d'évaluation
- 8 39 PDFDocument1 page8 39 PDFAbdulHaseebArifPas encore d'évaluation